Abstract
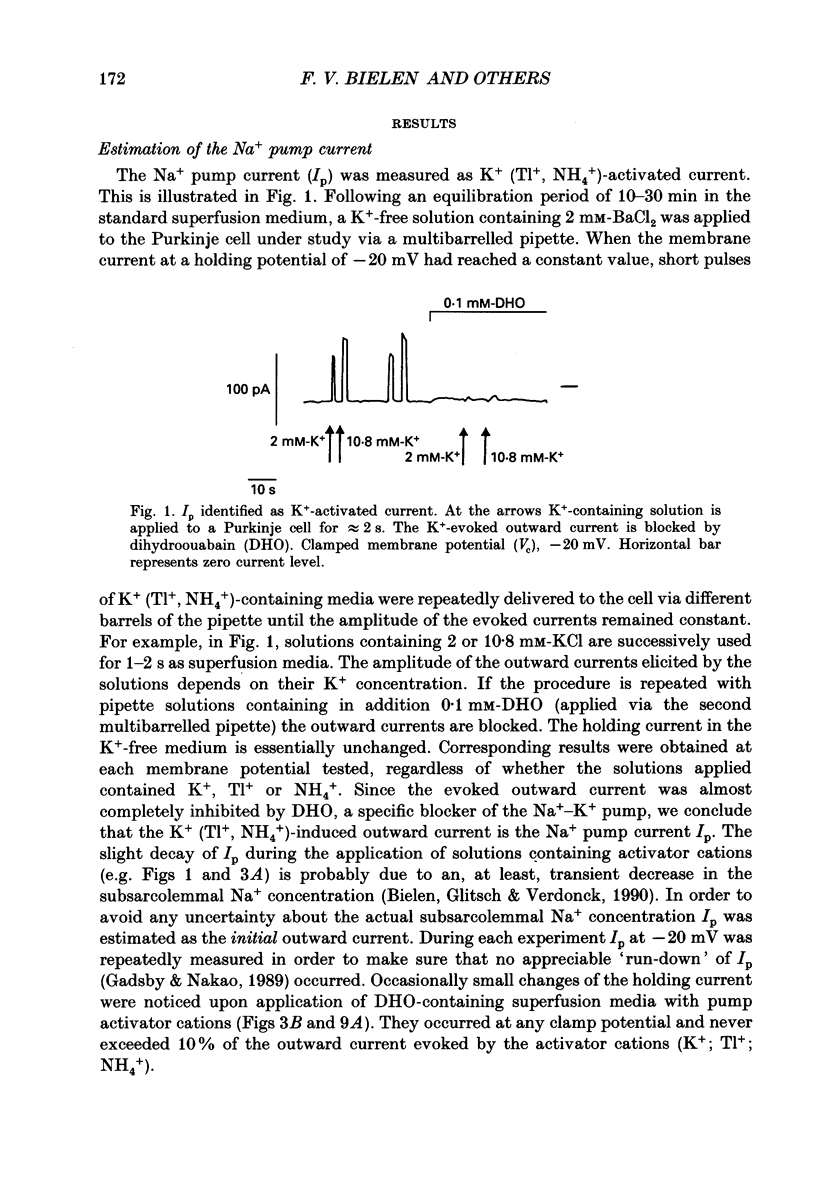
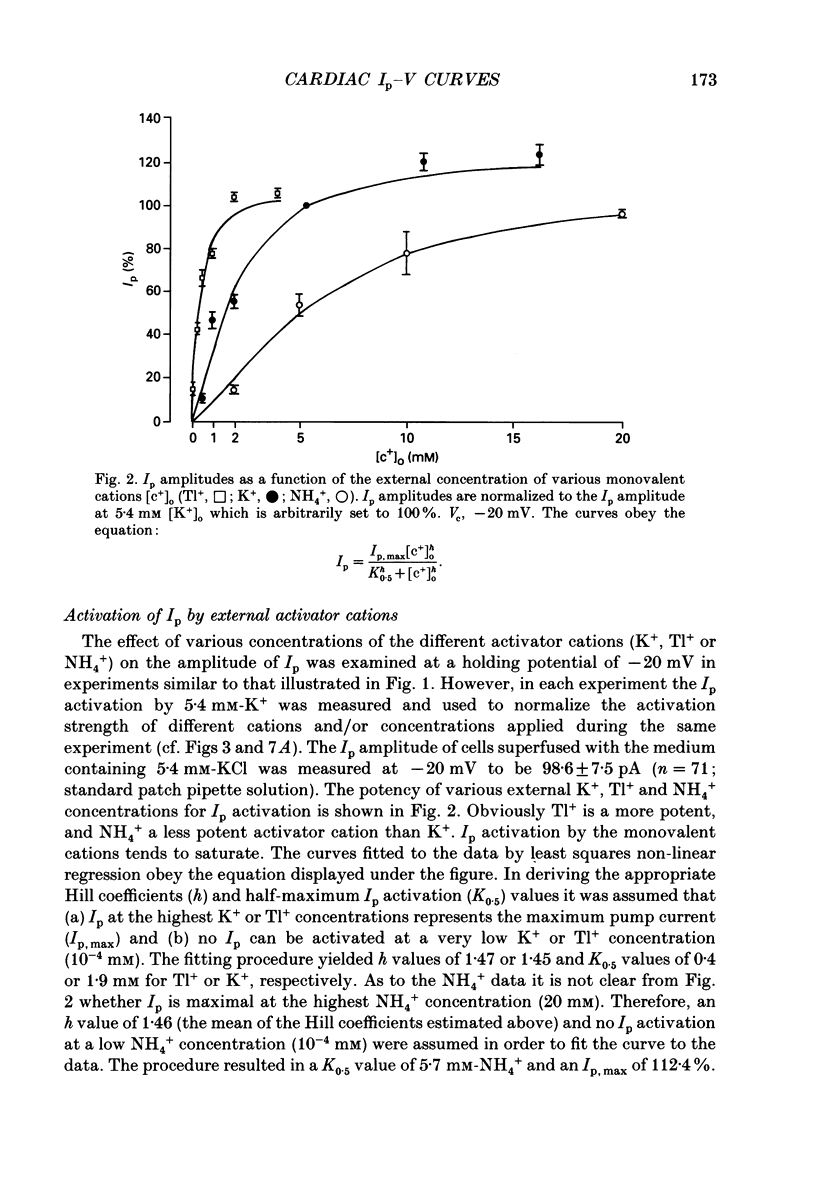
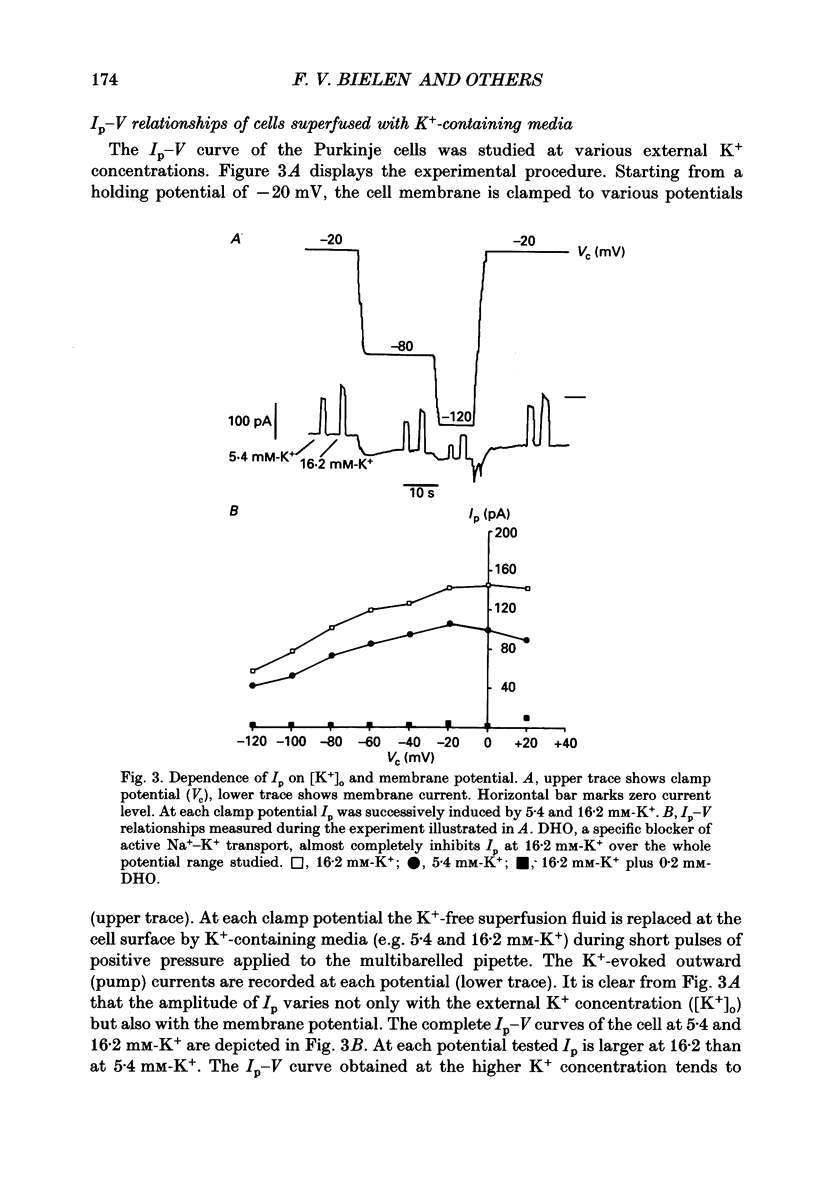
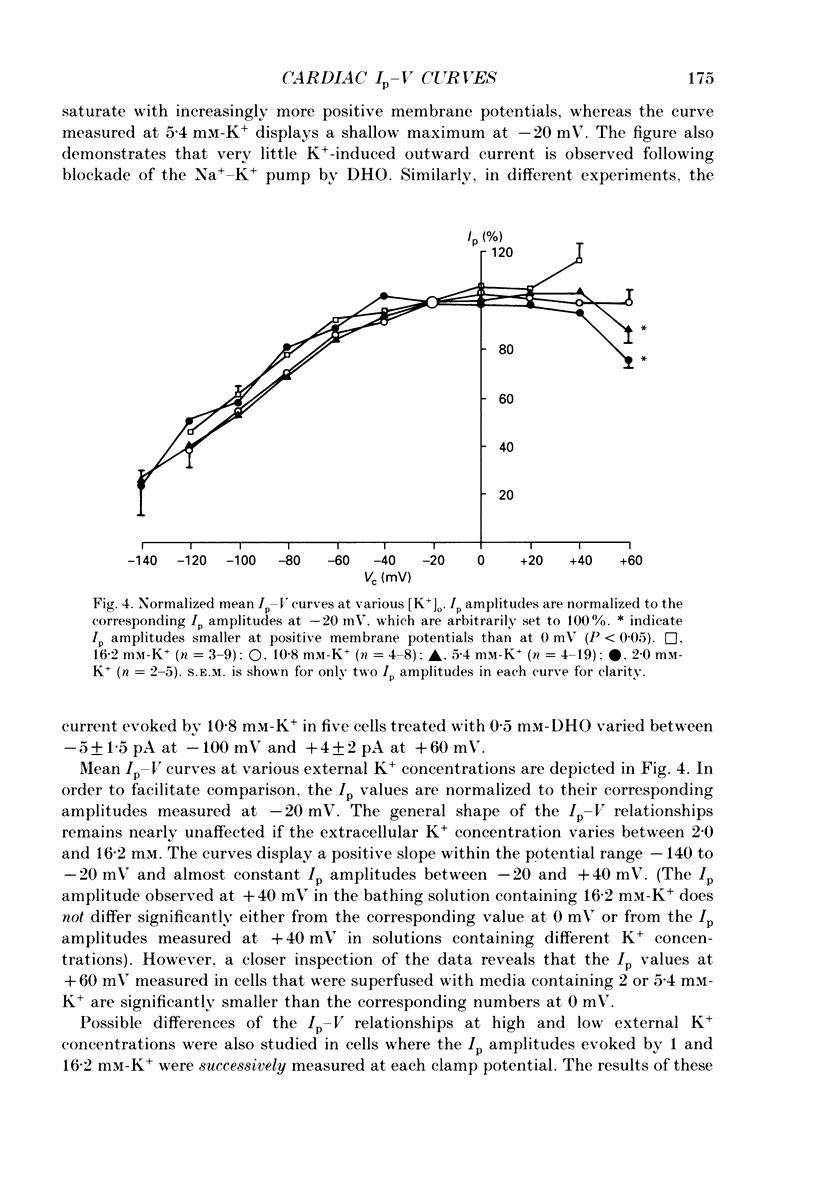
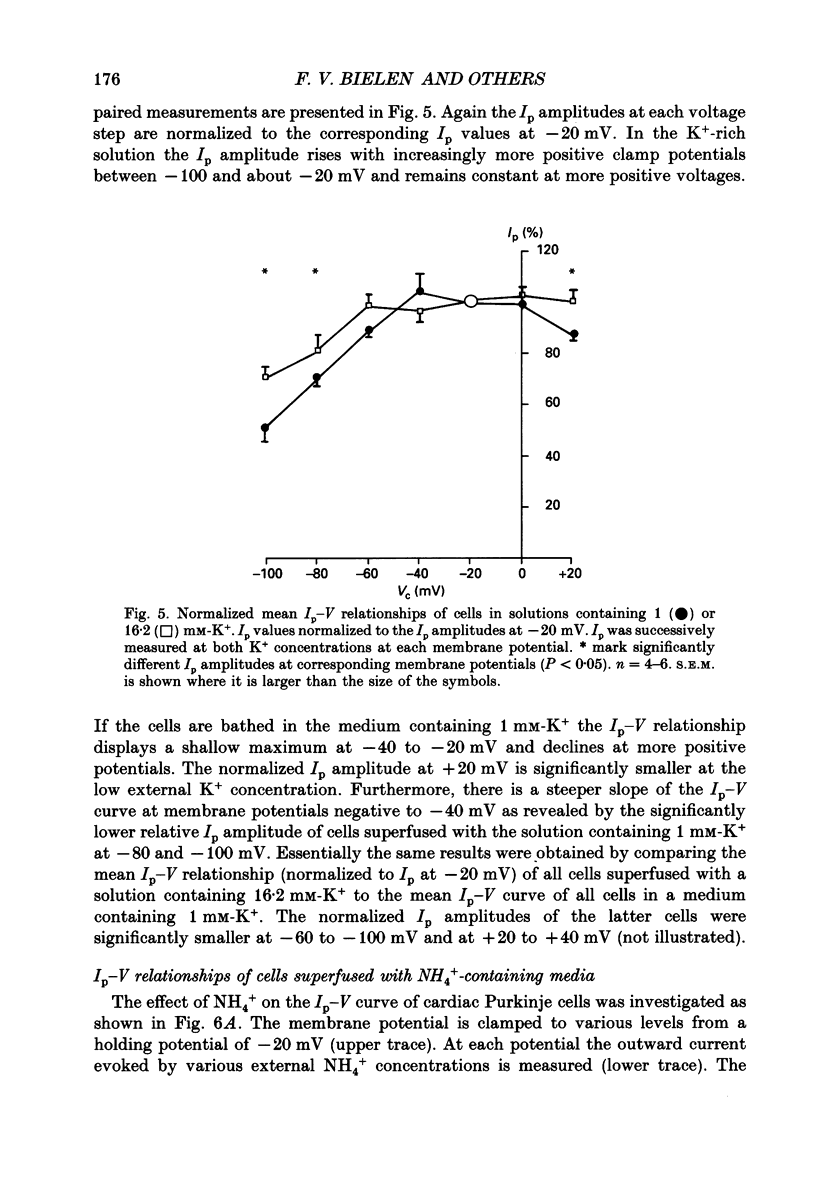
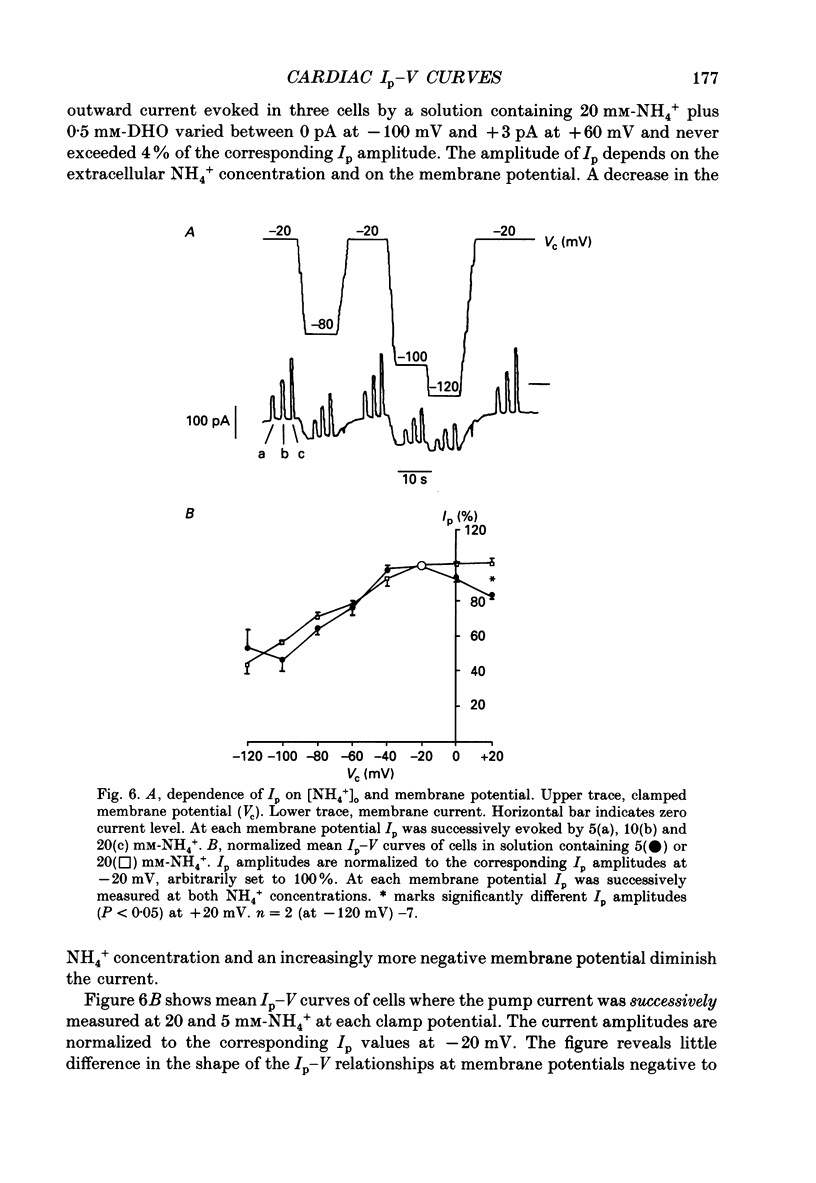
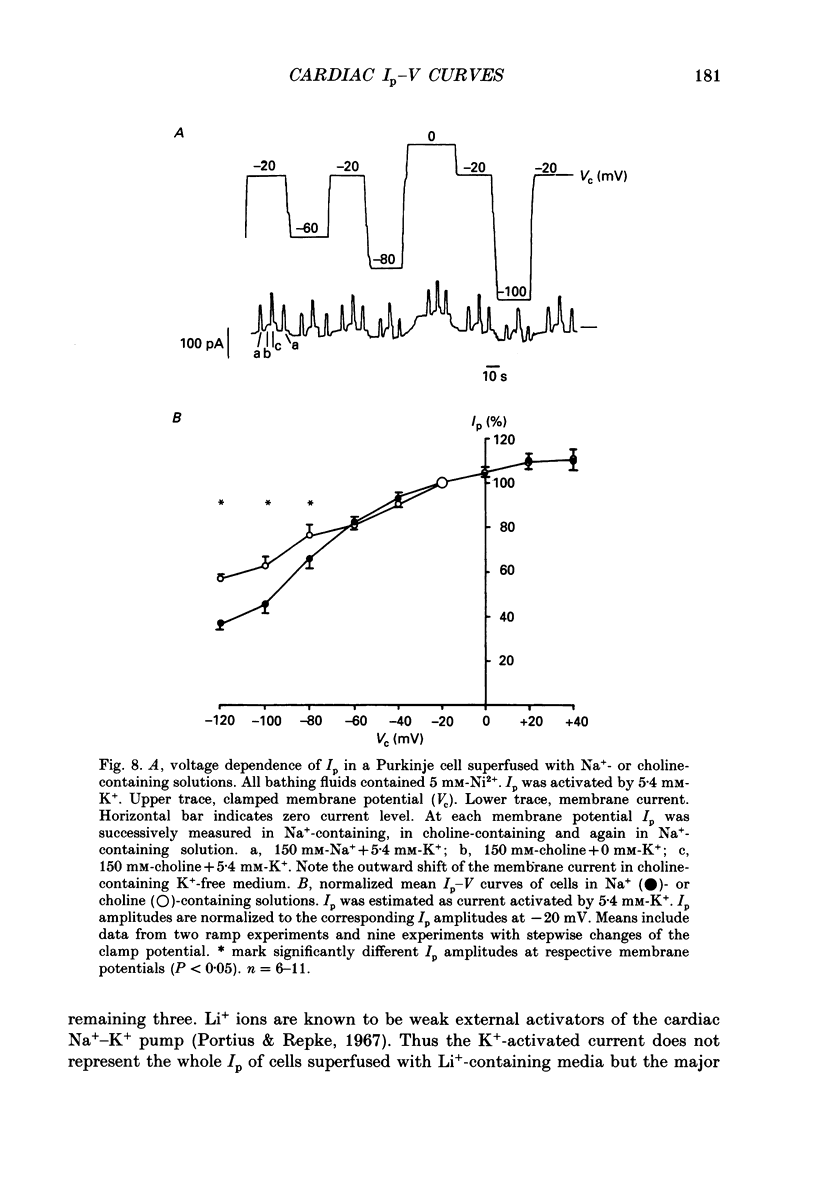
1. The effect of membrane potential and various extracellular monovalent cations on the Na+ pump current (Ip) was studied on isolated, single Purkinje cells of the rabbit heart by means of whole-cell recording. 2. Ip was identified as current activated by external K+ or its congeners NH4+ and Tl+. The current was blocked by dihydroouabain (1-5 x 10(-4) M) over the whole range of membrane potentials tested. 3. In Na(+)-containing solution half-maximum Ip activation (K0.5) occurred at 0.4 mM-Tl+, 1.9 mM-K+ and 5.7 mM-NH4+ (holding potential, -20 mV). 4. The pump current (Ip)-voltage (V) relationship of the cells in Na(+)-containing media with K+ or its congeners at the tested concentrations greater than K0.5 displayed a steep positive slope at negative membrane potentials between -120 and -20 mV. Little voltage dependence of Ip was observed at more positive potentials up to +40 mV. At even more positive potentials Ip measured at 2 and 5.4 mM-K+ decreased. 