Abstract
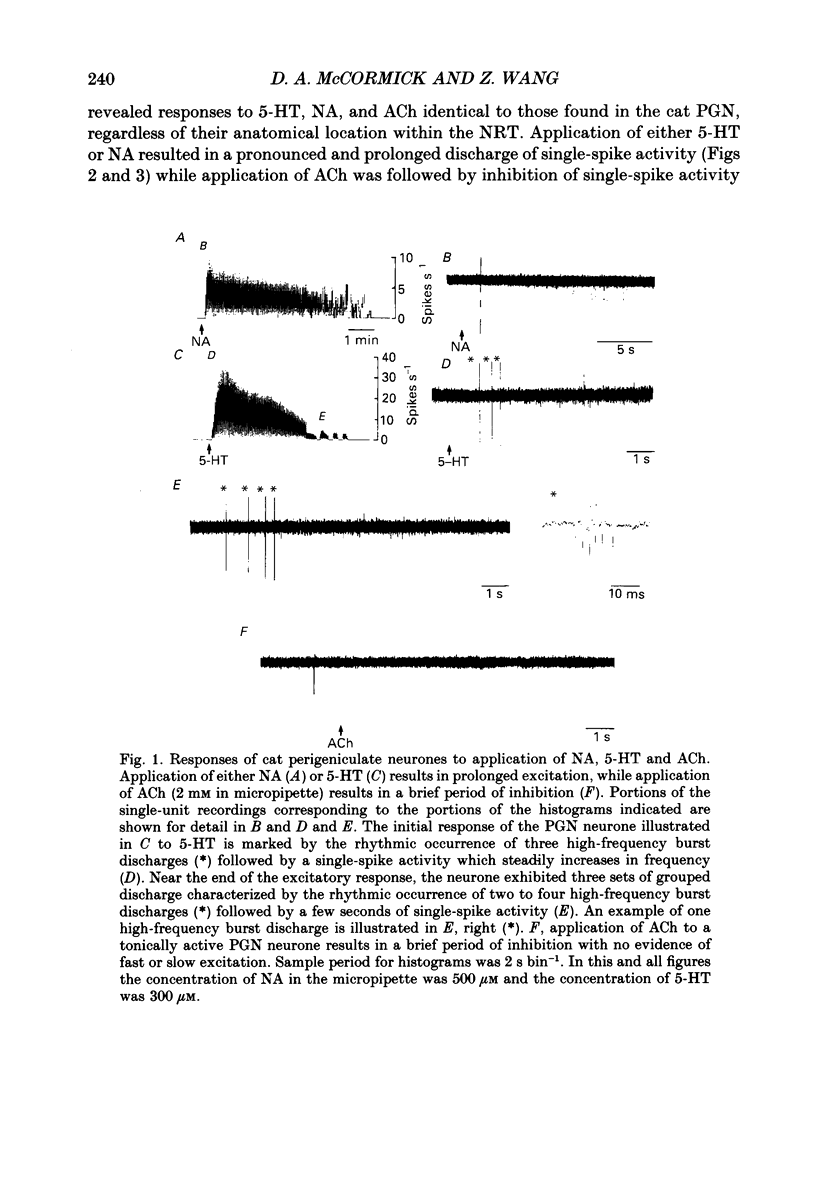
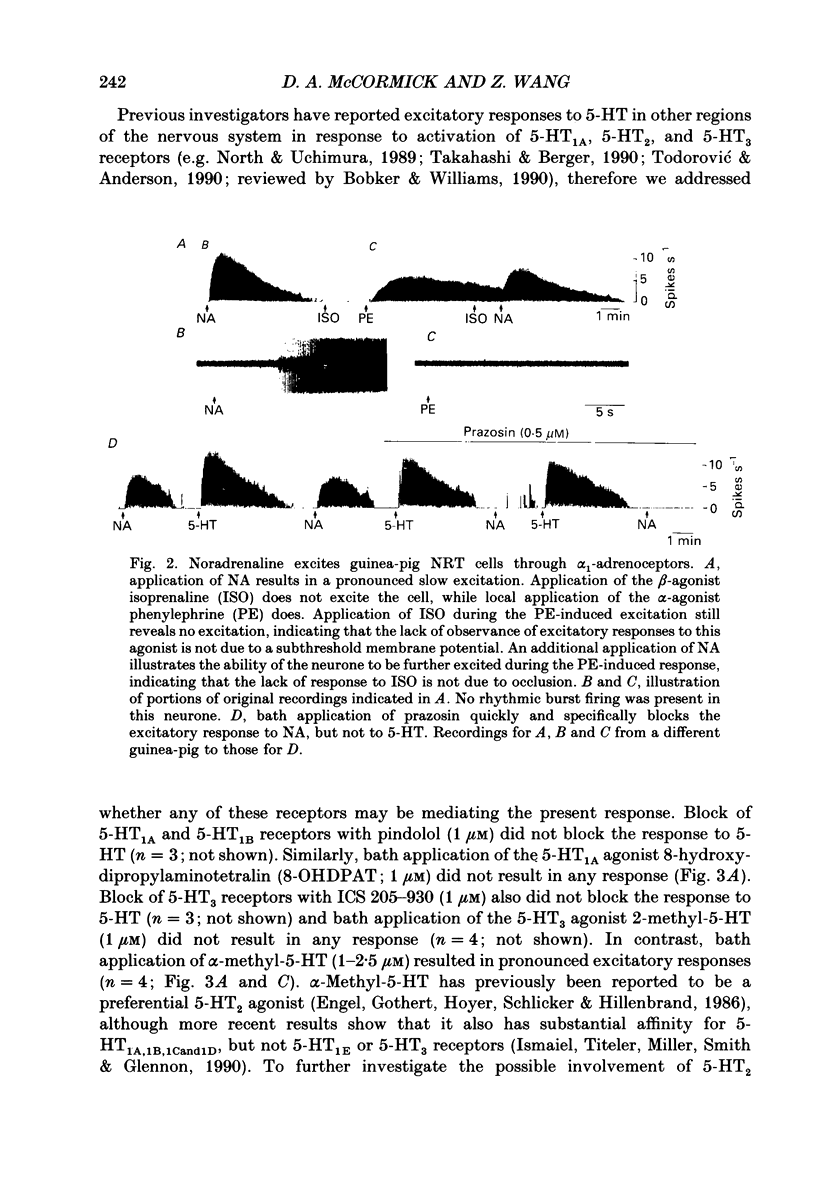
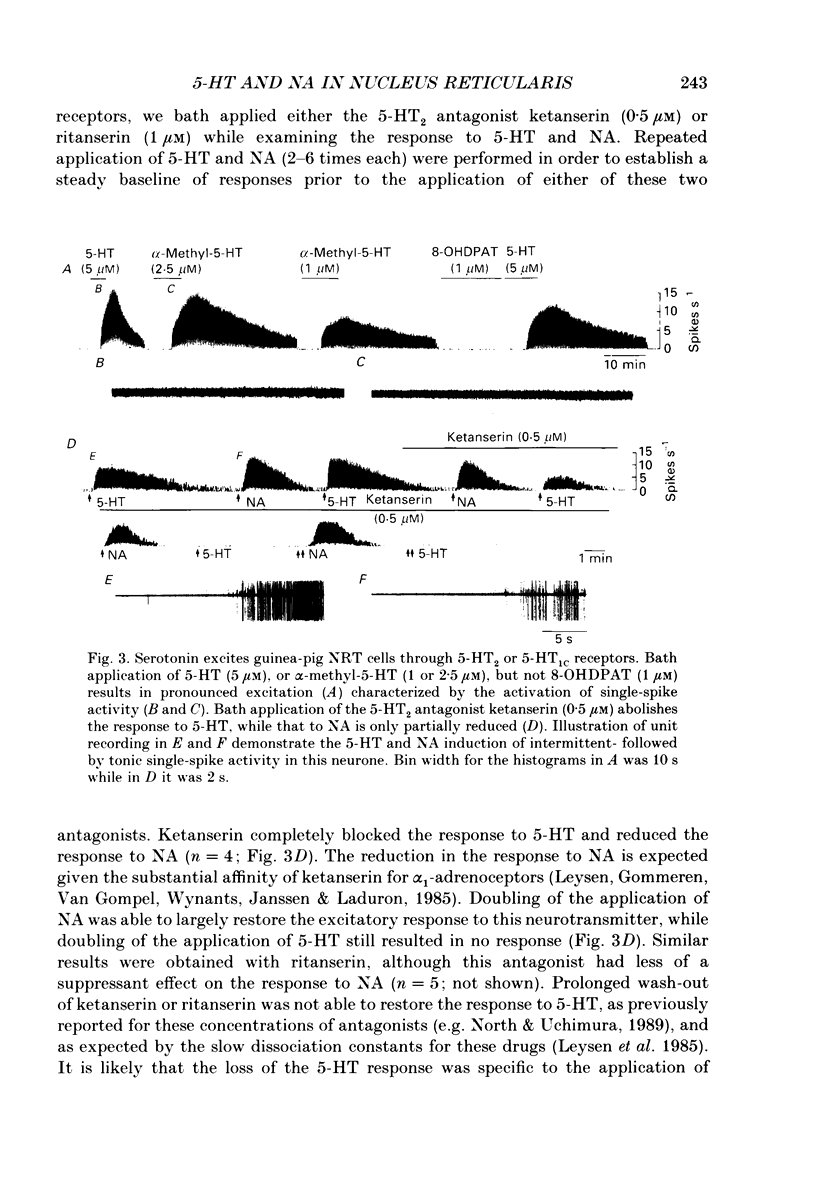
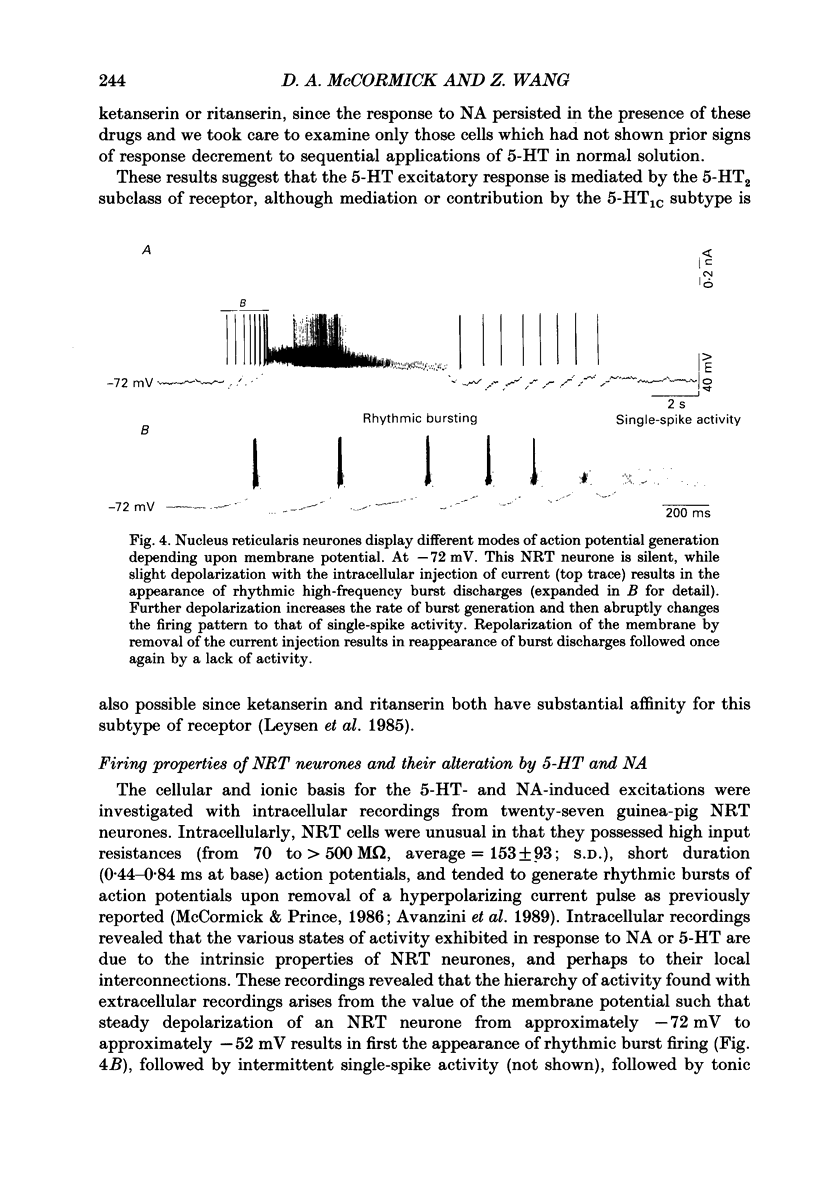
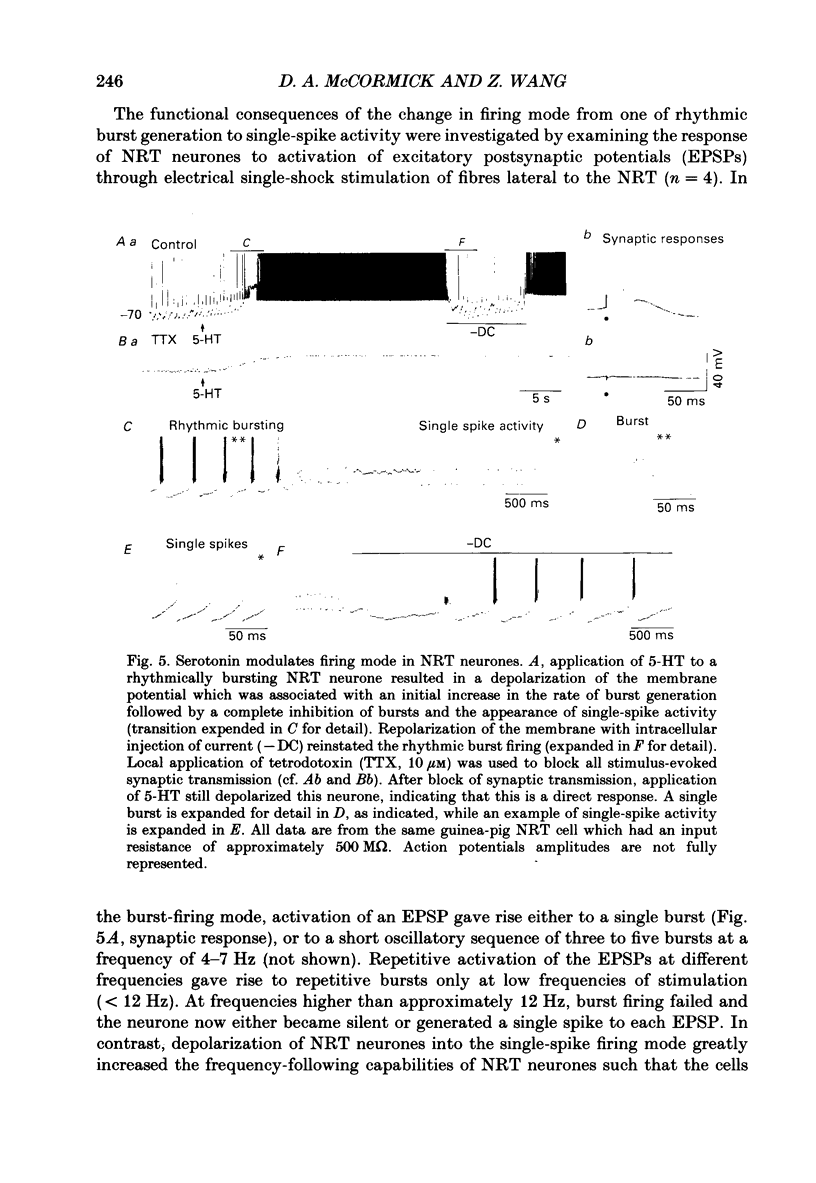
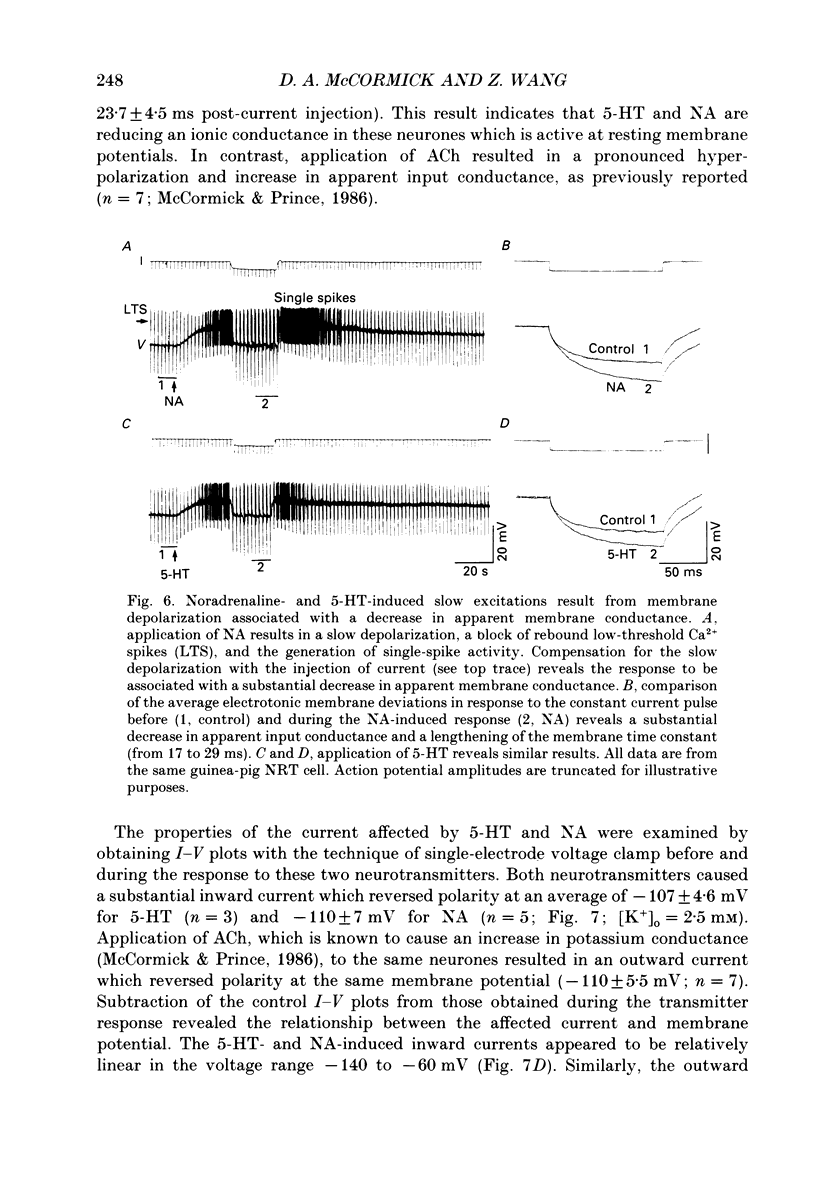
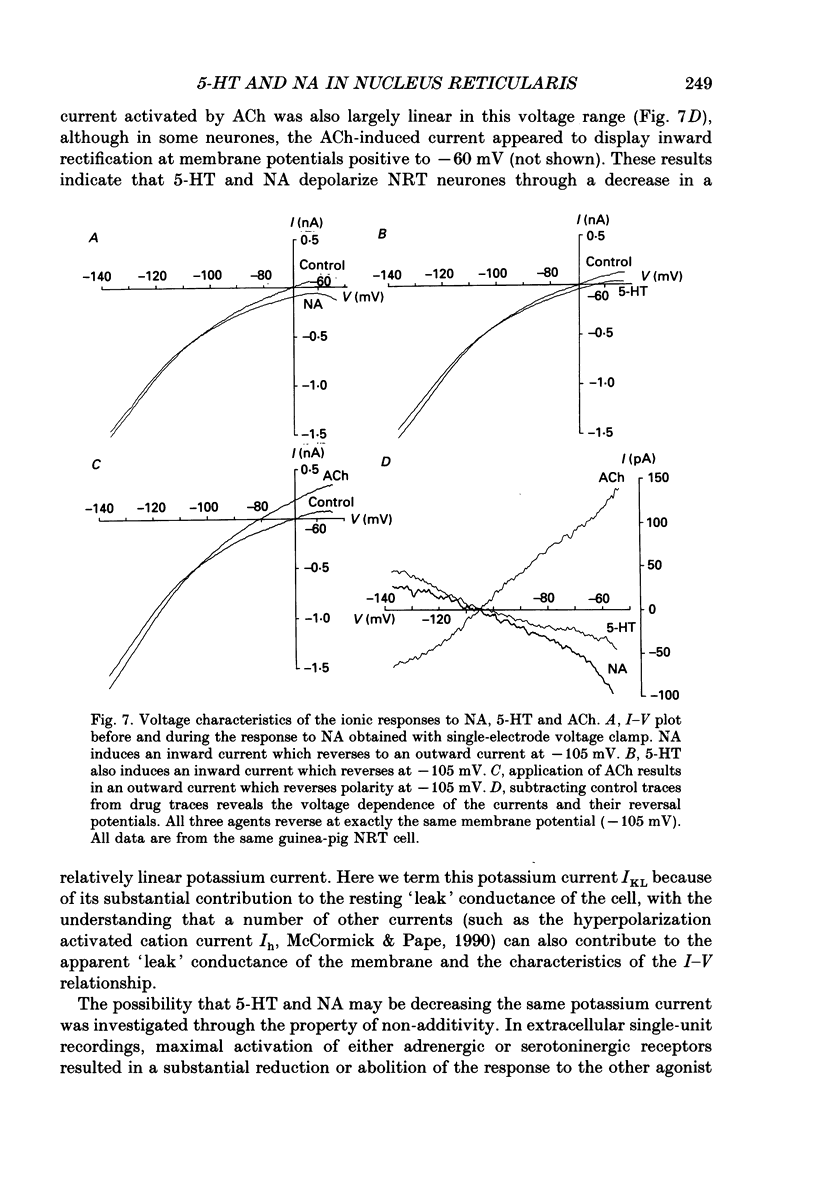
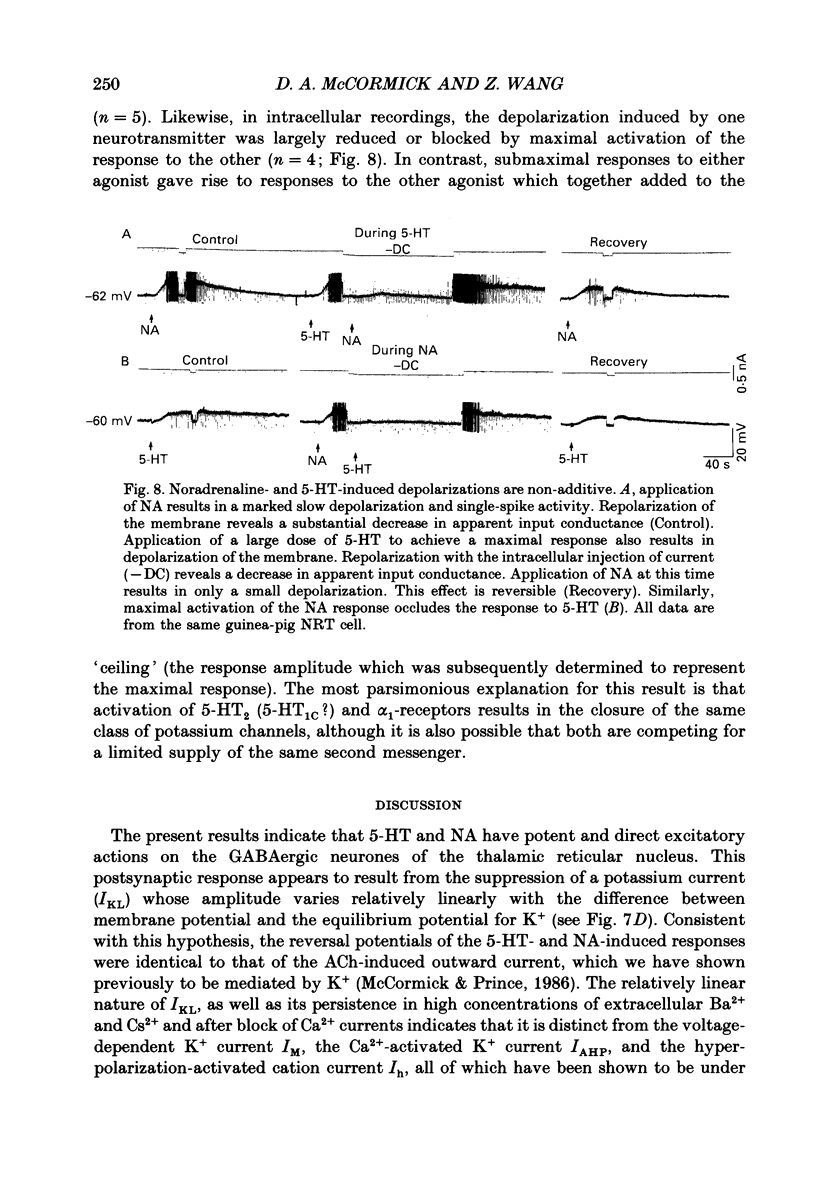
1. The actions of serotonin (5-HT) and noradrenaline (NA) in the cat perigeniculate nucleus (PGN) and the guinea-pig nucleus reticularis thalami (NRT) were investigated with extracellular and intracellular recordings obtained from neurones in thalamic slices maintained in vitro. 2. Single, local application of either 5-HT or NA resulted in pronounced (5-50 Hz) and prolonged (2-10 min) excitation associated with the occurrence of single-spike activity. Serotoninergic excitation was specifically blocked by the 5-HT2/5-HT1C antagonists ketanserin and ritanserin, but not by the 5-HT1A antagonist pindolol or the 5-HT3 antagonist ICS 205-930. Furthermore, the 5-HT response was mimicked by alpha-methyl-5-HT, but not by the 5-HT1A agonist 8-hydroxydipropylaminotetralin (8-OHDPAT) or the 5-HT3 agonist 2-methyl-5-HT. Together, these results indicate that this excitatory response is mediated through 5-HT2 receptors with the possible involvement of 5-HT1C receptors. 