Abstract
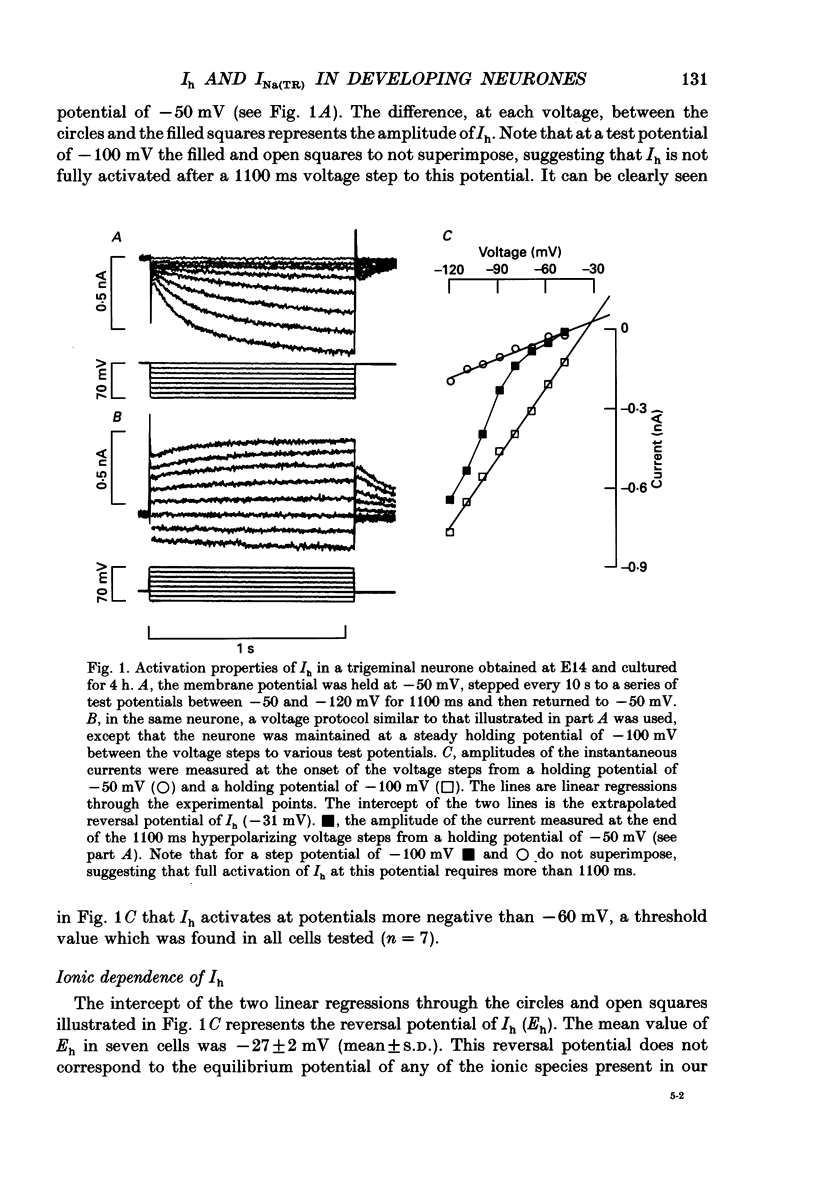
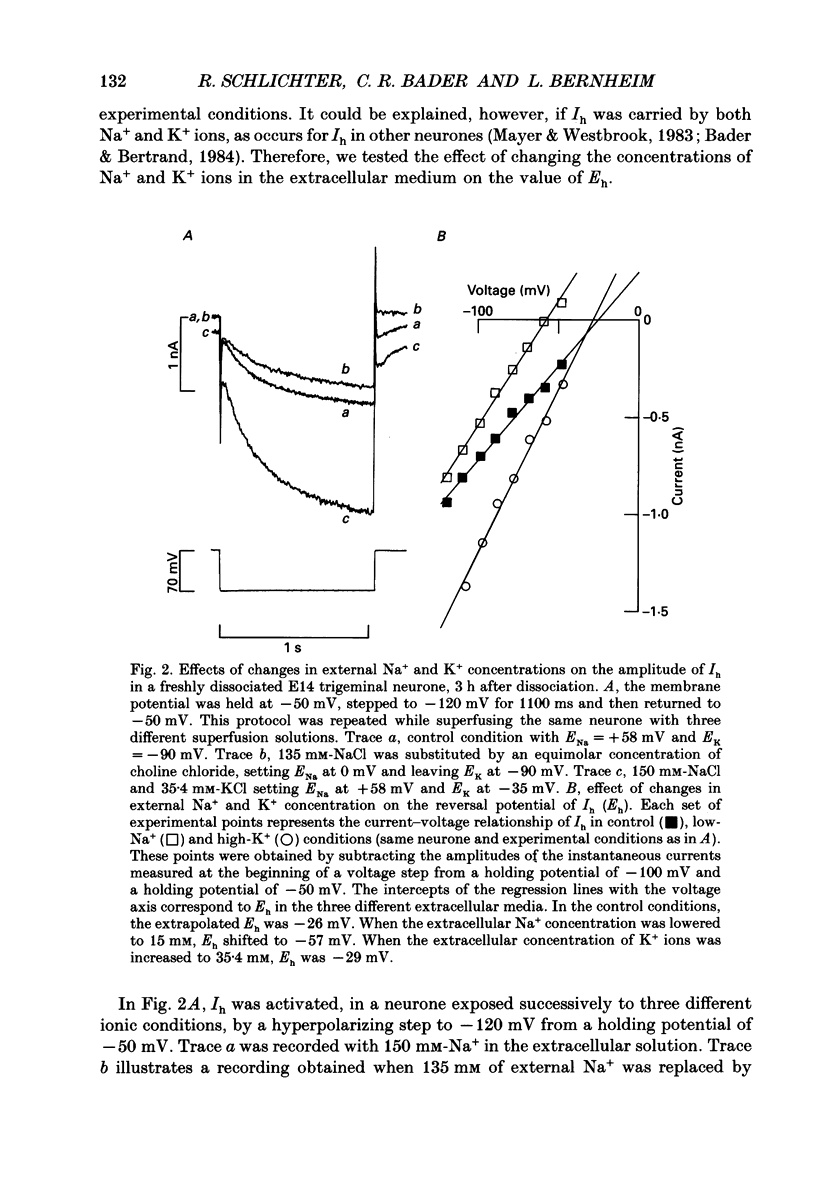
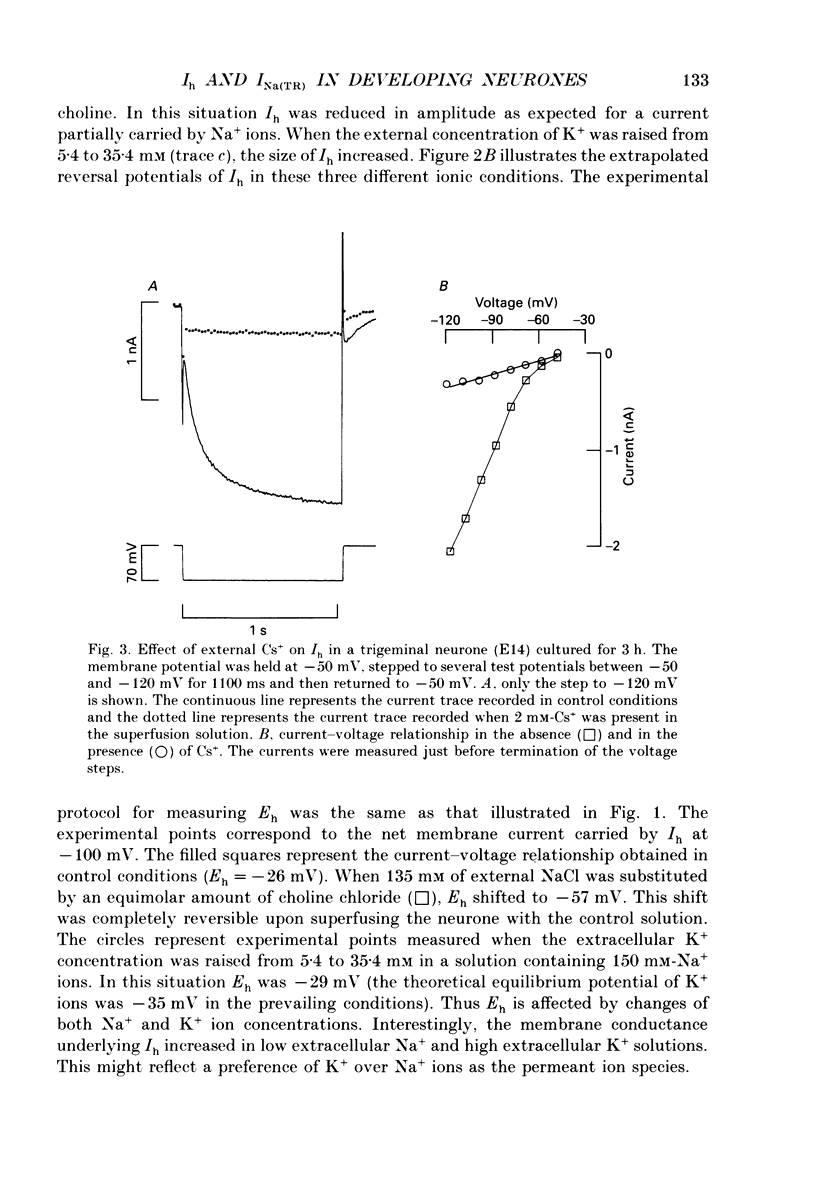
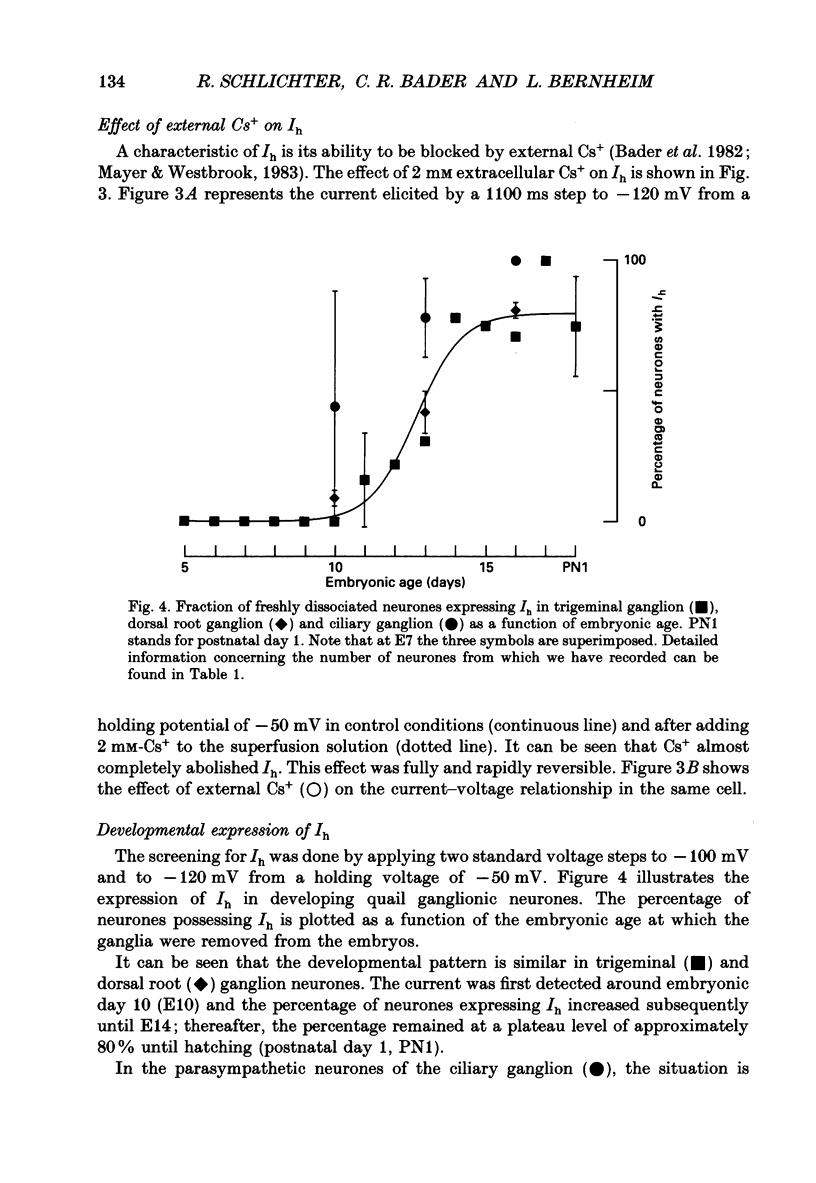
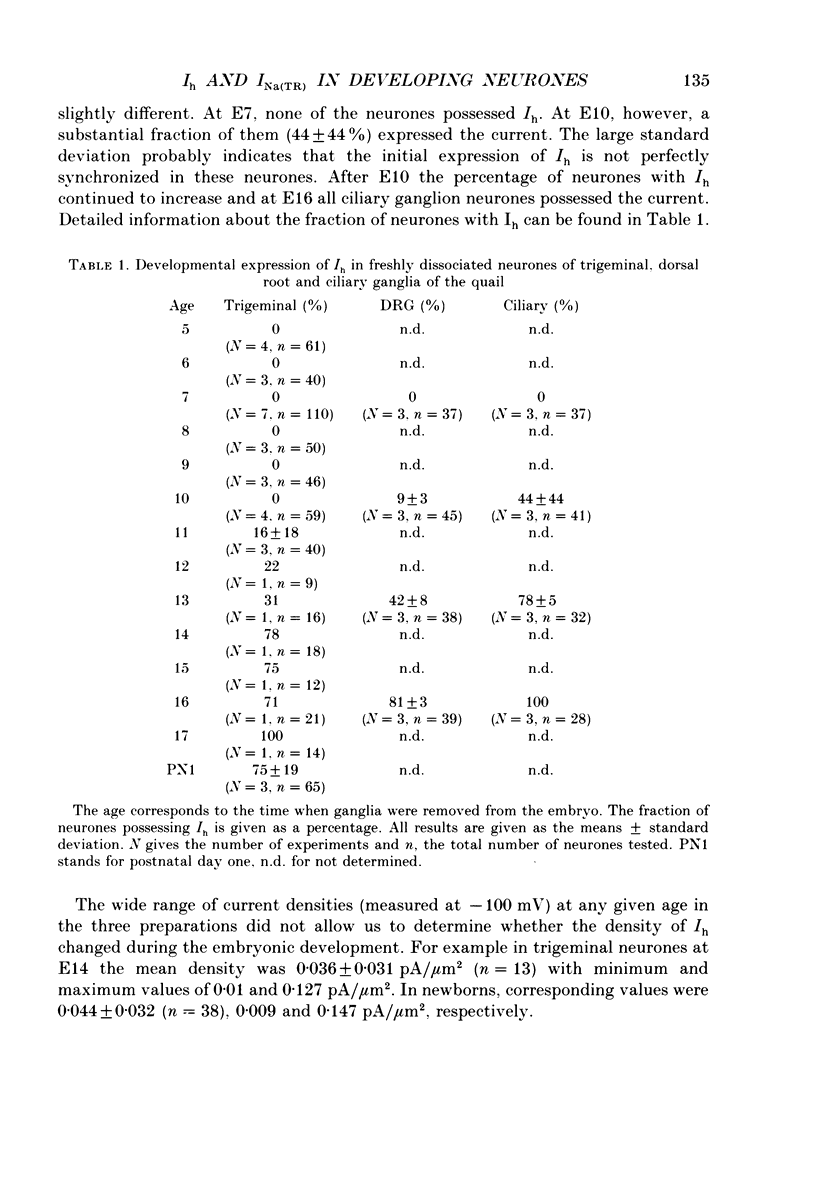
1. The developmental expression of an inwardly rectifying current activated by membrane hyperpolarization (Ih) and of a tetrodotoxin (TTX)-resistant Na+ current (INa(TR)) was studied using freshly dissociated ganglionic quail neurones of various embryonic ages. This work was carried out on parasympathetic (ciliary) and sensory (trigeminal and dorsal