Abstract
Blue light induced eye damage (BLED) belongs to modern diseases. It is an ophthalmic disease caused by prolonged exposure to electronic devices or screens containing a large amount of high-energy short waves (blue light). Specific symptoms include dryness and discomfort in the eyes, blurred vision, headache, insomnia, and in severe cases, it may also cause various eye diseases such as cataracts and glaucoma. At present, the development of health products and drugs for eye blue light injury faces many difficulties. Therefore, further exploration and research are needed on the pathogenesis, pathophysiology, and pharmacological mechanisms of blue light injury. Natural medicine ingredients and preparations have unique advantages in targeting eye blue light injury fatigue products due to their multi-component synergistic effects, overall regulation, and mild and safe characteristics. Starting from the disease-related mechanisms and pathophysiological characteristics of eye blue light injury, this article elucidates the pharmacological mechanisms of various drugs for treating eye blue light injury. At the same time, it reviews the research on in vitro cultured cell and animal model conditions for blue light injury eyes, in order to provide reference for subsequent blue light injury modeling experiments. And explore future research directions to provide new ideas and methods for the prevention and treatment of BLED.
Keywords: eye, blue light damage, pharmacological mechanism, modeling conditions, research direction
Graphical Abstract
1 Introduction
In recent years, with the popularity of electronic devices such as computers and smartphones, people are increasingly exposed to artificial light sources (Ouyang et al., 2020), and prolonged exposure to light has become a major challenge affecting public visual health. The phototoxicity of visible light, especially blue light, has been extensively studied. Research has shown that short wave blue light between 400 and 460 nm is the most harmful (Wang and Li, 2021), as it can induce symptoms such as eye fatigue, dryness, pain, blurred vision, headache, insomnia, etc. Because it can penetrate the cornea and lens, directly reaching the retina, causing irreversible photochemical damage, known as blue light hazard (Kara-Junior et al., 2011; van Norren and Vos, 2016). However, endogenous pretreatment can to some extent alleviate the damage of blue light to biological tissues. It is a natural protective mechanism in organisms, which can reduce the damage of harmful light exposure to photoreceptors and RPE cells by synthesizing photoprotective pigments (such as melanin and lipofuscin) to absorb blue light, activate antioxidant enzymes, neutralize free radicals, etc. The subthreshold micropulse yellow laser (SMYL) treatment effectively treats macular edema by stimulating retinal pigment epithelial (RPE) cells with light, reducing the number of inflammatory cytokines and hyper-reflective retinal spots (HRS) (Bonfiglio et al., 2022).
Blue light damage to the eyes has led to an increase in various ophthalmic diseases such as age-related macular degeneration (AMD) (Jin and Jeong, 2022; Xia et al., 2019), cataracts (Xiang et al., 2020), and keratitis (Li et al., 2020). Epidemiological studies have shown that AMD is a human retinal degenerative disease affecting people aged 55 and above in industrialized countries, and is the main cause of blindness. Studies have shown that long-term accumulation of light exposure (especially blue light) can lead to retinal degeneration, causing damage to RPE cells and photoreceptor cells. AMD is mainly caused by functional impairment and loss of RPE, indicating that blue light is involved in the generation and development of AMD. Among patients aged 75 and above, the risk of early AMD is 25%, and the risk of late AMD is 8%. As the population ages, the absolute number of AMD patients worldwide will increase (Thomas et al., 2021). It is expected that by 2040, there will be 288 million AMD patients worldwide, with Asia having the highest number of disease patients (Keenan et al., 2021).
