Abstract
We have identified a wall hydrolytic enzyme from Trichoderma reesei with potent ability to induce extension of heat-inactivated type I cell walls. It is a small (23-kD) endo-1,4-β-glucanase (Cel12A) belonging to glycoside hydrolase family 12. Extension of heat-inactivated walls from cucumber (Cucumis sativus cv Burpee Pickler) hypocotyls was induced by Cel12A after a distinct lag time and was accompanied by a large increase in wall plasticity and elasticity. Cel12A also increased the rate of stress relaxation of isolated walls at very short times (<200 ms; equivalent to reducing t0, a parameter that estimates the minimum relaxation time). Similar changes in wall plasticity and elasticity were observed in wheat (Triticum aestivum cv Pennmore Winter) coleoptile (type II) walls, which showed only a negligible extension in response to Cel12A treatment. Thus, Cel12A modifies both type I and II walls, but substantial extension is found only in type I walls. Cel12A has strong endo-glucanase activity against xyloglucan and (1→3,1→4)-β-glucan, but did not exhibit endo-xylanase, endo-mannase, or endo-galactanase activities. In terms of kinetics of action and effects on wall rheology, wall loosening by Cel12A differs qualitatively from the action by expansins, which induce wall extension by a non-hydrolytic polymer creep mechanism. The action by Cel12A mimics some of the changes in wall rheology found after auxin-induced growth. The strategy used here to identify Cel12A could be used to identify analogous plant enzymes that cause auxin-induced changes in cell wall rheology.
In many embryophytes, the growing cell wall consists of a scaffold of cellulose microfibrils coated with (and perhaps linked together by) xyloglucans and other hemicelluloses and embedded in pectic polysaccharides (McNeil et al., 1984; McCann and Roberts, 1991; Pauly et al., 1999). This so-called “type I” wall contrasts with “type II” walls (found in grasses and related species) where the cell walls have relatively low amounts of xyloglucan and pectin but a high proportion of arabinoxylans and, at certain stages of development, (1→3,1→4)-β-glucans (Carpita and Gibeaut, 1993). Growing walls are strong enough to withstand high turgor forces, yet can become pliant enough to expand as the cell grows. Such expansion is believed to require a selective loosening of the wall, thereby permitting movement or slippage of cellulose microfibrils in response to the mechanical forces generated by cell turgor (Cosgrove, 1997). However, the exact identity and arrangement of the polymers that give the wall its mechanical strength and pliancy are uncertain (Talbott and Ray, 1992a; Whitney et al., 1999).
A long-standing hypothesis for wall loosening proposes that hydrolysis or transglycosylation of matrix glucans enables the cell wall to enlarge by weakening the matrix that holds cellulose microfibrils together (Cleland, 1981; Hayashi et al., 1984; Fry, 1989; Masuda, 1990; Fry et al., 1992). Considerable circumstantial evidence in support of this idea has amassed, particularly in the case of xyloglucan and (1→3,1→4)-β-glucan metabolism during auxin-induced growth (Carpita and Gibeaut, 1993; Inouhe et al., 1997; Inouhe et al., 2000). Moreover, this hypothesis would seem to be an inevitable outcome of some current models of cell wall architecture, where cellulose microfibrils are thought to be directly linked together by xyloglucan chains that bind to the surface of two or more microfibrils, thereby forming a tethered network (Carpita and Gibeaut, 1993; Pauly et al., 1999).
Nevertheless, a key prediction of this hypothesis has not been met; namely, that the action of wall glucanases or transglycosylases directly leads to wall extension. Previous attempts to test this idea, by applying glucanases, transglycosylases, and mixtures of wall-degrading enzymes to isolated walls clamped in an extensometer, failed to find wall extension activity (McQueen-Mason et al., 1993; Cosgrove and Durachko, 1994; Cosgrove, 1997). In contrast, purification of proteins with marked wall extension activity led to the discovery of α-expansins (McQueen-Mason et al., 1992; Li et al., 1993; Cho and Kende, 1997) and later β-expansins (Cosgrove et al., 1997). Expansins promote wall creep (time-dependent, irreversible extension), evidently without hydrolysis of the major polysaccharides of the cell wall (McQueen-Mason and Cosgrove, 1995; Cosgrove, 2000a). Because some wall polysaccharide hydrolases may make the wall more responsive to expansin action (Cosgrove and Durachko, 1994), they may be considered indirect, or secondary, wall-loosening agents, whereas expansins have the properties of a primary wall-loosening agent, i.e. expansins can directly induce wall extension (Cosgrove, 1999). Nevertheless, if microfibrils are directly tethered together by hemicelluloses, as commonly proposed (Fry, 1989; Hayashi, 1989; Pauly et al., 1999), it remains enigmatic that wall polysaccharide hydrolases fail to cause wall extension in vitro.
In this study, we examined a number of commercial wall polysaccharide hydrolase mixtures for their ability to induce wall extension. Although most mixtures failed to induce cell wall creep, we found that it is possible to cause cell walls to extend in vitro by the action of a single endoglucanase, and that the character of such wall loosening is fundamentally different from expansin-induced wall loosening.
RESULTS
Cel12A Purification and Identification
We tested several commercial “cellulase” and “pectinase” preparations for wall extension activity by adding enzymes to heat-inactivated hypocotyls clamped at a constant tensile load. We specifically looked for enzymes that induced prolonged cell wall extension without breakage. This is the method originally used to identify expansins (McQueen-Mason et al., 1992), the only plant cell wall proteins currently known to have substantial wall extension activity in vitro (Cosgrove, 2000a). Most wall-degrading enzymes did not cause wall extension at low concentrations, but at high concentrations they often caused breakage, preceded by a short period of accelerating wall extension (Fig. 1). We interpret this latter behavior to be the result of massive degradation and mechanical failure of the wall.
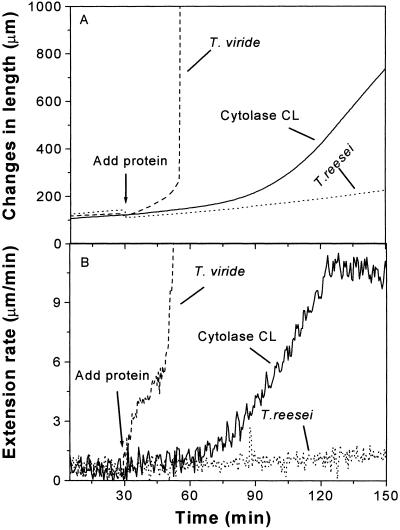
Figure 1.