root) ganglion neurones with the whole-cell configuration of the patch-clamp technique. 2. In sensory and parasympathetic neurones, Ih was activated at potentials more negative than -60 mV and displayed strong inward rectification. No sign of time- or voltage-dependent inactivation was apparent. Ih was carried by both Na+ and K+ ions and was selectively and reversibly blocked by extracellular Cs+. 3. During the development of sensory neurones, Ih was observed for the first time between embryonic day 10 (E10) and E11 and the percentage of neurones expressing the current increased subsequently, reaching a plateau level of about 80% at E14. In the parasympathetic neurones of the ciliary ganglion, Ih was already detected at E10 and the percentage of neurones possessing the current increased until E16, a stage at which all neurones were found to express Ih. 4. In the presence of TTX (1 microM), an inward Na+ current, INa(TR), was recorded in sensory neurones after E12. This current was activated at potentials more depolarized than -30 mV and its amplitude was maximal at +5 mV. INa(TR) showed time- and voltage-dependent inactivation. Half-maximal steady-state inactivation was observed at -40 mV. 5. INa(TR) was observed for the first time after E12 in sensory neurones and the percentage of neurones with INa(TR) increased until E14. Thereafter, 80% of the neurones had the current. In contrast, INa(TR) was never observed in the parasympathetic neurones of the ciliary ganglion during embryonic development. 6. Our results with parasympathetic and sensory neurones suggest that the expression of INa(TR) is linked to the phenotype and not to the embryonic origin of a neurone.