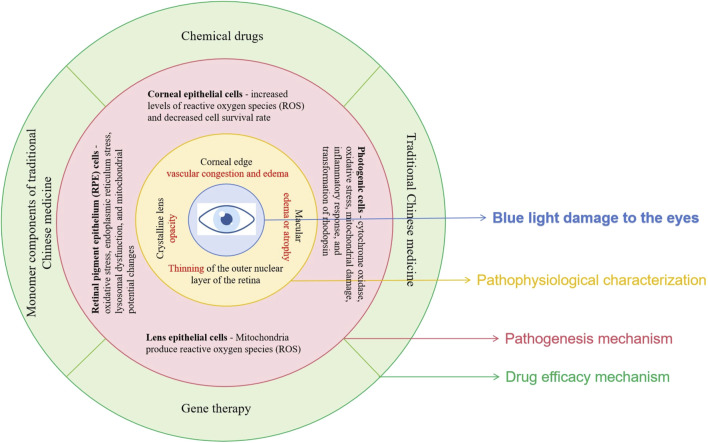
Many ophthalmic diseases caused by blue light damage can seriously affect people’s quality of life. To further investigate the pathogenesis and treatment methods of blue light damage, Based on the pathogenesis and pathophysiology of eye blue light injury, this article summarizes the pharmacological mechanisms of chemical small molecule drugs, natural drugs, and gene drugs related to the treatment of eye blue light injury. And summarize and organize the modeling conditions of cells and animals damaged by blue light, providing reference for subsequent blue light damage modeling experiments. Simultaneously exploring future research directions in order to lay the foundation for the application and development of natural medicine in the treatment of eye diseases.
2 Pathological characteristics and pathogenesis of blue light damage in the eyes
2.1 Pathophysiological characterization
The pathways of blue light damage mainly include oxidative stress, endoplasmic reticulum stress and oxidative DNA damage, inflammatory response, mitochondrial damage and cell apoptosis, lysosomal autophagy and vascular endothelial damage (Fan et al., 2022; Zhang H. et al., 2023). On the ocular surface, blue light irradiation can induce disordered autophagy levels in corneal stromal cells, affecting ocular surface function (Niwano et al., 2014). High energy and prolonged exposure to blue light can also penetrate the cornea and enter the lens, and the pathogenesis of cataracts may be closely related to oxidative damage to lens epithelial cells (Wang and Li, 2021; Liu, 2019). Blue light enters the retina, stimulating photoreceptors in the retina and photosensitive pigments in pigment epithelial cells, such as rhodopsin in photoreceptors and lipofuscin in pigment epithelial cells, producing a large amount of free radicals and reactive oxygen species (ROS). Lipid peroxidation is caused by ROS, which can lead to oxidative stress. Oxidative stress and photochemical damage can activate the apoptotic signaling pathway within cells, resulting in programmed cell death (Nan et al., 2023; Marie et al., 2018). In addition, the fluorescent group A2E of lipofuscin is activated by blue light, releasing free radicals and causing lipid peroxidation. This not only activates inflammatory reactions but also causes DNA breakage, while inhibiting the normal function of mitochondria and lysosomes (Moreira and Oliveira, 2011; Davies et al., 2001; Alaimo et al., 2019).
According to morphological observations, blue light irradiation can cause congestion and edema of corneal limbal blood vessels, dilation and thinning of the central part of the cornea, protrusion forward, and increased curvature. Long term exposure to blue light can cause the lens to become opaque, and the degree of opacity of the lens increases with prolonged exposure time. After blue light injury, the outer nuclear layer of the retina significantly thins, and due to degeneration and necrosis of some cells, the loss of outer nuclear layer cells decreases. Because ONL belongs to the photoreceptor cell layer, it often experiences a decrease in thickness and a decrease in the number of rod and cone cells when damaged, leading to problems such as decreased visual sensitivity, loss of field of view, and color vision abnormalities (Choi et al., 2016). Due to the release of pro-inflammatory cytokines, the permeability of blood vessels increases, and some harmful components in the blood can seep into the retina, leading to partial cell apoptosis. In addition, nuclear condensation and some irregular nuclei may occur, and fragmented nuclei can be seen (Shang et al., 2014). The pathological changes of blue light damage to the ocular surface, crystalline lens, and retina mentioned above may lead to various eye diseases, such as keratitis, dry eye syndrome, cataracts, myopia, and age-related macular degeneration (Figure 1).
FIGURE 1.