Induction of extension in heat-inactivated hypocotyl cell walls by various T. reesei cellulase preparations. A, Length traces. B, Rate traces. Walls were clamped in 50 mm sodium acetate, then enzyme solution was added (arrow). Cytolase CL, Diluted 1:2,000 with 50 mm sodium acetate, pH 4.5; T. viride cellulase: Boehringer Mannheim GmbH (Indianapolis; lot 13061420-13), 250 μg mL−1 in the same buffer; Trichoderma reesei cellulase: Sigma (St. Louis; C-8546), 50 μg mL−1 in the same buffer.
In contrast, sustained wall extension was caused by low concentrations of Cytolase CL, a wall-degrading enzyme preparation from the fungus T. reesei (Fig. 1). Unlike expansin, which induces extension immediately upon application, Cytolase CL induced extension only after a marked lag, greater than 30 min in these trials with the crude enzyme. The extension rate was sustained and comparable in magnitude to the extension rate of native cucumber (Cucumis sativus cv Burpee Pickler) cell walls and of walls treated with expansin (Cosgrove, 1989; McQueen-Mason et al., 1992). Cytolase CL was the only enzyme preparation we found with this kind of activity.
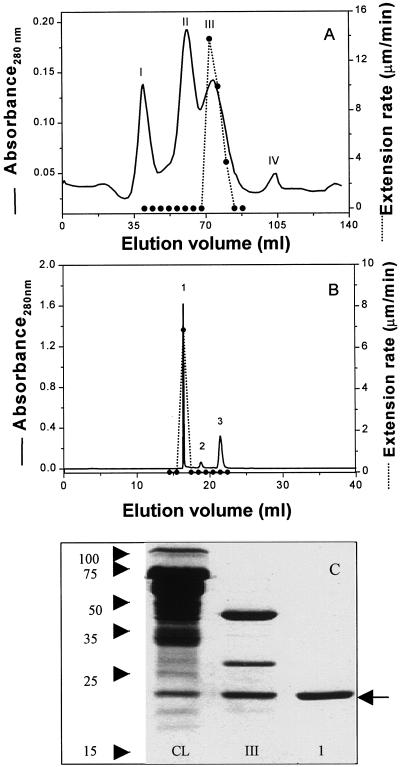
Fractionation of Cytolase CL by carboxy methyl (CM)-sepharose chromatography resulted in a single active fraction (peak III in Fig. 2A) containing three major proteins and traces of other proteins (Fig. 2C). The active fraction was further separated by HPLC (Fig. 2B) to obtain an active fraction (peak no. 1) with a single major protein of 23 kD (Fig. 2C). Table I summarizes the purification and yield of this activity. The purified fraction had hydrolytic activity against carboxymethyl cellulose (not shown). Other glucanases that hydrolyzed this cellulose derivative were also found in the Cytolase CL preparation, including cellobiohydrolase II (Cel6A; determined by protein sequencing), but they did not exhibit cell wall extension activity.
Figure 2.
Purification of an enzyme with wall extension activity. A, Fractionation of Cytolase CL by CM-sepharose chromatography yielded a single peak of activity (III). B, Peak III from A was fractionated by HPLC on a CM300 column, yielding a single peak of activity (no. 1). C, Coomassie-stained SDS-PAGE. CL, Cytolase CL; III, active peak III in A; 1, active peak no. 1 in B. Molecular size markers (in kilodaltons) are shown at left. Arrow indicates the active protein at approximately 23 kD.
Table I.
Summary of purification of wall extension-inducing protein from Cytolase CL
| Fraction | Total Protein | Total Enzyme | Specific Activity | Fold Purification | Yield |
|---|---|---|---|---|---|
| mg | units | units mg protein−1 | % | ||
| Crude enzyme | 485.6 | 20,667 | 42.6 | 1.0 | 100 |
| Fraction I from CM-sepharose | 3.35 | 901 | 269 | 6.3 | 4.4 |
| Fraction A from HPLC-CM300 | 0.76 | 749 | 984 | 23.1 | 3.6 |
Sequencing of the purified active protein (peak no. 1) found the amino terminus to be blocked, but a tryptic digest yielded the internal peptide SYQNSQIAIPQK, which exactly matches Cel12A, the family 12 endoglucanase from T. reesei. This enzyme is a minor endo-1,4-β-glucanase, variously called EG III, endoIV, and Cel12A (Vincken et al., 1997; Okada et al., 1998; Sandgren et al., 2001). We refer to it as Cel12A, following the recent nomenclature for cellulases (Henrissat et al., 1998). The cDNA for Cel12A (GenBank accession no. AB003694) predicts a protein of 23.5 kD, in conformity with our size estimate by SDS-PAGE (Fig. 2C). A previous study (Okada et al., 1998) found native Cel12A to be blocked at its amino terminus, again in conformity with our results.
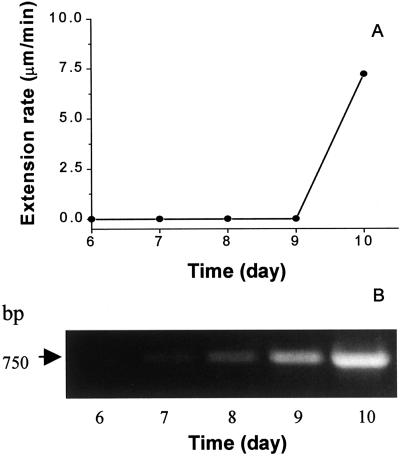
We examined T. reesei cultures to determine when the Cel12A gene was expressed and whether wall extension activity was detectable in such cultures at this time. As shown in Figure 3A, wall extension activity was detected in culture filtrates beginning on d 10, and this was coincident with the accumulation of Cel12A mRNA to a high level (Fig. 3B). These results are consistent with the conclusion that Cel12A has significant wall extension activity.
Figure 3.
Expression of extension activity and Cel12A mRNA in T. reesei cultures. A, Culture filtrates were assayed for wall extension activity using heat-inactivated hypocotyl walls. B, Reverse transcription (RT)-PCR analysis of Cel12A mRNA at varying culture times.
