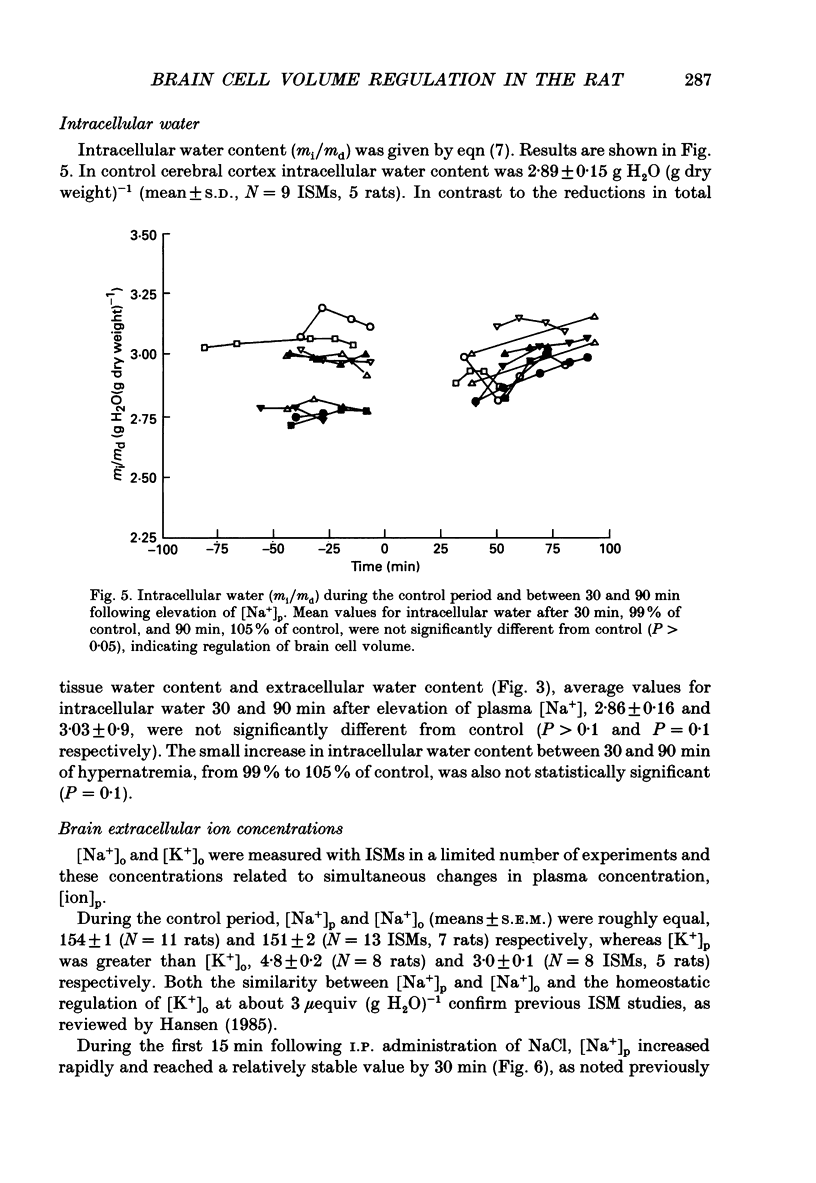
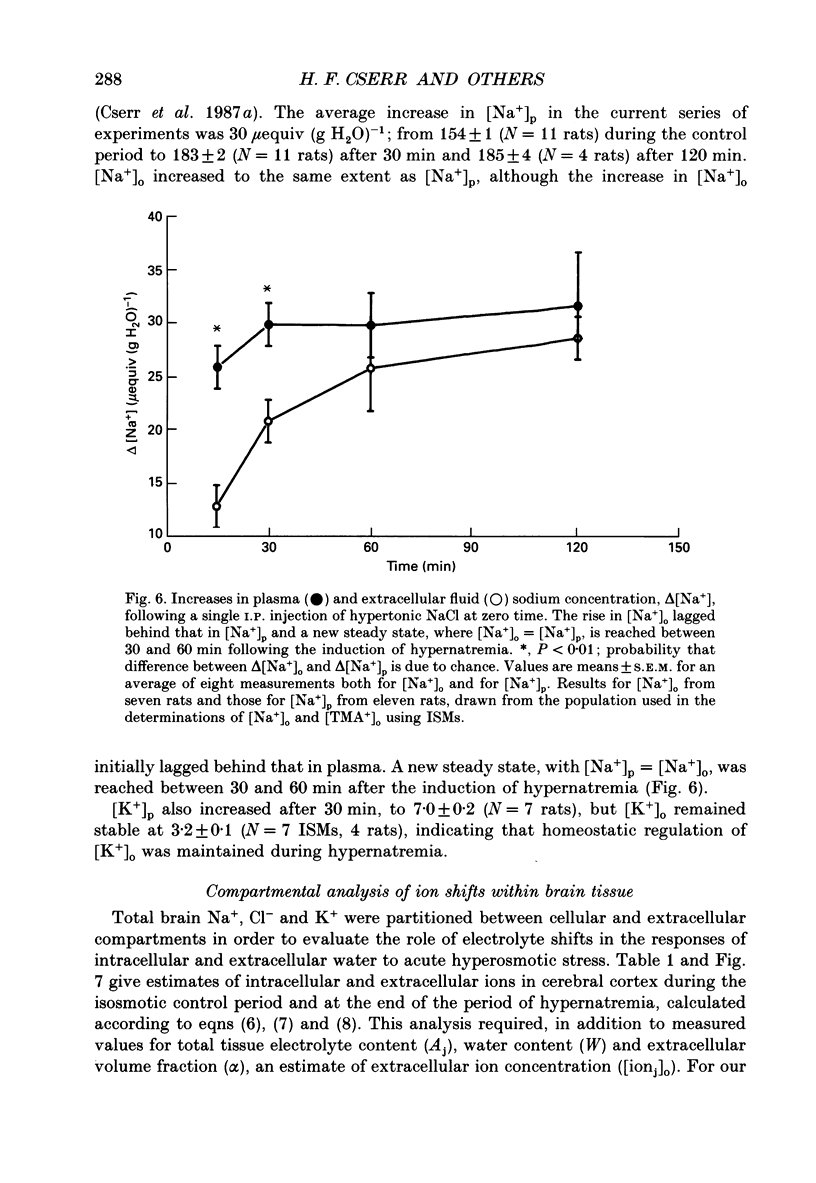
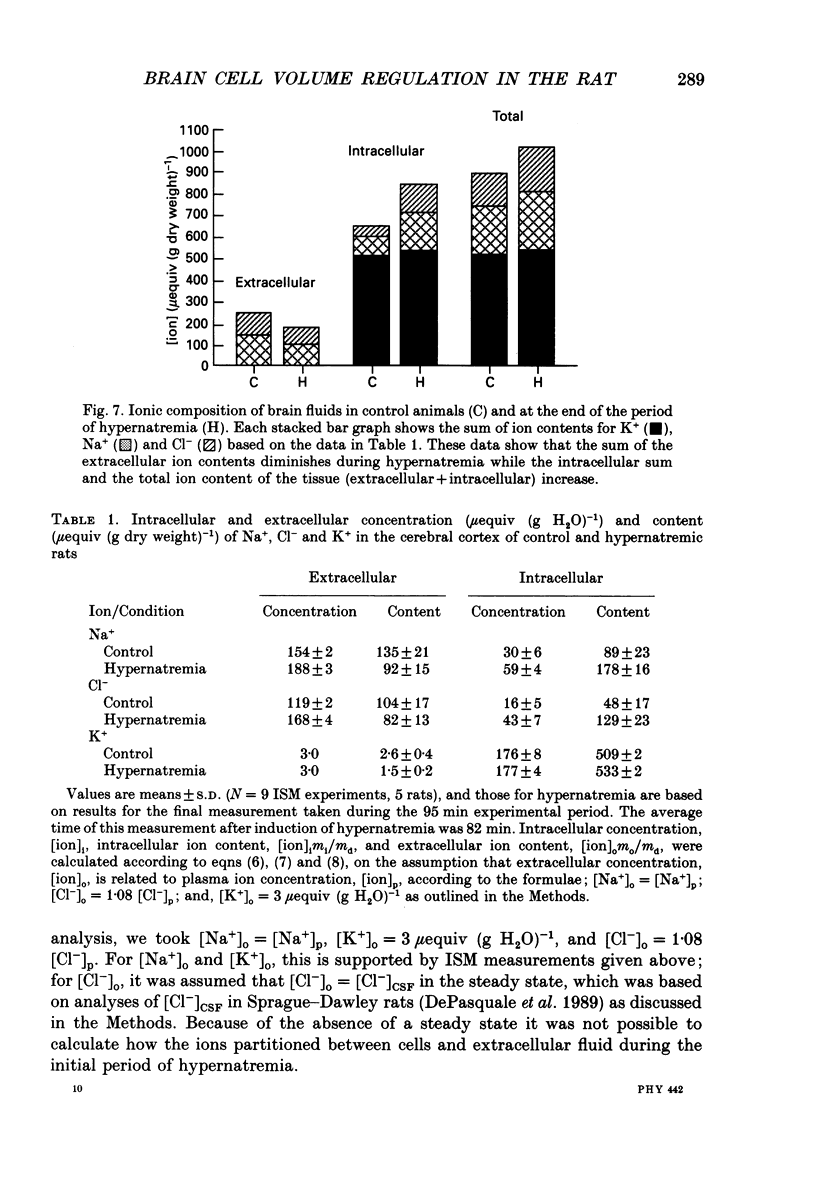
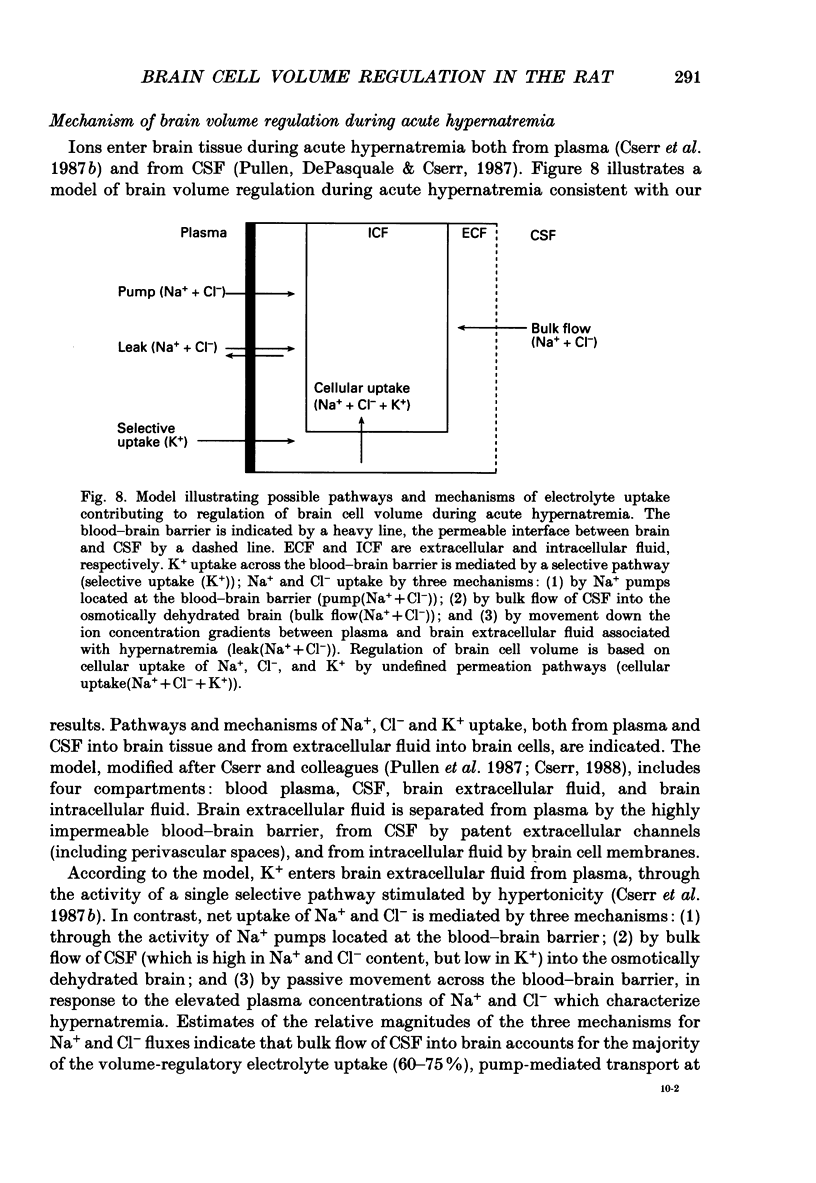
Abstract
1. Regulation of brain extracellular and intracellular water content, regarded as volume, and electrolytes in response to 90 min of hypernatremia has been studied in the cerebral cortex of rats under urethane anaesthetic. 2. Total tissue electrolytes and water were partitioned between extracellular and intracellular compartments based on measurements made in two series of experiments. In one, tissue samples were collected and analysed for total water, Na+, K+ and Cl-. In the other, tissue extracellular volume fraction, [Na+] and [K+] were measured in situ using ion-selective microelectrodes. 