5. Lowering the concentration of K+ or its congeners below the K0.5 value in Na(+)-containing solution induced a region of negative slope of the Ip-V curve at membrane potentials positive to -20 mV. 6. The shape of the Ip-V relationship remained unchanged when the K+ concentration (5.4 mM) of the Na(+)-containing medium was replaced by NH4+ or Tl+ concentrations of similar potency to activate Ip (20 mM-NH4+ or 2 mM-Tl+). 7. In Na(+)-free, choline-containing solution half-maximum Ip activation occurred at 0.13 mM-K+ (holding potential, -20 mV). 8. At negative membrane potentials the positive slope of the Ip-V curve was flatter in Na(+)-free than in Na(+)-containing media. A reduced voltage dependence of Ip persisted, regardless of whether choline ions or Li+ were used as a Na+ substitute. 9. Lowering the K+ concentration of the Na(+)-free, choline-containing solution to 0.05 mM evoked an extended region of negative slope in the Ip-V relationship at membrane potentials between -40 and +60 mV. 10. It is concluded that the apparent affinity of the Na(+)-K+ pump towards K+ in cardiac Purkinje cells depends on both the membrane potential and the extracellular Na+ concentration. 11. The region of negative slope of the Ip-V curve observed in cells which were superfused with media containing low concentrations of K+ or its congeners strongly suggests the existence of at least two voltage-sensitive steps in the cardiac Na(+)-K+ pump cycle.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



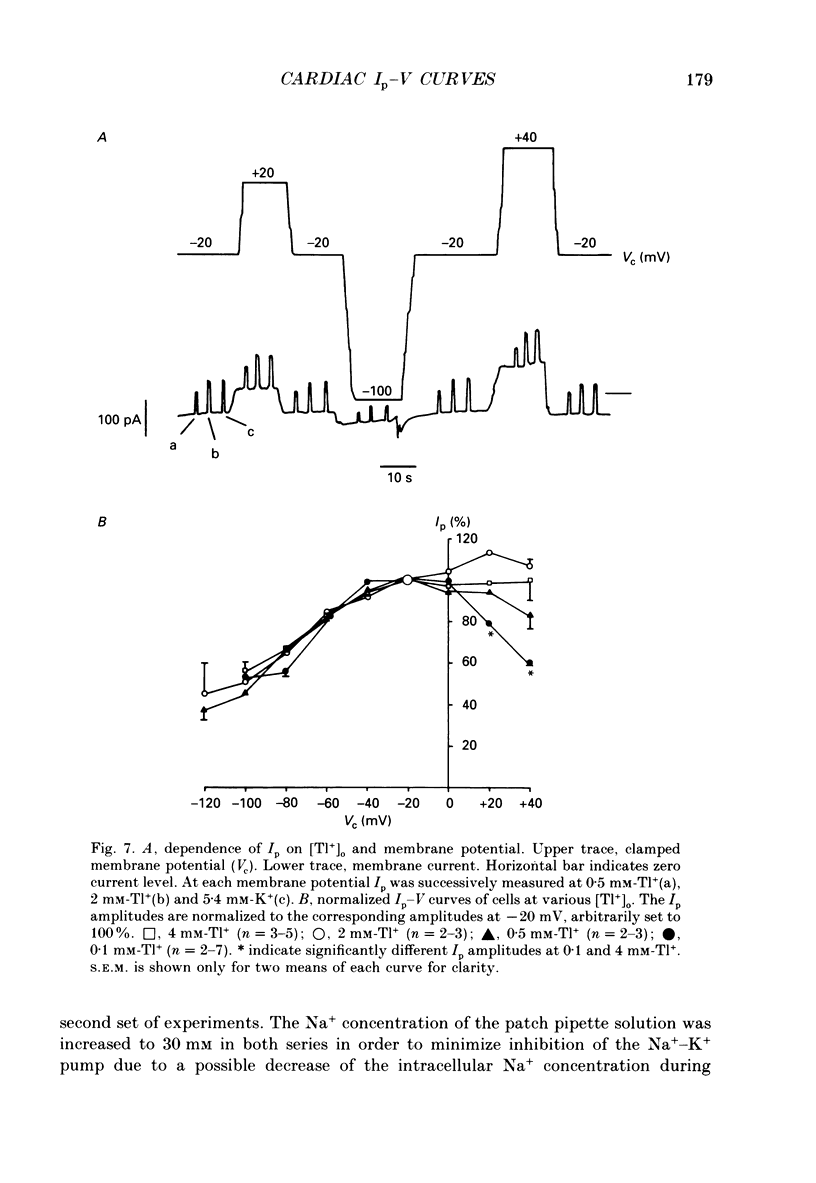








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apell H. J., Borlinghaus R., Läuger P. Fast charge translocations associated with partial reactions of the Na,K-pump: II. Microscopic analysis of transient currents. J Membr Biol. 1987;97(3):179–191. doi: 10.1007/BF01869221. [DOI] [PubMed] [Google Scholar]
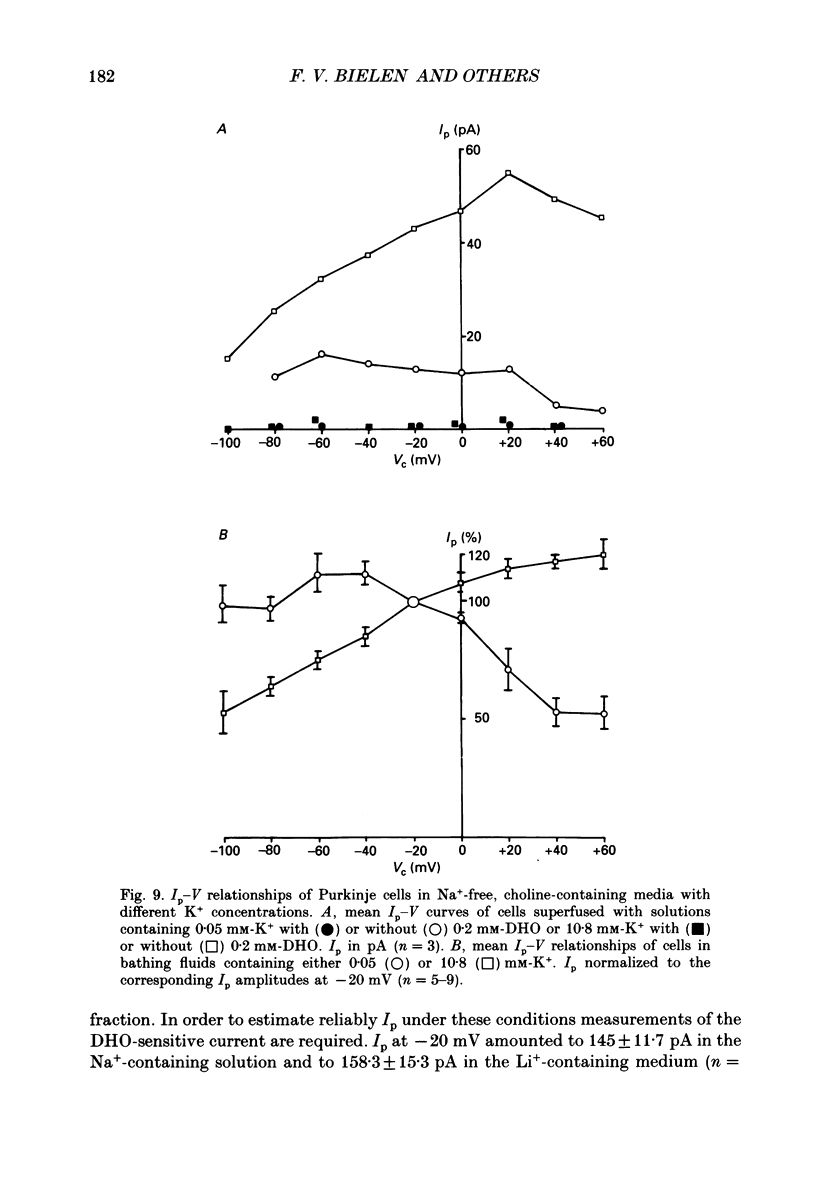
- Apell H. J. Electrogenic properties of the Na,K pump. J Membr Biol. 1989 Sep;110(2):103–114. doi: 10.1007/BF01869466. [DOI] [PubMed] [Google Scholar]