Changes in eyeball structure before and after blue light.
2.2 Pathogenesis
2.2.1 Effects of blue light on cornea and lens
Research has shown that blue light toxicity is not limited to the retina, but can also damage the ocular surface through oxidative stress, inflammatory response, and cell apoptosis, and is associated with the formation of ocular surface inflammation and dry eye syndrome (Lee et al., 2016; Chong, 2022). High energy blue light is close to ultraviolet light in the spectrum, so excessive exposure to blue light poses a great risk to the ocular surface. Blue light has high energy and can penetrate the cornea and lens to reach the retina. Due to its location in the anterior part of the eyeball, the cornea is the first point of contact for light entering the eye (Zhao et al., 2018). Therefore, blue light can directly act on corneal epithelial cells and endothelial cells, leading to a decrease in cell survival rate (Marek et al., 2018). At the same time, the content of reactive oxygen species (ROS) and the secretion of interleukin IL-1 β in the cornea will also increase, further mediating cellular oxidative damage and inflammatory response. The transparency of the crystalline lens decreases with age, leading to a gradual increase in absorbance within the blue light spectrum (Lee et al., 2016). Certain structural proteins, protein metabolites, and enzymes in the crystalline lens can absorb blue light, which can induce the production of reactive oxygen species (ROS) in the mitochondria of lens epithelial cells and lead to cell apoptosis through the transforming growth factor - β/Smad3 signaling pathway. This can lead to the gradual darkening and yellowing of the crystalline lens, which in turn can trigger cataracts (Cougnard-Gregoire et al., 2023). In addition, blue light damage can also trigger inflammatory reactions on the ocular surface, with damaged cells releasing inflammatory factors that attract the infiltration of inflammatory cells, further exacerbating tissue damage (Ouyang et al., 2023).
2.2.2 Effects of blue light on the retina
Research has shown that the mechanism of retinal blue light damage is mainly related to the production of free radicals, lipid peroxidation, lipofuscin, rhodopsin, Ca2+levels, and other factors. The main ones involved are RPE cells and photoreceptor cells (Qin et al., 2019). Short term (minutes to hours) exposure to high irradiance blue light can cause damage to RPE cells (Tosini et al., 2016). Lipofuscin accumulates extensively in RPE cells, and its toxicity is determined by the total content of its main fluorescent group, N-subretinoyl-N-retinol-ethanolamine (A2E). Blue light can accelerate the oxidation of cells by A2E, leading to oxidative stress response and inducing RPE cell apoptosis. Researchers can search for effective candidate drugs that may treat blue light damage based on this mechanism (Conti et al., 2021). Lipofuscin generates a large amount of ROS under blue light irradiation, which triggers endoplasmic reticulum stress (ER stress) in RPE cells, manifested by upregulation of GRP 78 and CHOP protein expression, and then induces cell apoptosis through activation of Caspase-3 and decreased expression of Bcl-2 (Zhao, 2015). Blue light irradiation leads to dysfunction of lysosomes, decreased ability of lysosome phagocytosis and autophagy, accumulation of lipofuscin in cells, disruption of epithelial barrier, and ultimately resulting in cell apoptosis; Blue light can also affect the intracellular Ca2+concentration in RPE cells, causing changes in mitochondrial transmembrane potential and leading to cell apoptosis (Yang et al., 2023). In addition, blue light irradiation can also cause damage to retinal vascular endothelium. Hypoxia inducible factor 1 α (HIF-1 α) is significantly expressed in RPE, and as the main hypoxia sensor, it can regulate the expression of vascular endothelial growth factor (VEGF). VEGF plays a key role in angiogenesis and vascular permeability, but its overexpression can damage endothelial function and epithelial mesenchymal transition (Zhang Y. et al., 2023). Research has found that the expression of HIF-1 α and VEGF is elevated in the retina of rabbits with light damage, indicating that light exposure can induce endothelial dysfunction in the retina (Wang et al., 2016). Therefore, the mechanism of blue light induced RPE cell apoptosis is related to oxidative stress, endoplasmic reticulum stress, lysosomal dysfunction, and mitochondrial potential changes.