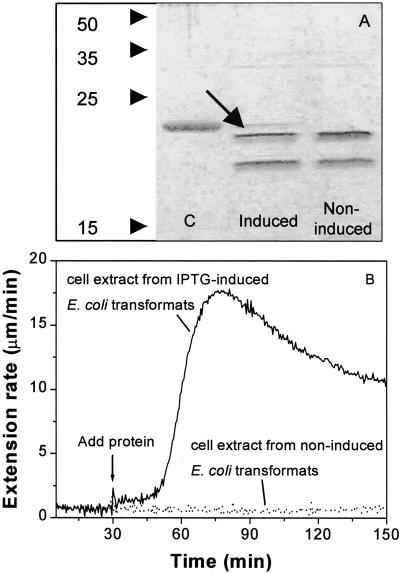
As a more direct test of this idea, we expressed Cel12A in Escherichia coli under the control of an inducible promoter (Fig. 4A). Bacterial extracts from uninduced cells harboring the expression plasmid lacked wall extension activity, whereas after induction bacterial extracts caused wall extension in a manner identical to that caused by Cytolase CL and purified Cel12A (Fig. 4B). These results confirm that Cel12A by itself is able to induce extension of plant cell walls.
Figure 4.
Expression of Cel12A in E. coli. A, SDS-PAGE analysis of proteins from E. coli harboring the Cel12A expression plasmid, with and without isopropylthio-β-d-galactoside (IPTG) induction. Each lane contains 0.3 μg of protein and was silver stained. Arrow indicates recombinant Cel12A. Lane C, Purified native Cel12A. Note: Most E. coli proteins were not soluble in the pH 4.5 extraction buffer. B, Wall extension activity of bacterial extracts from E. coli with the Cel12A expression plasmid, with and without IPTG induction.
Characteristics of Wall Loosening by Cel12A
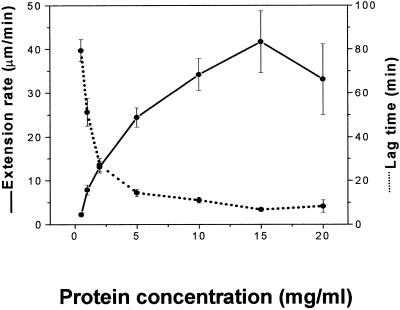
To examine the action of Cel12A further, we treated heat-inactivated cucumber walls with increasing concentrations of purified Cel12A (Fig. 5). Extension activity increased to a saturating rate of 42% h−1 (35 mm min−1). This is an exceptionally high rate, approximately four times greater than that obtained with saturating α-expansin in the same system or with “native” walls containing their endogenous wall-loosening proteins (Cosgrove, 1989; McQueen-Mason et al., 1992). The lag time before onset of rapid wall extension was reduced at higher Cel12A concentrations, but did not approach 0 min; instead, the minimum lag was 6 min (Fig. 5). During the lag, a small increase (approximately 1 μm min−1) in the extension rate was detectable (see Fig. 6). In comparison, α-expansin induces rapid wall extension without an appreciable lag (< 1 min; McQueen-Mason et al., 1992).
Figure 5.
Lag time and maximal rate of extension of heat-inactivated hypocotyl walls following treatment with varying concentrations of Cel12A purified from Cytolase CL. Means ± se of 16 measurements.
Figure 6.
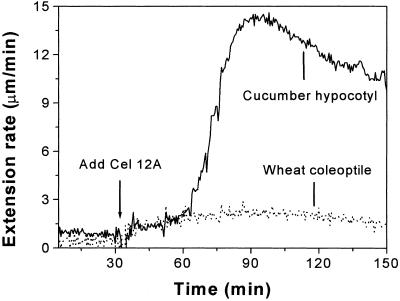
Wall extension induced by purified Cel12A in heat-inactivated hypocotyl (type I) walls and coleoptile (type II) walls. Cel12A was applied at 2 mg mL−1 to hypocotyl walls and 10 μg mL−1 to coleoptile walls. Cel12A was purified from Cytolase CL.
At 2 μg mL−1, purified Cel12A induced a high rate of extension in heat-inactivated cucumber hypocotyl walls, but its effect on the extension of heat-inactivated coleoptile walls was negligible. Even at a high concentration (10 μg mL−1) that caused nearly maximal extension rates in cucumber hypocotyls, the effect of Cel12A on coleoptile wall extension was very small (Fig. 6), comparable in magnitude with the tiny effect of Cel12A on cucumber hypocotyls during the lag time preceding onset of rapid extension. Thus, we conclude that Cel12A can induce high rates of extension in type I walls (cucumber hypocotyls), but only very low rates of extension in type II walls (wheat [Triticum aestivum cv Pennmore Winter] coleoptiles). In this respect, Cel12A is similar to α-expansin, which preferentially loosens type I walls (McQueen-Mason et al., 1992). However, wall extension induced by α-expansin lacks the pronounced lag seen with Cel12A.
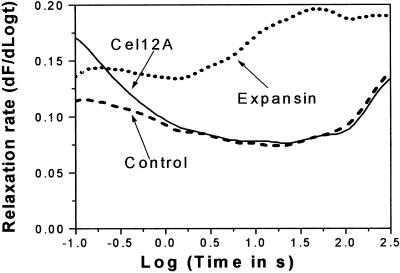
To explore further the distinctions between wall loosening by α-expansin and Cel12A, we measured additional wall rheological properties using stress/strain and stress relaxation methods. For stress relaxation assays, the wall was rapidly extended, then held to constant length and the subsequent decay in holding force was measured. The data are presented as the derivative of the decay in wall stress with respect to logarithmic time. Cel12A treatment did not change wall stress relaxation, except at very short times (<200 ms; Fig. 7). In contrast, α-expansin increased stress relaxation principally at longer times.
Figure 7.
Stress relaxation spectrum of walls pretreated with 2 μg mL−1 Cel12A or 50 μg mL−1 crude α-expansin or buffer alone (50 mm sodium acetate, pH 4.5) for 1 h at 27°C, 150 rpm shaking. Each curve is the mean of 10 replicates.