Full text
PDF



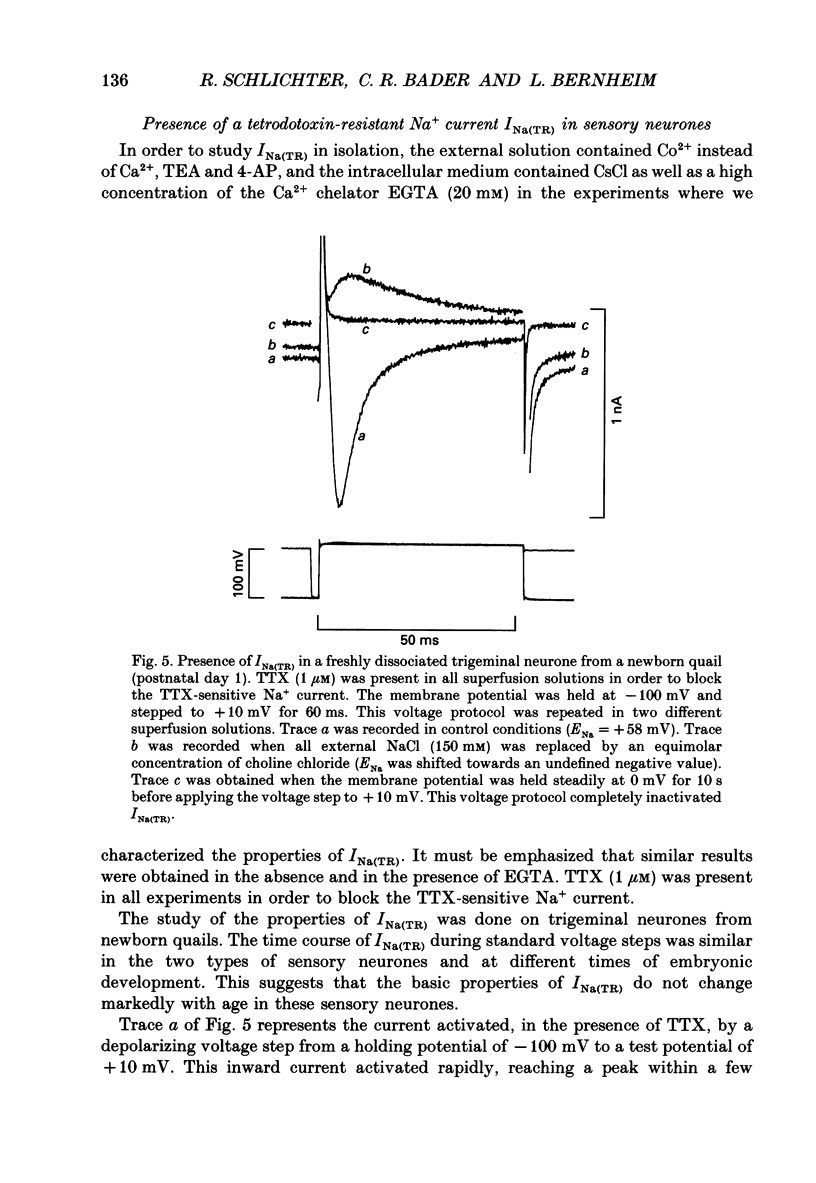
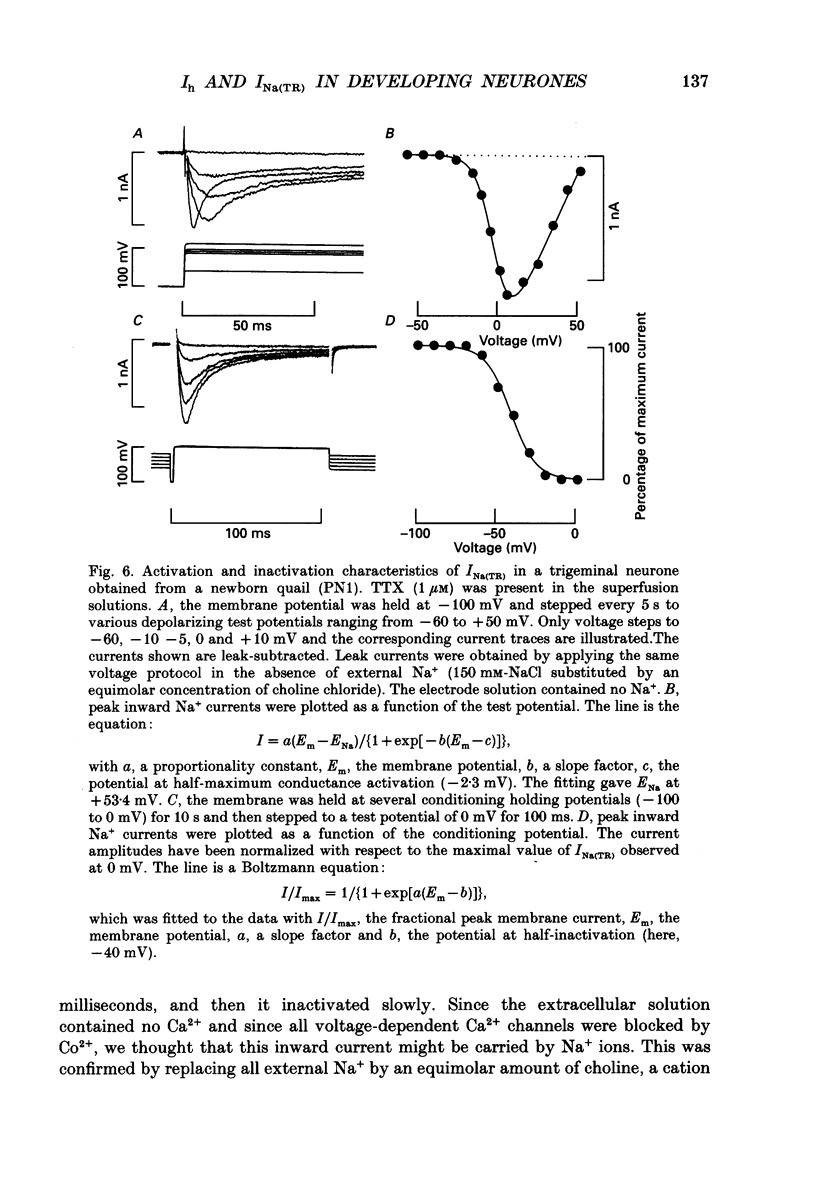
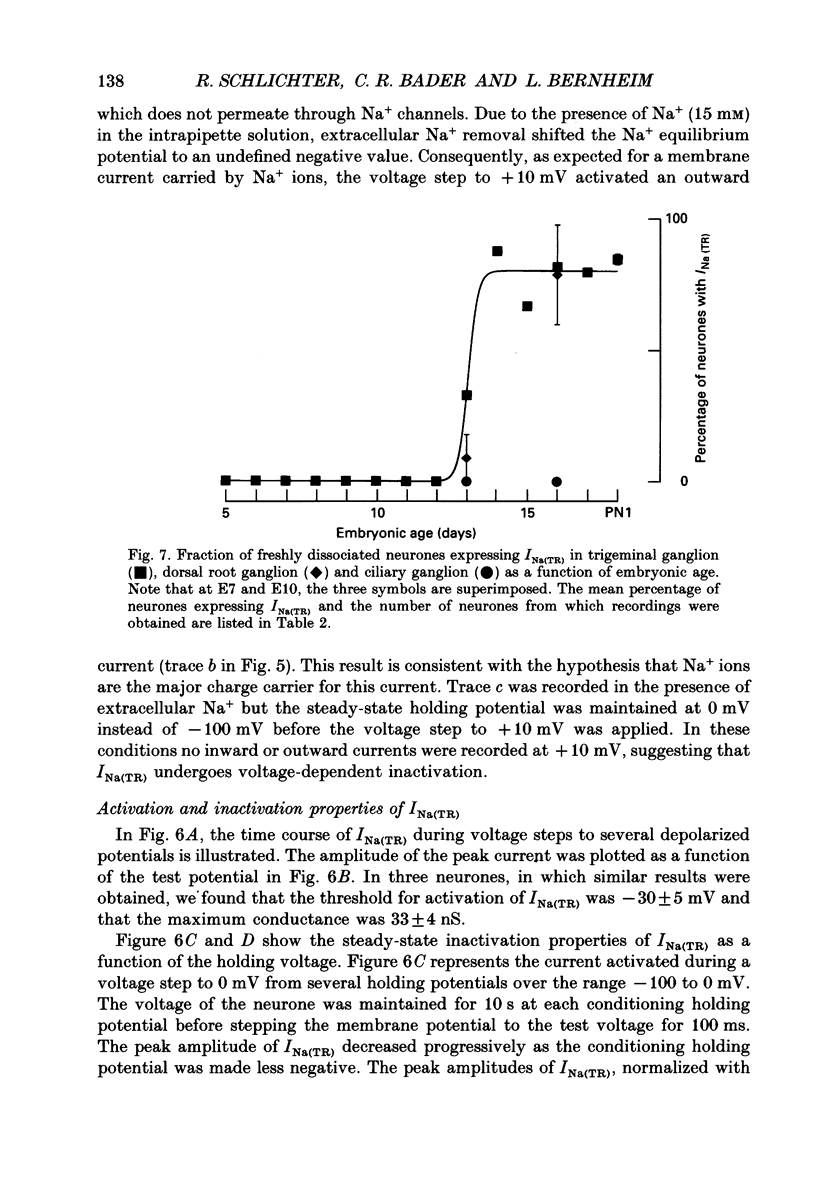







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J. The neural crest cell lineage problem: neuropoiesis? Neuron. 1989 Jul;3(1):1–12. doi: 10.1016/0896-6273(89)90110-4. [DOI] [PubMed] [Google Scholar]
- Baccaglini P. I., Cooper E. Electrophysiological studies of new-born rat nodose neurones in cell culture. J Physiol. 1982 Mar;324:429–439. doi: 10.1113/jphysiol.1982.sp014122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Dupin E., Kato A. C. Development of electrical membrane properties in cultured avian neural crest. 1983 Oct 27-Nov 2Nature. 305(5937):808–810. doi: 10.1038/305808a0. [DOI] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D. Effect of changes in intra- and extracellular sodium on the inward (anomalous) rectification in salamander photoreceptors. J Physiol. 1984 Feb;347:611–631. doi: 10.1113/jphysiol.1984.sp015086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schlichter R. Calcium-activated chloride current in cultured sensory and parasympathetic quail neurones. J Physiol. 1987 Dec;394:125–148. doi: 10.1113/jphysiol.1987.sp016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M., Bostock H., Grafe P., Martius P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol. 1987 Feb;383:45–67. doi: 10.1113/jphysiol.1987.sp016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barald K. F. Culture conditions affect the cholinergic development of an isolated subpopulation of chick mesencephalic neural crest cells. Dev Biol. 1989 Oct;135(2):349–366. doi: 10.1016/0012-1606(89)90185-1. [DOI] [PubMed] [Google Scholar]
- Bernheim L., Bader C. R., Bertrand D., Schlichter R. Transient expression of a Ca2+-activated Cl- current during development of quail sensory neurons. Dev Biol. 1989 Nov;136(1):129–139. doi: 10.1016/0012-1606(89)90136-x. [DOI] [PubMed] [Google Scholar]
- Bertrand D., Bader C. R. DATAC: a multipurpose biological data analysis program based on a mathematical interpreter. Int J Biomed Comput. 1986 May;18(3-4):193–202. doi: 10.1016/0020-7101(86)90016-4. [DOI] [PubMed] [Google Scholar]