3. Noradrenergic excitation was specifically blocked by the alpha 1-antagonist prazosin, but not by the beta-antagonist propranolol or the alpha 2-antagonist yohimbine. Similarly, the response was mimicked by the alpha-agonist phenylephrine, but not by the beta-agonist isoprenaline. These results indicate that the noradrenergic excitation is mediated by alpha 1-adrenoceptors. 4. Block of synaptic transmission either by lowering external calcium concentration ([Ca2+]o) to 0.5 mM and raising external magnesium concentration ([Mg2+]o) to 10 mM or by local application of tetrodotoxin failed to block the excitatory or depolarizing response to 5-HT or NA indicating that these responses are direct and not mediated through the release of other neurotransmitters. 5. Intracellular recordings revealed that the 5-HT- and NA-induced excitations are mediated by a pronounced slow depolarization associated with an apparent decrease in input conductance and an increase in the membrane time constant. Current versus voltage plots obtained under voltage clamp before and during the presence of 5-HT and NA revealed that these neurotransmitters induced an inward current which reversed to an outward current at -107 and -110 mV, respectively, in 2.5 mM external potassium concentration ([K+]o). This reversal potential was identical to that associated with an increase in potassium conductance activated by acetylcholine (-110 mV) in the same neurones. Plots of the amplitude of the 5-HT- or NA-induced current versus membrane potential revealed a linear relationship in the voltage range from -140 to -60 mV.