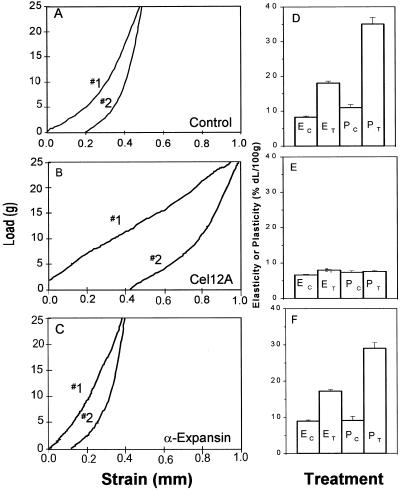
For stress/strain analyses, walls were extended in two cycles, with concomitant measurement of wall tension (Fig. 8, A–C). The second extension is reversible and is used to calculate the wall elastic compliance (E, the ratio of ▵length/▵force at the end of the extension), whereas the first extension includes an irreversible component, expressed as a plastic compliance (P, irreversible ▵length/▵force). Cel12A treatment of hypocotyl walls increased P nearly 3-fold and E nearly 2-fold (Fig. 8, B and D). In contrast, α-expansin had no effect on P and only a negligible effect on E (Fig. 8, C and E). Thus, the action of Cel12A and α-expansin differed significantly with regard to kinetics of action, stress relaxation, and stress/strain behavior.
Figure 8.
Stress/strain analysis of cell walls treated with Cel12A and α-expansin. A through C, Representative stress/strain curves of hypocotyl walls that were pre-incubated for 1 h at 27°C, 150 rpm shaking, with buffer alone (A), 2 μg mL−1 Cel12A (B), and 50 μg mL−1 crude α-expansin (C). The first extension (no. 1) is used to calculate the sum of the plastic (P) and elastic (E) compliances; the second extension (no. 2) is used to calculate the elastic compliance. D through F, Mean compliances of heat-inactivated walls ± se (units = percent change in length per 100-g force). D, Hypocotyl walls treated with Cel12; E, hypocotyl walls treated with α-expansin; F, coleoptile walls treated with Cel12. n = 15 (D) or 16 (E and F).
Cel12A treatment also increased P and E in coleoptile (type II) walls (Fig. 8F), as it did in hypocotyl walls. This was an unexpected result because Cel12A induced very little extension of coleoptile walls (Fig. 6). These changes in wall plasticity and elasticity evidently are not sufficient to cause substantial wall extension in coleoptiles.
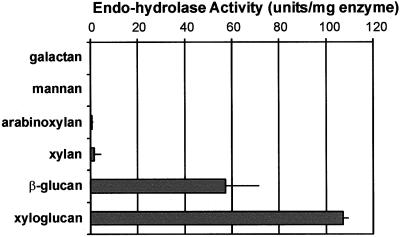
To assess the likely wall targets of Cel12A, we tested its ability to solubilize dye-coupled cross-linked polysaccharides derived from plant cell walls (Fig. 9). This assay is typically used to assess endo-type hydrolytic activity and has the virtues of great sensitivity and simplicity (McCleary, 1995). Cel12A had strong endo-hydrolytic activity against the two glucans tested [tamarind xyloglucan and barley (1→3,1→4)-β-glucan], but was inactive against oat xylan, wheat arabinoxylan, carob galactomannan, and potato galactan. All of these wall polysaccharides have a backbone made up of (1→4)-β-linked sugars. Consistent with these results, Cel12A caused clearing of Congo-Red-stained gels containing xylogucan, (1→3,1→4)-β-glucan, and carboxymethyl cellulose, but failed to cause clearing of gels containing xylan and arabinoxylan (data not shown). Likewise, when Cel12A activity was assessed by release of reducing sugars, it was found to hydrolyze xyloglucan, but was ineffective in glucomannan and arabinoxylan hydrolysis (data not shown). These results indicate that Cel12A hydrolyzes (1→4)-β-glucans, but it is inactive against other kinds of (1→4)-β-glycans. We propose that the changes in cell wall plasticity and cell wall extension caused by Cel12A result from the hydrolysis of cell wall (1→4)-β-glucans.
Figure 9.
Endo-hydrolase activity of Cel12A against cell wall polysaccharides, using dye-coupled cross-linked polysaccharides. Means + se of two measurements.
DISCUSSION
We have identified an enzyme (Cel12A) from T. reesei with a remarkable ability to make heat-inactivated hypocotyl (type I) walls extensible. The only proteins previously known with activity in this kind of assay were expansins (Cosgrove, 2000b). Cel12A is an endo-(1→4)-β-glucanase belonging to glycoside hydrolase family 12. This group of enzymes, formerly called family H (see http://afmb.cnrs-mrs.fr/∼pedro/CAZY/ghf.html) currently contains more than 20 fungal and bacterial enzymes with endoglucanase (EC 3.2.1.4) and xyloglucan hydrolase (EC not defined) activity. Cel12A does not have significant sequence similarity to expansin and its action on the wall appears to be fundamentally different from the action of expansin. In particular, Cel12A induces wall extension only after a distinctive lag time, it causes marked changes in the plastic and elastic compliances of the cell wall, and it enhances stress relaxation only at very short times (<200 ms). In contrast, expansin induces wall extension with a negligible lag (<1 min, probably diffusion limited). It accelerates wall stress relaxation at times >1 s, and it has negligible effects on wall plastic and elastic compliances. Because of these differences, we do not consider Cel12A to be an expansin, although it does have activity characteristic of a primary wall-loosening agent (Cosgrove, 1999).
The differences between expansin and Cel12A in their wall rheological effects and kinetics indicate fundamental differences in the way these two proteins loosen cell walls. Expansin alters the wall in a faster but subtler way by mediating stress-dependent slippage of the matrix polysaccharides without major change in wall structure (McQueen-Mason and Cosgrove, 1994; Cosgrove, 2000a). In contrast, Cel12A hydrolyzes an important structural polysaccharide of the cell wall, thereby leading to a major structural weakening of the wall (most readily detected as an increase in wall plasticity and elasticity). Because Cel12A readily hydrolyzes wall glucans such as xyloglucan and (1→3,1→4)-β-glucan, but not xylans, mannans, or galactans, we infer that glucan hydrolysis is the probable mechanism for Cel12A's effects on the wall. However, a curious and unexplained aspect of Cel12A action is its minimum lag time of 6 min before onset of rapid wall extension (Fig. 5), even at saturating Ce112A concentrations. Six minutes is a long time for enzymatic action. This minimum lag time suggests to us that a relatively inaccessible glucan must be attacked by Cel12A before the wall is able to extend.