3. Osmotically induced water loss from cerebral cortex was less than that predicted for ideal osmotic behaviour, revealing a degree of volume regulation, and this regulation was associated with net tissue uptake of Na+, Cl- and K+. 4. Total water content was 3.77 g H2O (g dry weight)-1 in control cortex and this decreased by 7% after 30 min of hypernatremia and then remained relatively stable at this value. Control extracellular water content, based on an extracellular volume fraction of 0.18, was 0.88 g H2O (g dry weight)-1. Control intracellular water content, estimated as the difference between total and extracellular water contents, was 2.89 g H2O (g dry weight)-1. After 30 min of hypernatremia, extracellular water content decreased by an average of 27% but intracellular water did not change. This indicates selective regulation of cell volume. By 90 min the extracellular water content had decreased by 47% and the loss in extracellular water content appeared to be accompanied by a roughly equivalent increase in intracellular water content. The intracellular volume increase, however, was not statistically significant. The tortuosity of the extracellular space averaged 1.57 and increased to 1.65 during the hypernatremia. 5. Brain extracellular fluid and plasma [Na+] were roughly equal in control tissue. Both increased by 30 mu equiv (g H2O)-1 as a result of the hypernatremia, although extracellular [Na+] lagged behind the plasma value during much of the first 60 min of hypernatremia. Extracellular [K+] was homeostatically regulated at 3 mu equiv (g H2O)-1 independent of changes in plasma electrolytes. 6. Estimates of extracellular and intracellular ion content (mu equiv (g dry weight)-1) indicate that extracellular Na+, Cl- and K+ content decreased during hypernatremia, by 32, 21 and 42% respectively, whereas intracellular ion content increased by 100, 169 and 5% respectively. 