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K. Modulation of a transient outward current in serotonergic neurones by alpha 1-adrenoceptors. Nature. 1985 Jun 6;315(6019):501–503. doi: 10.1038/315501a0. [DOI] [PubMed] [Google Scholar]
- Andrade R., Nicoll R. A. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987 Dec;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G., Bloom F. E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981 Aug;1(8):876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzini G., de Curtis M., Panzica F., Spreafico R. Intrinsic properties of nucleus reticularis thalami neurones of the rat studied in vitro. J Physiol. 1989 Sep;416:111–122. doi: 10.1113/jphysiol.1989.sp017752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobker D. H., Williams J. T. Ion conductances affected by 5-HT receptor subtypes in mammalian neurons. Trends Neurosci. 1990 May;13(5):169–173. doi: 10.1016/0166-2236(90)90042-9. [DOI] [PubMed] [Google Scholar]
- Colino A., Halliwell J. V. Differential modulation of three separate K-conductances in hippocampal CA1 neurons by serotonin. Nature. 1987 Jul 2;328(6125):73–77. doi: 10.1038/328073a0. [DOI] [PubMed] [Google Scholar]
- Constanti A., Adams P. R., Brown D. A. Who do barium ions imitate acetylcholine? Brain Res. 1981 Feb 9;206(1):244–250. doi: 10.1016/0006-8993(81)90125-6. [DOI] [PubMed] [Google Scholar]
- Cropper E. C., Eisenman J. S., Azmitia E. C. An immunocytochemical study of the serotonergic innervation of the thalamus of the rat. J Comp Neurol. 1984 Mar 20;224(1):38–50. doi: 10.1002/cne.902240104. [DOI] [PubMed] [Google Scholar]
- De Lima A. D., Singer W. The serotoninergic fibers in the dorsal lateral geniculate nucleus of the cat: distribution and synaptic connections demonstrated with immunocytochemistry. J Comp Neurol. 1987 Apr 15;258(3):339–351. doi: 10.1002/cne.902580303. [DOI] [PubMed] [Google Scholar]
- Engel G., Göthert M., Hoyer D., Schlicker E., Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D., Penny G. R., Schmechel D. E. Glutamic acid decarboxylase-immunoreactive neurons and terminals in the lateral geniculate nucleus of the cat. J Neurosci. 1984 Jul;4(7):1809–1829. doi: 10.1523/JNEUROSCI.04-07-01809.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser C. R., Vaughn J. E., Barber R. P., Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980 Nov 3;200(2):341–354. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- Hu B., Steriade M., Deschênes M. The effects of brainstem peribrachial stimulation on perigeniculate neurons: the blockage of spindle waves. Neuroscience. 1989;31(1):1–12. doi: 10.1016/0306-4522(89)90026-2. [DOI] [PubMed] [Google Scholar]