The saturating rate of wall extension at high enzyme concentrations (Fig. 5) is also a notable feature of Cel12A action. If cellulose microfibrils were held together solely by glucan tethers, one would not expect the extension rate to level off at high enzyme concentrations, but instead to increase as a higher proportion of the glucan tethers were broken. The saturation behavior suggests that at high enzyme concentrations wall extension is limited by another process, for instance, viscous retardation of microfibril movement by pectins or other wall matrix components.
It is also notable that Cel12A substantially increased wall plasticity and elasticity in coleoptiles, where it caused very little wall extension. These results indicate that glucans, such as xyloglucan and/or (1→3,1→4)-β-glucan, make important structural contributions to the coleoptile (type II) cell wall, but that glucan hydrolysis alone is not sufficient to allow the coleoptile wall to extend. A likely explanation (S. Yuan and D.J. Cosgrove, unpublished data) is that the coleoptile wall contains two significant polysaccharide networks that in parallel bind microfibrils together, and both networks must be loosened to permit significant wall extension. Because Cel12A affects only the glucan network, the wall is unable to extend under Cel12A action. These results also have implications for previous studies of coleoptile walls, where auxin-induced changes in wall rheology (plasticity and minimum relaxation time) were interpreted to mean the wall was more extensible (Yamamoto et al., 1970; Cleland, 1984; Masuda, 1990). Our results show that increases in plasticity are not sufficient to cause the coleoptile wall to extend in vitro, and thus the connection between an increase in wall plasticity and an ability to extend is an indirect one in the coleoptile.
The effects of Cel12A mimic changes in wall rheology found in excised tissues after growth induction by auxin. Auxin treatment leads to a gradual (1–3 h) increase in wall plasticity and elasticity, seen in stress/strain (“Instron”) analysis (Cleland, 1984), and an enhancement of stress relaxation at very short times (Yamamoto et al., 1970; Masuda, 1990). The stress relaxation of wall specimens has been studied in greatest detail by Masuda, Yamamoto, and coworkers, who have typically presented their data in terms of an idealized model of wall relaxation (Yamamoto et al., 1970), where the parameter they called t0 (minimum relaxation time) roughly corresponds to stress relaxation behavior at very short times (20–200 ms). Auxin treatment of excised segments for 1 to several h results in a gradual reduction in t0 (Yamamoto et al., 1970; Masuda, 1990). This reduction in t0 corresponds—to a first approximation—to the enhancement of stress relaxation at short times observed in Cel12A-treated walls (Fig. 7). Our results with Cel12A suggest that the reduction in t0 and the increase in plasticity reflect the same changes in wall structure and, furthermore, that the structural modifications of the wall caused by Cel12A may be similar to those resulting from auxin treatment.
In dicots, auxin treatment leads to changes in xyloglucan size distribution (Nishitani and Masuda, 1983; Talbott and Ray, 1992b; Hoson et al., 1993), but the specific enzymes involved in these wall modifications have not yet been identified with certainty. In this context, it is important to point out that plants are not known to contain family 12 glycoside hydrolases and therefore plant homologs of Cel12A probably do not exist. The Arabidopsis genome, for example, does not contain a homolog of Cel12A. However, plants do have many wall hydrolases, some of which may have the specific wall-loosening activities shown here for Cel12A. Some candidate wall-loosening enzymes have emerged from previous studies (Fry et al., 1992; Okazawa et al., 1993; Fry, 1995; Matsumoto et al., 1997; Takeda et al., 1997; Thomas et al., 2000), but so far none has been shown to be able to cause walls to extend in vitro (McQueen-Mason et al., 1993; Cosgrove, 1999). Therefore, direct assays of analogous plant enzymes are needed to establish whether or not they have wall extension activity and may play a direct or indirect wall-loosening role.
Finally, it is worth pointing out that the lag time and the effects on wall plasticity by Cel12A conform to the conventional expectations of a wall loosening enzyme, i.e. one that hydrolyzes matrix polysaccharides, thereby weakening the network that holds cellulose microfibrils in place. The minimum lag time (6 min) before onset of wall extension suggests to us that a relatively inaccessible (1→4)-β-glucan must be digested before the wall becomes extensible. How this might relate to different xyloglucan domains within the cell wall (Pauly et al., 1999) remains to be investigated. Cel12A action contrasts markedly with expansin action, which does not cause wall breakdown. Cel12A action also contrasts with the activity of most other wall-degrading enzymes that we have tested, which do not elicit prolonged wall extension in vitro. Cel12A thus appears to have a selective action that is lacking in many other endo-1,4-β-glucanases. This selective action may reside in Cel12A's ability to gain access to and hydrolyze key structural glucans in the wall, which are not attacked by other endo-1,4-β-glucanases. In this regard, it is notable that Cel12A is used commercially as a “depilling” agent to remove fuzz from cotton clothing, whereas more aggressive cellulases do not have this selective action. Cel12A's unusual actions on wall extension and on depilling may have a common underlying mechanism of action, which at this time is not well understood. Another study (S. Yuan, L.-C. Li, and D.J. Cosgrove, unpublished data) will examine the enzymatic basis for Cel12A's induction of wall extension.
MATERIALS AND METHODS
Plant Materials
Seeds of cucumber (Cucumis sativus cv Burpee Pickler, W. Atlee Burpee Co., Warminster, PA) were sown on Kimpak Seed Germination Paper K-22 (Seedburo Equipment Company, Chicago), soaked with distilled water, and grown in the dark at 27°C for 4 d. For extension assays, hypocotyls approximately 7 cm long were excised and frozen at −20°C. Seeds of wheat (Triticum aestivum cv Pennmore Winter, from the Pennsylvania State University Department of Agronomy) were germinated in the dark in wet vermiculite for 3 d (coleoptile length approximately 3 cm). The coleoptiles were excised and frozen at − 20°C.