7. It is concluded that during acute hypernatremia the extracellular space decreases in volume through the loss of water and electrolytes while the intracellular compartment maintains its water content and gains electrolytes.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


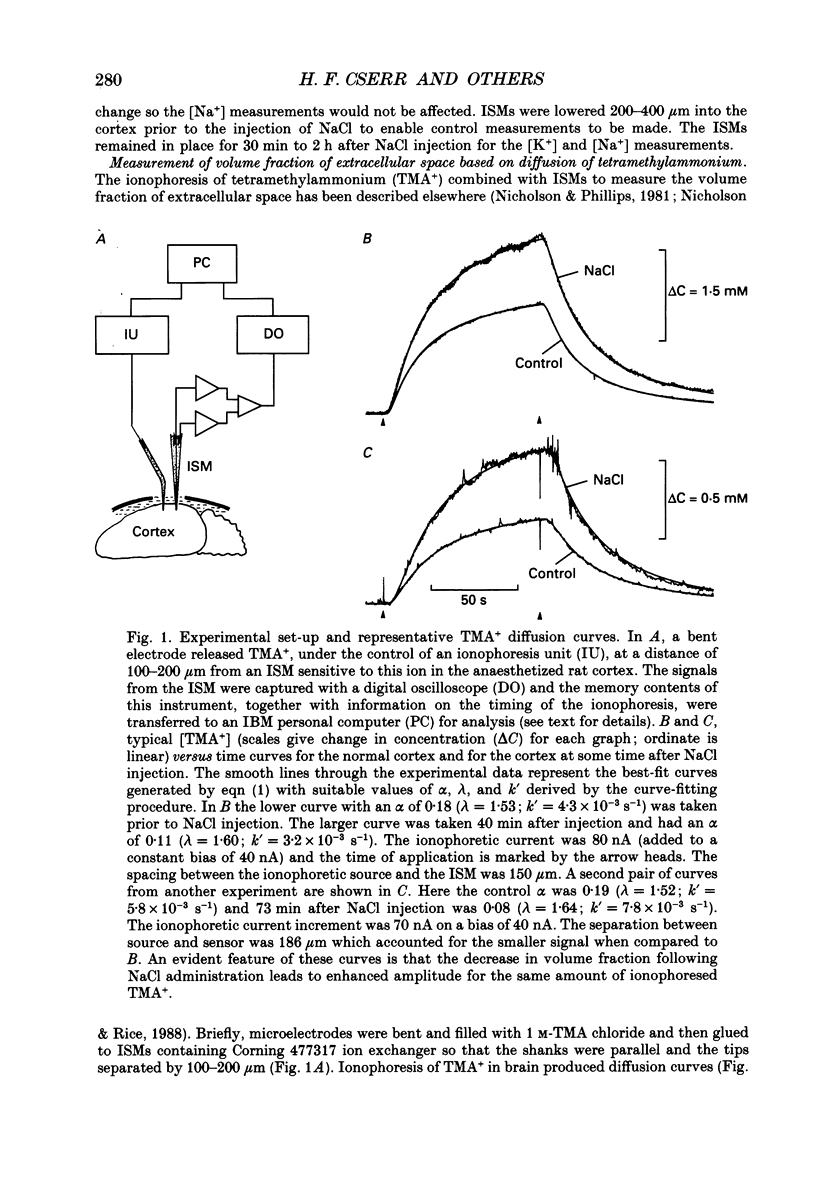



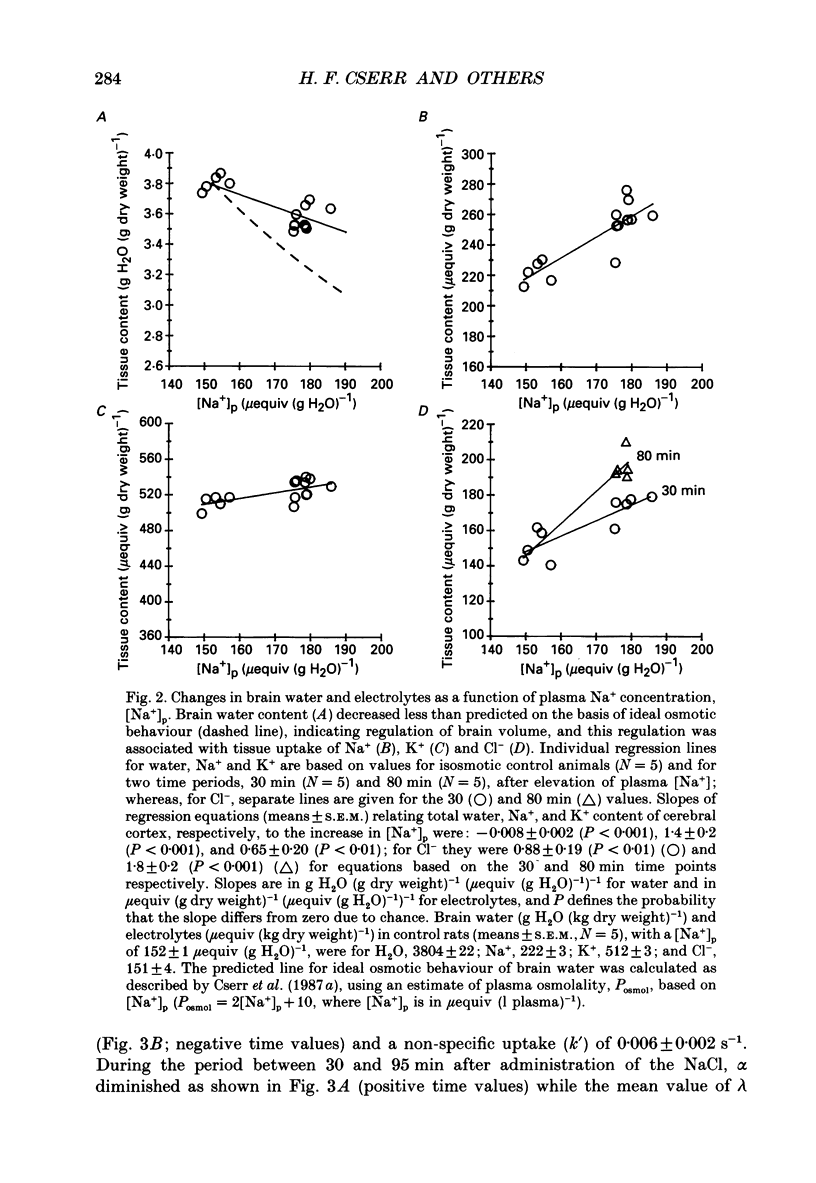
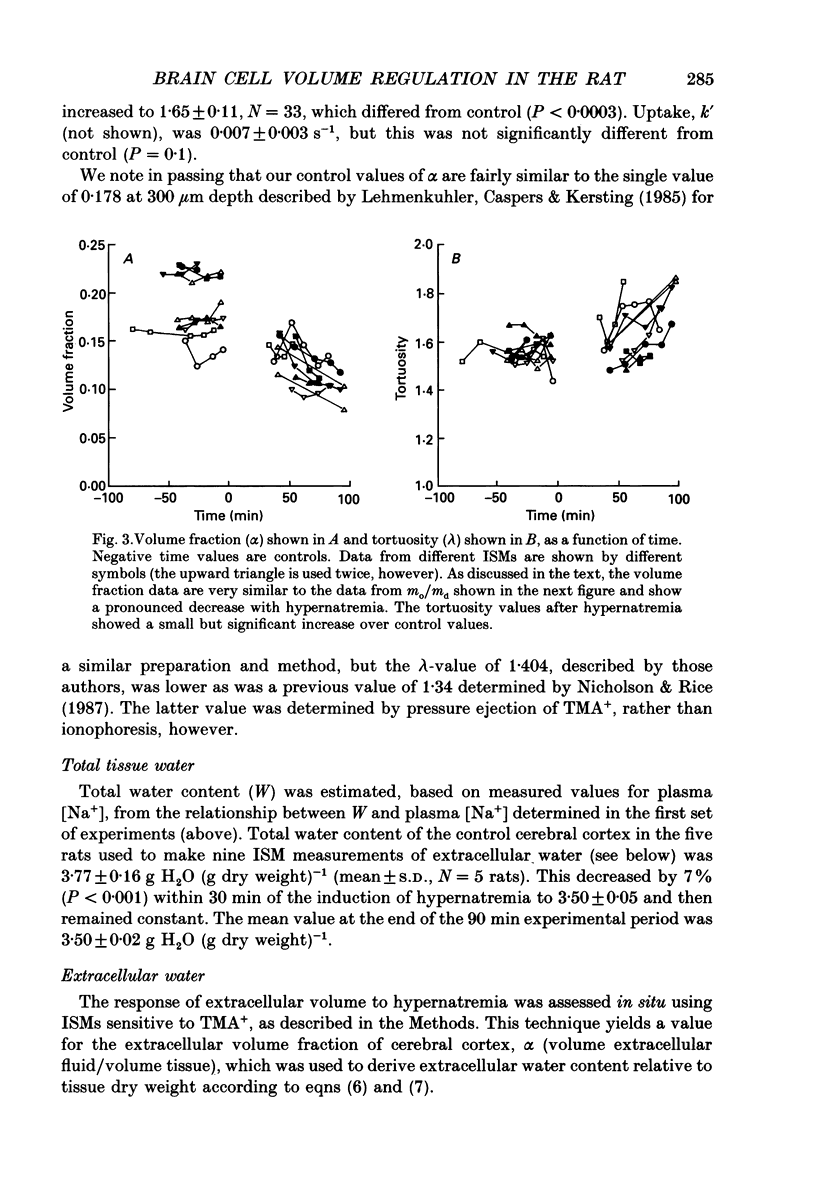
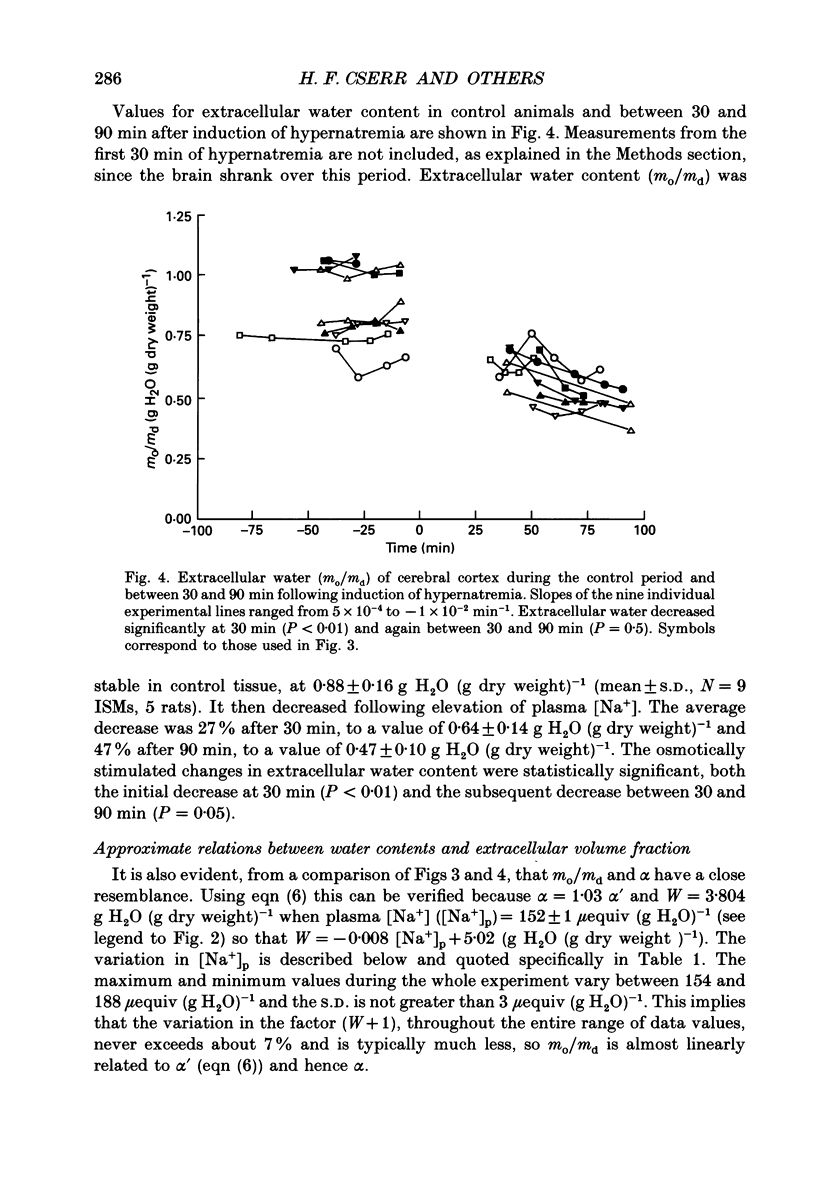





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coles J. A., Orkand R. K. Changes in sodium activity during light stimulation in photoreceptors, glia and extracellular space in drone retina. J Physiol. 1985 May;362:415–435. doi: 10.1113/jphysiol.1985.sp015686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr H. F., Bradbury M. W., Mackie K., Moody E. J. Control of extracellular ions in skate brain during osmotic disturbances. Am J Physiol. 