Long term (several days to several weeks) exposure to low irradiance blue light can affect the wavelength activated photoreceptors (Tosini et al., 2016). High intensity blue light can cause irreversible inhibition of cytochrome oxidase, damage mitochondrial function and trigger cell apoptosis, while reducing sodium potassium ATPase activity, causing ion imbalance and increased osmotic pressure inside and outside the cell, leading to cell edema and organic damage, ultimately causing photoreceptor degeneration (Osborne et al., 2017). The degree of damage to photoreceptor cells (including cone cells and rod cells) exposed to blue light is higher, mainly due to the upregulation of retinal reactive oxygen species (ROS) production by blue light, which leads to mitochondrial damage in photoreceptor cells through oxidative stress. ROS induces the activation of mitogen activated kinase (MAPK), downregulates the phosphorylation level of extracellular regulatory protein kinase (p-ERK), upregulates activated nuclear transcription factor (NF-KB), activates autophagy pathway, and leads to apoptosis of retinal photoreceptor cells. After exposure to light, the expression level of pro-inflammatory factor miR-155 increases in the retina, while the expression level of anti-inflammatory factor SHIP1 decreases, leading to degeneration of retinal photoreceptors. Activation of NLRP3 inflammasome mediates the production of caspase-1 and 1L-1 β, leading to damage to retinal photoreceptor cells (Qin et al., 2019). Due to the presence of rhodopsin in rod cells and S-opsin in cone cells (Wheway et al., 2019), the conversion of rhodopsin by blue light can cause damage to both cone and rod cells, leading to blurred vision in the human eye. Due to the presence of rhodopsin, the photon capture ability of the retina is significantly enhanced under blue light irradiation, leading to an increase in the number of light induced cell deaths. Short wavelength LED light causes aggregation of short wavelength visual proteins, leading to degeneration of cone cells in the short term and severe damage to photoreceptor cells (Nakanishi et al., 2013). Blue light stimulation can activate the activity of prostaglandin synthase G/H. Prostaglandin synthase G/H is located in the inner and outer segments of rod and cone cells, promoting prostaglandin synthesis. It acts as a pigment group to absorb blue light, triggering oxidative reactions and producing a large amount of oxygen free radicals, leading to retinal damage and cell apoptosis (Xu and Wang, 2007). Therefore, the damage mechanism of blue light on photoreceptors is related to cytochrome oxidase, oxidative stress, mitochondrial damage, inflammatory response, and conversion of rhodopsin (Figure 2).
FIGURE 2.
Mechanism of retinal blue light damage (Cougnard-Gregoire et al., 2023) (Quoted from Ophthalmology and therapy Journal).
3 Common models for research on blue light damage to eye diseases
3.1 Summary of cell modeling conditions
The replication of disease models through ex vivo cells has the advantages of short experimental time, convenient operation, easy observation, good controllability, and reduced use of experimental animals. Currently, most studies on the mechanism of blue light induced retinopathy and drug anti blue light activity also use in vitro experiments. Therefore, selecting a suitable cell model is crucial.
The retina plays an important role in visual transduction, with retinal photoreceptors and RPE cells involved in visual formation. Photoreceptors are divided into rod cells and cone cells. Rod cells contain rhodopsin, while cone cells contain opsin (Wheway et al., 2019). When exposed to blue light, both rhodopsin and opsin undergo varying degrees of damage, leading to retinal degeneration. RPE cells are crucial for vision, maintaining the vitality and function of photoreceptors by engulfing detached outer segments of photoreceptors (Hellinen et al., 2019). In addition, they have multiple functions such as supporting the neuroretina and choroid in the eye, as well as immune suppression (Sugita et al., 2021), and are commonly used in ophthalmic research and general epithelial cell research (Hazim et al., 2019).
Based on the physiological structure of the retina and the characteristics of blue light induced retinal lesions, mouse retinal photoreceptor cells (661W cells) and human retinal pigment epithelial cells (ARPE-19 cells) are commonly used cell models for light damage (Figure 3).