Wall Extension Assays
Frozen hypocotyls or coleoptiles were abraded with carborundum, washed in water, inactivated by boiling water for 15 s, pressed to remove excess water, clamped in a constant load extensometer (Cosgrove, 1989) with a 20-g load, and incubated in 50 mm sodium acetate, pH 4.5, at 27°C. Enzymes were added individually or in combination and changes in wall length were recorded at 30-s intervals (McQueen-Mason et al., 1992). Crude microbial cellulase and pectinase preparations from Sigma, Boehringer Mannheim, and CalBioChem (San Diego) were tested. Cytolase CL was from Gist-brocades International B.V. (Lille, France).
Stress/Strain (Instron) Assays
Wall specimens were prepared as above, treated for a given time with 50 mm sodium acetate, pH 4.5, with or without Cel12A or α-expansin, then stored on ice. Each specimen was clamped in a tensile tester with a 3-mm distance between the clamps (Cosgrove, 1989) and reversibly extended in two cycles at 3 mm per min until a force of 25g was reached, then immediately returned to the original length. For each of the two extensions, the stress/strain data were fitted to a second-degree polynomial by the least-squares method and the slope (compliance) at the end of the cycle was calculated from the fitted polynomial. Plastic and elastic compliances are expressed as percent extension per 100g force and are corrected for the extension that occurs during the measurement.
Stress Relaxation Assays
Wall specimens were prepared as above, treated for a given time with 50 mm sodium acetate, pH 4.5, with or without Cel12A or α-expansin, then stored on ice. Each specimen was clamped in a tensile tester (Cosgrove, 1989) with a 5-mm distance between the clamps and extended at a rate of 170 mm per min until a force of 20 g was attained. Thereafter, the clamps were held at a constant distance and the holding force was recorded during the subsequent 5 min by a microcomputer with a minimum sampling time of 2 ms, gradually increasing to 2 s. The relaxation rate is displayed as the derivative of the force with respect to log time.
Extraction of α-Expansin
Cucumber hypocotyls were grown as above and extracted for wall protein as described previously (McQueen-Mason et al., 1992). Protein was precipitated by addition of 0.113 g mL−1 (NH4)2SO4 and discarded. Protein precipitated by addition of another 0.260 g mL−1 (NH4)2SO4 was retained as the crude α-expansin fraction and stored at −80°C.
Cel12A Purification and Identification
Ten milliliters of Cytolase CL was diluted 10× with buffer A (20 mm sodium acetate, pH 4.5), and loaded onto a CM-Sepharose Fast Flow column (Sigma; 1 × 17 cm, 4°C) pre-equilibrated with buffer A. Protein fractions were eluted at a flow rate of 0.4 mL min−1 with a linear gradient of 0 to 0.5 m NaCl in buffer A. The active fraction (no. III) was desalted by membrane ultrafiltration and loaded onto a SynChropak CM300 HPLC column (250 × 4.6 mm, Micra Scientific, Darien, IL). Proteins were eluted at a flow rate of 1 mL min−1 with a 0 to 0.15 m NaCl linear gradient in 10 mm sodium acetate (pH 4.5). Fractions were tested for wall extension activity after dilution 1:50 with 50 mm sodium acetate, pH 4.5.
For Cel12A sequencing, HPLC-purified protein was further separated by SDS-PAGE, then either electroblotted onto a polyvinylidene difluoride membrane for sequencing of the amino terminus, or digested with trypsin, followed by HPLC separation of peptides and sequencing. All protein sequencing was done by the Wistar Protein Microsequencing Facility (Philadelphia).
Trichoderma reesei Culture and RNA Analysis
T. reesei strain QM9414 (American Type Culture Collection 26921) was grown on Vogel's medium agar with 1% (w/v) lactose (Sandhu and Kalra, 1982). For assays of Cel12A activity and mRNA accumulation, a mycelium disc (approximately 5-mm diameter) was taken from 4-d-old colonies and used to inoculate 50 mL of Vogel's medium with 1% (w/v) lactose (Sandhu and Kalra, 1982) in 250-mL flasks. The cultures were grown for up to 10 d as a stationary culture at 27°C. For activity assays, culture filtrates were diluted 1:1 with 50 mm sodium acetate, pH 4.0, and assayed directly. For Cel12A mRNA analysis, total RNA was extracted from mycelia grown in 25 mL of culture broth, using the RNeasy Plant Mini Kit (Qiagen, Valencia, CA) and digested with RNAase-free DNAase I on a Qiagen RNeasy Mini Spin Column, following the manufacturer's instructions. First strand cDNAs were synthesized with oligo(dT)12-18 primer from total RNA using the Superscript pre-amplification system (Gibco BRL, Rockville, MD). Five milligrams of total RNA was used in a 20-μL RT reaction for first stand cDNA synthesis. For cDNA amplification, 1 μL of the RT product was added to a 20-μL PCR mixture and amplified by 27 cycles of PCR using sequence-specific primers for Cel12A (sense, 5′-GTCGGATCCAT-GAAGTTCCTTCAAGTCCTC -3′; and antisense, 5′-AGTT-GAAAGCTTAGTTGATAGATGCGGTCC-3′) and Display TAQ FL DNA polymerase (PGC Scientifics Co., Gaithersburg, MD). The RT-PCR product was determined by 1% (w/v) agarose gel electrophoresis.
Cel12A Expression in Escherichia coli
The Cel12A expression plasmid (available upon request) was constructed by cloning the RT-PCR product encoding mature Cel12A (without signal peptide) from T. reesei into pETBlue-2 (Novagen, Madison, WI). cDNA was synthesized from total RNA with oligo(dT) primer and Superscript (Gibco BRL). Cel12A cDNA was amplified with sequence-specific primers (sense, 5′-CAATTACCATGGCCCAAACCAGCTGTG-3′; and antisense, 5′-AGTTGAAA-GCTTAGTTGATAGATGCGGTCC-3′). The PCR product was digested with NcoI and HindIII and ligated into pETBlue-2, creating pETBlue-2-mcel12A. The added NcoI site at the 5′ end of the Cel12A cDNA resulted in two extra amino acids (Met-Ala-) at the N terminus of the recombinant protein. For expression, pETBlue-2-mcel12A was transformed into E. coli Tuner (DE3) pLacI cells (Novagen). Transformants were grown at 37°C in LB-1% (w/v) Glc supplemented with 50 μg mL−1 carbenicillin and 34 μg mL−1 chloramphenicol, to an absorbance (600 nm) of 0.5 to 1.0, then IPTG was added to the culture medium (1-mm final concentration) for induction of recombinant protein synthesis. After 2 to 3 h, the cells were pelleted and resuspended in 10 mm Tris-HCl (pH 7.5) containing 100 μg mL−1 lysozyme and 1 mm phenylmethanesulfonyl fluoride. The mixture was incubated at 30°C for 15 min and adjusted with concentrated stocks to 50 mm sodium acetate buffer (pH 4.5) and 10 mm dithiothreitol. The cells were sonicated on ice, pelleted, and the resulting supernatant was used at full strength in wall extension assays.