- Ismaiel A. M., Titeler M., Miller K. J., Smith T. S., Glennon R. A. 5-HT1 and 5-HT2 binding profiles of the serotonergic agents alpha-methylserotonin and 2-methylserotonin. J Med Chem. 1990 Feb;33(2):755–758. doi: 10.1021/jm00164a046. [DOI] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. E., Beaudet A. Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyltransferase and tyrosine hydroxylase immunohistochemical study. J Comp Neurol. 1987 Jul 1;261(1):15–32. doi: 10.1002/cne.902610103. [DOI] [PubMed] [Google Scholar]
- Kayama Y., Negi T., Sugitani M., Iwama K. Effects of locus coeruleus stimulation on neuronal activities of dorsal lateral geniculate nucleus and perigeniculate reticular nucleus of the rat. Neuroscience. 1982 Mar;7(3):655–666. doi: 10.1016/0306-4522(82)90071-9. [DOI] [PubMed] [Google Scholar]
- Levey A. I., Hallanger A. E., Wainer B. H. Cholinergic nucleus basalis neurons may influence the cortex via the thalamus. Neurosci Lett. 1987 Feb 10;74(1):7–13. doi: 10.1016/0304-3940(87)90042-5. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Gommeren W., Van Gompel P., Wynants J., Janssen P. F., Laduron P. M. Receptor-binding properties in vitro and in vivo of ritanserin: A very potent and long acting serotonin-S2 antagonist. Mol Pharmacol. 1985 Jun;27(6):600–611. [PubMed] [Google Scholar]