- Bahinski A., Nakao M., Gadsby D. C. Potassium translocation by the Na+/K+ pump is voltage insensitive. Proc Natl Acad Sci U S A. 1988 May;85(10):3412–3416. doi: 10.1073/pnas.85.10.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. S., Datyner N. B., Gintant G. A., Mulrine N. K., Pennefather P. Properties of an electrogenic sodium-potassium pump in isolated canine Purkinje myocytes. J Physiol. 1987 Feb;383:251–267. doi: 10.1113/jphysiol.1987.sp016407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P., Gadsby D. C., Rakowski R. F. Voltage dependence of the Na-K pump. Annu Rev Physiol. 1988;50:225–241. doi: 10.1146/annurev.ph.50.030188.001301. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. The role of the sodium pump in the effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:279–301. doi: 10.1113/jphysiol.1979.sp012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendler K., Grell E., Haubs M., Bamberg E. Pump currents generated by the purified Na+K+-ATPase from kidney on black lipid membranes. EMBO J. 1985 Dec 1;4(12):3079–3085. doi: 10.1002/j.1460-2075.1985.tb04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Kimura J., Noma A. Voltage dependence of Na/K pump current in isolated heart cells. Nature. 1985 May 2;315(6014):63–65. doi: 10.1038/315063a0. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Nakao M. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):511–537. doi: 10.1085/jgp.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. The Na/K pump of cardiac cells. Annu Rev Biophys Bioeng. 1984;13:373–398. doi: 10.1146/annurev.bb.13.060184.002105. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Krahn T., Pusch H. The dependence of sodium pump current on internal Na concentration and membrane potential in cardioballs from sheep Purkinje fibres. Pflugers Arch. 1989 May;414(1):52–58. doi: 10.1007/BF00585626. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Krahn T., Verdonck F. Activation of the Na pump current by external K and Cs ions in cardioballs from sheep Purkinje fibres. Pflugers Arch. 1989 May;414(1):99–101. doi: 10.1007/BF00585634. [DOI] [PubMed] [Google Scholar]
- Goldshlegger R., Karlish S. J., Rephaeli A., Stein W. D. The effect of membrane potential on the mammalian sodium-potassium pump reconstituted into phospholipid vesicles. J Physiol. 