Cel12A Activity against Different Wall Polysaccharides
The polysaccharide preference of Cel12A for endo-hydolase activity was assessed by measuring the solubilization of azurine-coupled cross-linked polysaccharides (Megazyme International, Bray, Ireland). For each assay, 10 mg of insoluble dye-coupled polysaccharide was mixed with 0.25 mL of 25 mm sodium acetate, pH 4.5, ±Cel12A (final enzyme concentration of 0.1 μg mL−1). The mixture was incubated for 60 min at 37°C with agitation, then 1.0 mL of 2% (w/v) Tris base was added to stop the reaction. The mixture was centrifuged to pellet the residual insoluble polysaccharide and the absorbance of the supernatant was measured at 590 nm. Enzyme activity was measured in units, defined as the amount of enzyme resulting in an absorbency of 0.24 at 590 nm in a 1-cm cuvette.
Endo-hydrolyase activity was also assessed by Congo Red staining of polysaccharides embedded into agarose gels (Wood et al., 1988). For this assay, 0.1% (w/v) polysaccharide was incorporated into a layer of 1% (w/v) agarose gel containing 50 mm sodium acetate, pH 4.5. Approximately 1 mg of Cel12 was applied to the agar surface in 5 mL sodium acetate buffer and the plate was incubated overnight. Breakdown of the polysaccharide was detected by staining with 0.1% (w/v) Congo Red, followed by destaining in 1 m NaCl. Unstained regions indicated polysaccharide breakdown.
Cel12A hydrolysis of tamarind xyloglucan, Konjac glucomannan, and wheat arabinoxylan (Megazyme) was also measured by colorimetric determination of reducing sugars producing during digestion with Cel12A. Polysaccharide powder was dissolved or suspended in 50 mm sodium acetate, pH 5.5, to a concentration of 0.5 mg mL−1. Cel12A was added to a final concentration of 0.1 mg mL−1 and the mixture was incubated at 37°C for 0 and 2 h, followed by reaction with p-hydroxybenzoic acid hydrazide and absorbance measurement at 410 nm (Lever, 1972). The increase in absorbance between zero time and 2 h was taken as an indicator of polysaccharide hydrolysis. Glc solutions were used as standard equivalents of reducing sugars.
ACKNOWLEDGMENTS
We thank Lian-Chao Li, Mark Shieh, and Daniel M. Durachko for advice and technical assistance.
Footnotes
This work was supported by a grant from the U.S. Department of Energy.
LITERATURE CITED
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Cho HT, Kende H. Expansins in deepwater rice internodes. Plant Physiol. 1997;113:1137–1143. doi: 10.1104/pp.113.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland RE. Wall extensibility: hormones and wall extension. In: Tanner W, Loewus FA, editors. Encyclopedia of Plant Physiology, New Series: Plant Carbohydrates II: Extracellular Carbohydrates. Berlin: Springer; 1981. pp. 225–276. [Google Scholar]
- Cleland RE. The Instron technique as a measure of immediate-past wall extensibility. Planta. 1984;160:514–520. doi: 10.1007/BF00411139. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Characterization of long-term extension of isolated cell walls from growing cucumber hypocotyls. Planta. 1989;177:121–130. [PubMed] [Google Scholar]
- Cosgrove DJ. Relaxation in a high-stress environment: the molecular bases of extensible cell walls and cell enlargement. Plant Cell. 1997;9:1031–1041. doi: 10.1105/tpc.9.7.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:391–417. doi: 10.1146/annurev.arplant.50.1.391. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000a;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Expansive growth of plant cell walls. Plant Physiol Biochem. 2000b;38:1–16. doi: 10.1016/s0981-9428(00)00164-9. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ, Bedinger P, Durachko DM. Group I allergens of grass pollen as cell wall-loosening agents. Proc Natl Acad Sci USA. 1997;94:6559–6564. doi: 10.1073/pnas.94.12.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ, Durachko DM. Autolysis and extension of isolated walls from growing cucumber hypocotyls. J Exp Bot. 1994;45:1711–1719. doi: 10.1093/jxb/45.special_issue.1711. [DOI] [PubMed] [Google Scholar]
- Fry SC. Cellulases, hemicelluloses and auxin-stimulated growth: a possible relationship. Physiol Plant. 1989;75:532–536. [Google Scholar]
- Fry SC. Polysaccharide-modifying enzymes in the plant cell wall. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:497–520. [Google Scholar]
- Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem J. 1992;282:821–828. doi: 10.1042/bj2820821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T. Xyloglucans in the primary cell wall. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:139–168. [Google Scholar]
- Hayashi T, Wong YS, Maclachlan G. Pea xyloglucan and cellulose: II. Hydrolysis by pea endo-1,4-β-glucanases. Plant Physiol. 1984;75:605–610. doi: 10.1104/pp.75.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrissat B, Teeri TT, Warren RAJ. A scheme for designating enzymes that hydrolyze the polysaccharides in the cell walls of plants. FEBS Lett. 1998;425:352–354. doi: 10.1016/s0014-5793(98)00265-8. [DOI] [PubMed] [Google Scholar]
- Hoson T, Sone Y, Misaki A, Masuda Y. Role of xyloglucan breakdown in epidermal cell walls for auxin-induced elongation of azuki bean epicotyl segments. Physiol Plant. 1993;87:142–147. [Google Scholar]
- Inouhe M, Hayashi K, Thomas BR, Nevins DJ. Exo- and endoglucanases of maize coleoptile cell walls: their interaction and possible regulation. Int J Biol Macromol. 2000;27:157–162. doi: 10.1016/s0141-8130(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Inouhe M, Mcclellan M, Nevins DJ. Developmental regulation of polysaccharide metabolism and growth in the primary cells walls of maize. Int J Biol Macromol. 1997;21:21–28. doi: 10.1016/s0141-8130(97)00036-6. [DOI] [PubMed] [Google Scholar]