- McCormick D. A. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 1989 Jun;12(6):215–221. doi: 10.1016/0166-2236(89)90125-2. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Feeser H. R. Functional implications of burst firing and single spike activity in lateral geniculate relay neurons. Neuroscience. 1990;39(1):103–113. doi: 10.1016/0306-4522(90)90225-s. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. 1986 Jan 30-Feb 5Nature. 319(6052):402–405. doi: 10.1038/319402a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J Neurophysiol. 1988 Mar;59(3):978–996. doi: 10.1152/jn.1988.59.3.978. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8098–8102. doi: 10.1073/pnas.86.20.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. H., Foote S. L. Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J Comp Neurol. 1986 Jan 1;243(1):117–138. doi: 10.1002/cne.902430110. [DOI] [PubMed] [Google Scholar]
- Mulle C., Madariaga A., Deschênes M. Morphology and electrophysiological properties of reticularis thalami neurons in cat: in vivo study of a thalamic pacemaker. J Neurosci. 1986 Aug;6(8):2134–2145. doi: 10.1523/JNEUROSCI.06-08-02134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- North R. A., Uchimura N. 5-Hydroxytryptamine acts at 5-HT2 receptors to decrease potassium conductance in rat nucleus accumbens neurones. J Physiol. 1989 Oct;417:1–12. doi: 10.1113/jphysiol.1989.sp017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape H. C., McCormick D. A. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989 Aug 31;340(6236):715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Shosaku A., Kayama Y., Sumitomo I., Sugitani M., Iwama K. Analysis of recurrent inhibitory circuit in rat thalamus: neurophysiology of the thalamic reticular nucleus. Prog Neurobiol. 1989;32(2):77–102. doi: 10.1016/0301-0082(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984 Nov;320(1):1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschênes M., Domich L., Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985 Dec;54(6):1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Steriade M., Domich L., Oakson G., Deschênes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987 Jan;57(1):260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- Steriade M., Domich L., Oakson G. Reticularis thalami neurons revisited: activity changes during shifts in states of vigilance. J Neurosci. 1986 Jan;6(1):68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Llinás R. R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988 Jul;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Steriade M., Parent A., Hada J. Thalamic projections of nucleus reticularis thalami of cat: a study using retrograde transport of horseradish peroxidase and fluorescent tracers. J Comp Neurol. 1984 Nov 10;229(4):531–547. doi: 10.1002/cne.902290407. [DOI] [PubMed] [Google Scholar]