- Blair L. A. The timing of protein synthesis required for the development of the sodium action potential in embryonic spinal neurons. J Neurosci. 1983 Jul;3(7):1430–1436. doi: 10.1523/JNEUROSCI.03-07-01430.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobker D. H., Williams J. T. Serotonin augments the cationic current Ih in central neurons. Neuron. 1989 Jun;2(6):1535–1540. doi: 10.1016/0896-6273(89)90041-x. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., Feltz A. Patch-clamp study of the tetrodotoxin-resistant sodium current in group C sensory neurones. Neurosci Lett. 1984 Oct 12;51(2):241–246. doi: 10.1016/0304-3940(84)90558-5. [DOI] [PubMed] [Google Scholar]
- Bowers C. W. A cadmium-sensitive, tetrodotoxin-resistant sodium channel in bullfrog autonomic axons. Brain Res. 1985 Aug 5;340(1):143–147. doi: 10.1016/0006-8993(85)90783-8. [DOI] [PubMed] [Google Scholar]
- Carr V. M., Simpson S. B., Jr Proliferative and degenerative events in the early development of chick dorsal root ganglia. I. Normal development. J Comp Neurol. 1978 Dec 15;182(4):727–739. doi: 10.1002/cne.901820410. [DOI] [PubMed] [Google Scholar]
- Fukuda J., Kameyama M. Tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in tissue-cultured spinal ganglion neurons from adult mammals. Brain Res. 1980 Jan 20;182(1):191–197. doi: 10.1016/0006-8993(80)90844-6. [DOI] [PubMed] [Google Scholar]
- Gallego R. The ionic basis of action potentials in petrosal ganglion cells of the cat. J Physiol. 1983 Sep;342:591–602. doi: 10.1113/jphysiol.1983.sp014870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann K., Dietzel I. D., Lux H. D., Huck S., Rohrer H. Development of inward currents in chick sensory and autonomic neuronal precursor cells in culture. J Neurosci. 1988 Oct;8(10):3722–3732. doi: 10.1523/JNEUROSCI.08-10-03722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V., Brunso-Bechtold J. K., Yip J. W. Neuronal death in the spinal ganglia of the chick embryo and its reduction by nerve growth factor. J Neurosci. 1981 Jan;1(1):60–71. doi: 10.1523/JNEUROSCI.01-01-00060.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Lawson S. N. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol. 1985 Feb;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer E. J., Macdonald R. L. Calcium- and sodium-dependent action potentials of mouse spinal cord and dorsal root ganglion neurons in cell culture. J Neurophysiol. 1982 Apr;47(4):641–655. doi: 10.1152/jn.1982.47.4.641. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G., Weight F. F. Na+ and Ca2+ currents of acutely isolated adult rat nodose ganglion cells. J Neurophysiol. 1986 Mar;55(3):527–539. doi: 10.1152/jn.1986.55.3.527. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-I. Sodium currents. Neuroscience. 1981;6(12):2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Interactions between neurons and their targets during in vivo synaptogenesis. Fed Proc. 1978 May 15;37(7):2016–2022. [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Synaptic transmission and cell death during normal ganglionic development. J Physiol. 1974 Sep;241(3):737–749. doi: 10.1113/jphysiol.1974.sp010681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Trypsin inhibits the action of tetrodotoxin on neurones. Nature. 1977 Feb 24;265(5596):751–753. doi: 10.1038/265751a0. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Sodium conductance in calcium channels of guinea-pig ventricular cells induced by removal of external calcium ions. Pflugers Arch. 1986 Nov;407(5):465–475. doi: 10.1007/BF00657502. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Yoshida S., Yonezawa T. Tetrodotoxin sensitivity and Ca component of action potentials of mouse dorsal root ganglion cells cultured in vitro. Brain Res. 1978 Oct 6;154(1):69–82. doi: 10.1016/0006-8993(78)91052-1. [DOI] [PubMed] [Google Scholar]
- Mayer M. L. Selective block of inward but not outward rectification in rat sensory neurones infected with herpes simplex virus. J Physiol. 1986 Jun;375:327–338. doi: 10.1113/jphysiol.1986.sp016119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCobb D. P., Best P. M., Beam K. G. Development alters the expression of calcium currents in chick limb motoneurons. Neuron. 1989 Jun;2(6):1633–1643. doi: 10.1016/0896-6273(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Narayanan C. H., Narayanan Y. On the origin of the ciliary ganglion in birds studied by the method of interspecific transplantation of embryonic brain regions between quail and chick. J Embryol Exp Morphol. 1978 Oct;47:137–148. [PubMed] [Google Scholar]
- Nerbonne J. M., Gurney A. M. Development of excitable membrane properties in mammalian sympathetic neurons. J Neurosci. 1989 Sep;9(9):3272–3286. doi: 10.1523/JNEUROSCI.09-09-03272.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd D. K. RNA synthesis dependence of action potential development in spinal cord neurones. Nature. 1983 Jun 16;303(5918):619–621. doi: 10.1038/303619a0. [DOI] [PubMed] [Google Scholar]