- Lever M. A new reaction for colorimetric determination of carbohydrates. Anal Biochem. 1972;47:273–279. doi: 10.1016/0003-2697(72)90301-6. [DOI] [PubMed] [Google Scholar]
- Li Z-C, Durachko DM, Cosgrove DJ. An oat coleoptile wall protein that induces wall extension in vitro and that is antigenically related to a similar protein from cucumber hypocotyls. Planta. 1993;191:349–356. [Google Scholar]
- Masuda Y. Auxin-induced cell elongation and cell wall changes. Bot Mag Tokyo. 1990;103:345–370. [Google Scholar]
- Matsumoto T, Sakai F, Hayashi T. A xyloglucan-specific endo-1,4-β-glucanase isolated from auxin-treated pea stems. Plant Physiol. 1997;114:661–667. doi: 10.1104/pp.114.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MC, Roberts K. Architecture of the primary cell wall. In: Lloyd C, editor. Cytoskeletal Basis of Plant Growth and Form. London: Academic Press; 1991. pp. 109–129. [Google Scholar]
- McCleary BV. Measurement of polysaccharide-degrading enzymes in plants using chromogenic and colorimetric substrates. In: Skerrit JH, Appels R, editors. New Diagnostics in Crop Sciences. Oxon, UK: CAB International; 1995. pp. 277–301. [Google Scholar]
- McNeil M, Darvill AG, Fry SC, Albersheim P. Structure and function of the primary cell walls of plants. Ann Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- McQueen-Mason S, Cosgrove DJ. Disruption of hydrogen bonding between wall polymers by proteins that induce plant wall extension. Proc Natl Acad Sci USA. 1994;91:6574–6578. doi: 10.1073/pnas.91.14.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason S, Cosgrove DJ. Expansin mode of action on cell walls: analysis of wall hydrolysis, stress relaxation, and binding. Plant Physiol. 1995;107:87–100. doi: 10.1104/pp.107.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason S, Durachko DM, Cosgrove DJ. Two endogenous proteins that induce cell wall expansion in plants. Plant Cell. 1992;4:1425–1433. doi: 10.1105/tpc.4.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason S, Fry SC, Durachko DM, Cosgrove DJ. The relationship between xyloglucan endotransglycosylase and in vitro cell wall extension in cucumber hypocotyls. Planta. 1993;190:327–331. doi: 10.1007/BF00196961. [DOI] [PubMed] [Google Scholar]
- Nishitani K, Masuda Y. Auxin-induced changes in the cell wall xyloglucans: effects of auxin on the two different subfractions of xyloglucans in the epicotyl cell wall of Vigna angularis. Plant Cell Physiol. 1983;24:345–355. [Google Scholar]
- Okada H, Tada K, Sekiya T, Yokoyama K, Takahashi A, Tohda H, Kumagai H, Morikawa Y. Molecular characterization and heterologous expression of the gene encoding a low-molecular-mass endoglucanase from Trichoderma reesei QM9414. Appl Environ Microbiol. 1998;64:555–563. doi: 10.1128/aem.64.2.555-563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa K, Sato Y, Nakagawa T, Asada K, Kato I, Tomita E, Nishitani K. Molecular cloning and cDNA sequencing of endoxyloglucan transferase, a novel class of glycosyltransferase that mediates molecular grafting between matrix polysaccharides in plant cell walls. J Biol Chem. 1993;268:25364–25368. [PubMed] [Google Scholar]
- Pauly M, Albersheim P, Darvill A, York WS. Molecular domains of the cellulose/xyloglucan network in the cell walls of higher plants. Plant J. 1999;20:629–639. doi: 10.1046/j.1365-313x.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- Sandgren M, Shaw A, Ropp TH, Wu S, Bott R, Cameron AD, Stahlberg J, Mitchinson C, Jones TA. The X-ray crystal structure of the Trichoderma reesei family 12 endoglucanase 3, Cel12A, at 1.9 A resolution. J Mol Biol. 2001;308:295–310. doi: 10.1006/jmbi.2001.4583. [DOI] [PubMed] [Google Scholar]
- Sandhu DK, Kalra MK. Production of cellulase, xylanase and pectinase by Trichoderma longibrachitum on different substrates. Trans Br Mycol Soc. 1982;19:409–413. [Google Scholar]
- Takeda T, Sakai F, Hayashi T. Expression of gene encoding endo-1,4-β-glucanase in suspension-cultured poplar (Poplus alba L.) cells. Biosci Biotechnol Biochem. 1997;61:907–908. doi: 10.1271/bbb.61.907. [DOI] [PubMed] [Google Scholar]
- Talbott LD, Ray PM. Molecular size and separability features of pea cell wall polysaccharides: implications for models of primary wall structure. Plant Physiol. 1992a;92:357–368. doi: 10.1104/pp.98.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Ray PM. Changes in molecular size of previously deposited and newly synthesized pea cell wall matrix polysaccharides. Plant Physiol. 1992b;98:369–379. doi: 10.1104/pp.98.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BR, Inouhe M, Simmons CR, Nevins DJ. Endo-1,3;1,4-beta-glucanase from coleoptiles of rice and maize: role in the regulation of plant growth. Int J Biol Macromol. 2000;27:145–149. doi: 10.1016/s0141-8130(00)00110-0. [DOI] [PubMed] [Google Scholar]
- Vincken J-P, Beldman G, Voragen AG. Substrate specificity of endoglucanases: what determines xyloglucanase activity? Carbohydr Res. 1997;298:299–310. doi: 10.1016/s0008-6215(96)00325-4. [DOI] [PubMed] [Google Scholar]
- Whitney SEC, Gothard MGE, Mitchell JT, Gidley MJ. Roles of cellulose and xyloglucan in determining the mechanical properties of primary plant cell walls. Plant Physiol. 1999;121:657–663. doi: 10.1104/pp.121.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PJ, Erfles TD, Teather RM. Use of complex formation between Congo Red and polysaccharides in detection and assay of polysaccharide hydrolases. Meth Enzymol. 1988;160:59–86. [Google Scholar]
- Yamamoto R, Shinozaki K, Masuda Y. Stress-relaxation properties of plant cell walls with special reference to auxin action. Plant Cell Physiol. 1970;11:947–956. [Google Scholar]