1983 Dec;245(6):R853–R859. doi: 10.1152/ajpregu.1983.245.6.R853. [DOI] [PubMed] [Google Scholar]
- Cserr H. F., DePasquale M., Patlak C. S. Regulation of brain water and electrolytes during acute hyperosmolality in rats. Am J Physiol. 1987 Sep;253(3 Pt 2):F522–F529. doi: 10.1152/ajprenal.1987.253.3.F522. [DOI] [PubMed] [Google Scholar]
- Cserr H. F., DePasquale M., Patlak C. S. Volume regulatory influx of electrolytes from plasma to brain during acute hyperosmolality. Am J Physiol. 1987 Sep;253(3 Pt 2):F530–F537. doi: 10.1152/ajprenal.1987.253.3.F530. [DOI] [PubMed] [Google Scholar]
- DePasquale M., Patlak C. S., Cserr H. F. Brain ion and volume regulation during acute hypernatremia in Brattleboro rats. Am J Physiol. 1989 Jun;256(6 Pt 2):F1059–F1066. doi: 10.1152/ajprenal.1989.256.6.F1059. [DOI] [PubMed] [Google Scholar]
- Hansen A. J. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985 Jan;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Hebert S. C. Hypertonic cell volume regulation in mouse thick limbs. I. ADH dependency and nephron heterogeneity. Am J Physiol. 1986 Jun;250(6 Pt 1):C907–C919. doi: 10.1152/ajpcell.1986.250.6.C907. [DOI] [PubMed] [Google Scholar]
- Holliday M. A., Kalayci M. N., Harrah J. Factors that limit brain volume changes in response to acute and sustained hyper- and hyponatremia. J Clin Invest. 1968 Aug;47(8):1916–1928. doi: 10.1172/JCI105882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempski O., Chaussy L., Gross U., Zimmer M., Baethmann A. Volume regulation and metabolism of suspended C6 glioma cells: an in vitro model to study cytotoxic brain edema. Brain Res. 1983 Nov 21;279(1-2):217–228. doi: 10.1016/0006-8993(83)90180-4. [DOI] [PubMed] [Google Scholar]