1987 Jun;387:331–355. doi: 10.1113/jphysiol.1987.sp016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaire A. V., Schwarz W. Voltage dependence of the rheogenic Na+/K+ ATPase in the membrane of oocytes of Xenopus laevis. J Membr Biol. 1986;91(1):43–51. doi: 10.1007/BF01870213. [DOI] [PubMed] [Google Scholar]
- Mogul D. J., Rasmussen H. H., Singer D. H., Ten Eick R. E. Inhibition of Na-K pump current in guinea pig ventricular myocytes by dihydroouabain occurs at high- and low-affinity sites. Circ Res. 1989 Jun;64(6):1063–1069. doi: 10.1161/01.res.64.6.1063. [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. Voltage dependence of Na translocation by the Na/K pump. Nature. 1986 Oct 16;323(6089):628–630. doi: 10.1038/323628a0. [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):539–565. doi: 10.1085/jgp.94.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski R. F., Gadsby D. C., De Weer P. Stoichiometry and voltage dependence of the sodium pump in voltage-clamped, internally dialyzed squid giant axon. J Gen Physiol. 1989 May;93(5):903–941. doi: 10.1085/jgp.93.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski R. F., Vasilets L. A., LaTona J., Schwarz W. A negative slope in the current-voltage relationship of the Na+/K+ pump in Xenopus oocytes produced by reduction of external [K+]. J Membr Biol. 1991 Apr;121(2):177–187. doi: 10.1007/BF01870531. [DOI] [PubMed] [Google Scholar]
- Rephaeli A., Richards D. E., Karlish S. J. Electrical potential accelerates the E1P(Na)----E2P conformational transition of (Na,K)-ATPase in reconstituted vesicles. J Biol Chem. 1986 Sep 25;261(27):12437–12440. [PubMed] [Google Scholar]
- Reynolds J. A., Johnson E. A., Tanford C. Incorporation of membrane potential into theoretical analysis of electrogenic ion pumps. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6869–6873. doi: 10.1073/pnas.82.20.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scamps F., Carmeliet E. Delayed K+ current and external K+ in single cardiac Purkinje cells. Am J Physiol. 1989 Dec;257(6 Pt 1):C1086–C1092. doi: 10.1152/ajpcell.1989.257.6.C1086. [DOI] [PubMed] [Google Scholar]
- Schuurmans Stekhoven F., Bonting S. L. Transport adenosine triphosphatases: properties and functions. Physiol Rev. 1981 Jan;61(1):1–76. doi: 10.1152/physrev.1981.61.1.1. [DOI] [PubMed] [Google Scholar]
- Schwarz W., Gu Q. B. Characteristics of the Na+/K+-ATPase from Torpedo californica expressed in Xenopus oocytes: a combination of tracer flux measurements with electrophysiological measurements. Biochim Biophys Acta. 1988 Nov 22;945(2):167–174. doi: 10.1016/0005-2736(88)90479-8. [DOI] [PubMed] [Google Scholar]
- Schweigert B., Lafaire A. V., Schwarz W. Voltage dependence of the Na-K ATPase: measurements of ouabain-dependent membrane current and ouabain binding in oocytes of Xenopus laevis. Pflugers Arch. 1988 Oct;412(6):579–588. doi: 10.1007/BF00583758. [DOI] [PubMed] [Google Scholar]
- Stimers J. R., Shigeto N., Lieberman M. Na/K pump current in aggregates of cultured chick cardiac myocytes. J Gen Physiol. 1990 Jan;95(1):61–76. doi: 10.1085/jgp.95.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürmer W., Bühler R., Apell H. J., Läuger P. Charge translocation by the Na,K-pump: II. Ion binding and release at the extracellular face. J Membr Biol. 1991 Apr;121(2):163–176. doi: 10.1007/BF01870530. [DOI] [PubMed] [Google Scholar]