- Steriade M., Parent A., Paré D., Smith Y. Cholinergic and non-cholinergic neurons of cat basal forebrain project to reticular and mediodorsal thalamic nuclei. Brain Res. 1987 Apr 7;408(1-2):372–376. doi: 10.1016/0006-8993(87)90408-2. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Berger A. J. Direct excitation of rat spinal motoneurones by serotonin. J Physiol. 1990 Apr;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorović S., Anderson E. G. 5-HT2 and 5-HT3 receptors mediate two distinct depolarizing responses in rat dorsal root ganglion neurons. Brain Res. 1990 Mar 12;511(1):71–79. doi: 10.1016/0006-8993(90)90226-2. [DOI] [PubMed] [Google Scholar]
- Trulson M. E., Jacobs B. L. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979 Mar 9;163(1):135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Hendrickson A. E. Serotonergic axons in the monkey's lateral geniculate nucleus. Vis Neurosci. 1988;1(1):125–133. doi: 10.1017/s0952523800001061. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Sasa M., Takaori S. Serotonin-mediated inhibition from dorsal raphe nucleus of neurons in dorsal lateral geniculate and thalamic reticular nuclei. Brain Res. 1984 Jan 2;290(1):95–105. doi: 10.1016/0006-8993(84)90739-x. [DOI] [PubMed] [Google Scholar]