- Lohr J. W., Grantham J. J. Isovolumetric regulation of isolated S2 proximal tubules in anisotonic media. J Clin Invest. 1986 Nov;78(5):1165–1172. doi: 10.1172/JCI112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr J. W., McReynolds J., Grimaldi T., Acara M. Effect of acute and chronic hypernatremia on myoinositol and sorbitol concentration in rat brain and kidney. Life Sci. 1988;43(3):271–276. doi: 10.1016/0024-3205(88)90317-7. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Phillips J. M. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol. 1981 Dec;321:225–257. doi: 10.1113/jphysiol.1981.sp013981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C., Rice M. E. Calcium diffusion in the brain cell microenvironment. Can J Physiol Pharmacol. 1987 May;65(5):1086–1091. doi: 10.1139/y87-170. [DOI] [PubMed] [Google Scholar]
- Pollock A. S., Arieff A. I. Abnormalities of cell volume regulation and their functional consequences. Am J Physiol. 1980 Sep;239(3):F195–F205. doi: 10.1152/ajprenal.1980.239.3.F195. [DOI] [PubMed] [Google Scholar]
- Pullen R. G., DePasquale M., Cserr H. F. Bulk flow of cerebrospinal fluid into brain in response to acute hyperosmolality. Am J Physiol. 1987 Sep;253(3 Pt 2):F538–F545. doi: 10.1152/ajprenal.1987.253.3.F538. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A., HOOPER N. K., CUSICK J. T. Brain electrolytes and cortical impedance. Am J Physiol. 1961 Jul;201:139–143. doi: 10.1152/ajplegacy.1961.201.1.139. [DOI] [PubMed] [Google Scholar]
- Wiig H., Reed R. K. Rat brain interstitial fluid pressure measured with micropipettes. Am J Physiol. 1983 Feb;244(2):H239–H246. doi: 10.1152/ajpheart.1983.244.2.H239. [DOI] [PubMed] [Google Scholar]