- O'Dowd D. K., Ribera A. B., Spitzer N. C. Development of voltage-dependent calcium, sodium, and potassium currents in Xenopus spinal neurons. J Neurosci. 1988 Mar;8(3):792–805. doi: 10.1523/JNEUROSCI.08-03-00792.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom B. R., Holz R. W. Ionic determinants of excitability in cultured mouse dorsal root ganglion and spinal cord cells. Brain Res. 1977 Nov 18;136(3):445–453. doi: 10.1016/0006-8993(77)90069-5. [DOI] [PubMed] [Google Scholar]
- Ribera A. B., Spitzer N. C. A critical period of transcription required for differentiation of the action potential of spinal neurons. Neuron. 1989 Jan;2(1):1055–1062. doi: 10.1016/0896-6273(89)90229-8. [DOI] [PubMed] [Google Scholar]
- Rudy B., Kirschenbaum B., Rukenstein A., Greene L. A. Nerve growth factor increases the number of functional Na channels and induces TTX-resistant Na channels in PC12 pheochromocytoma cells. J Neurosci. 1987 Jun;7(6):1613–1625. doi: 10.1523/JNEUROSCI.07-06-01613.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt P. C., Spain W. J., Crill W. E. Influence of anomalous rectifier activation on afterhyperpolarizations of neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1988 Feb;59(2):468–481. doi: 10.1152/jn.1988.59.2.468. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C., Lamborghini J. E. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld C. E., Wallis D. I. Properties of visceral primary afferent neurons in the nodose ganglion of the rabbit. J Neurophysiol. 1985 Aug;54(2):245–260. doi: 10.1152/jn.1985.54.2.245. [DOI] [PubMed] [Google Scholar]
- Straznicky C., Rush R. A. The effect of nerve growth factor on developing primary sensory neurons of the trigeminal nerve in chick embryos. Anat Embryol (Berl) 1985;171(1):91–95. doi: 10.1007/BF00319058. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Matsuda Y., Samejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. J Neurophysiol. 1978 Sep;41(5):1096–1106. doi: 10.1152/jn.1978.41.5.1096. [DOI] [PubMed] [Google Scholar]
- Ziller C., Fauquet M., Kalcheim C., Smith J., Le Douarin N. M. Cell lineages in peripheral nervous system ontogeny: medium-induced modulation of neuronal phenotypic expression in neural crest cell cultures. Dev Biol. 1987 Mar;120(1):101–111. doi: 10.1016/0012-1606(87)90108-4. [DOI] [PubMed] [Google Scholar]
- d'Amico-Martel A., Noden D. M. An autoradiographic analysis of the development of the chick trigeminal ganglion. J Embryol Exp Morphol. 1980 Feb;55:167–182. [PubMed] [Google Scholar]