FIGURE 3.

Schematic diagram of cell modeling.
3.1.1 661W cell model
661W cells are derived from retinal tumors formed in transgenic mice expressing SV40 large T antigen under the control of the IRBP (photoreceptor retinol binding protein) promoter. They are currently widely used in the study of glaucoma (Sayyad et al., 2017), macular degeneration (Huang et al., 2022), intraretinal neurodegeneration (Kunimi et al., 2021), and blue light induced retinal lesions. A study used a 661W cell model to investigate the improvement of blue light induced subcellular damage by swiftgrass extract. After drug pretreatment for 1 h, 661W cells were exposed to blue light with a wavelength of 464 nm and a light intensity of 0.38 mW/cm2 for 24 h. It was found that swiftgrass extract protected cells from blue light induced cell death by inhibiting the increase in ROS production, and had a protective effect on mitochondria and lysosomes (Yamazaki et al., 2024); At the same time, ARPE-19 cells and 661W cells were used to investigate the anti blue light activity of Dendrobium polysaccharides. The modeling conditions were irradiated with 2500lux light for 3 h, 6 h, and 16 h. After 6 h of illumination, there was a significant difference in cell survival rate between the model group and the control group, indicating successful modeling (Hsu et al., 2024); The anti blue light activity of astaxanthin was investigated using a 661W cell model. LED blue light tubes with a wavelength of 450 ± 20 nm were selected and placed in an incubator. After pretreatment with astaxanthin at different concentrations for 1 h, the cells were exposed to a light intensity of 2000lux for 24 h. After modeling was completed, the cells were further cultured in a complete medium containing the drug for 24–48 h, and the anti blue light activity of astaxanthin was evaluated by measuring ROS levels and mitochondrial damage degree (Lin et al., 2020).
3.1.2 ARPE-19 cell model
The ARPE-19 cell line is derived from adult retinal pigment epithelial (RPE) explants, and ARPE-19 cells can be extensively expanded, providing a relatively stable source of retinal epithelial cells for functional and genetic research (Golconda et al., 2023). Lipofuscin is a byproduct of RPE cells engulfing the outer segment of photoreceptor cells, and its main fluorescent group is N-vinylidene retinyl-N-vinylethanolamine (A2E). When A2E is exposed to blue light, it is oxidized, inducing ROS production, oxidative stress, inflammatory response, etc., leading to RPE cell damage (Cho et al., 2023). Compared with 661W cells, ARPE-19 cells are more widely used in blue light damage research. Evaluate the anti blue light damage and retinal protective activity of the main active ingredient, hesperidin, in chrysanthemum using ARPE-19 cells. After pretreatment with A2E, expose the cells to blue light at a wavelength of 430 nm for 2 h, and the cell survival rate decreases by 50%, indicating the successful establishment of a light damage model (Feng et al., 2021); A study comparing two types of RPE cells, hTERT-RPE1 and ARPE-19, found that compared to hTERT-RPE1 cells, ARPE-19 cells have a stronger barrier function formed by tight junctions between cells. However, after being irradiated with blue light at a wavelength of 468 nm and a light intensity of 118.1 W/m2 for 4 h, the barrier function was impaired (Feng et al., 2021). The modeling conditions for the blue light damaged cell model are shown in (Table 1).
TABLE 1.
Summary of modeling conditions for ARPE-19 cell model with blue light damage.
| Wavelength | Light intensity | Light time | Other conditions | Cell survival rate (%) | References |
|---|---|---|---|---|---|
| 430 nm | 6000 lux | 10–min | A2E pretreatment, drug pretreatment, and incubation for 6 h after modeling | 47.8 | Cho et al. (2023) |
| 430 nm | 2 h | A2E pretreatment, drug pretreatment | 50 | Feng et al. (2021) | |
| 468 nm | 118.1 W/m2 | 4 h | - | Cellular barrier function impaired | Ozkaya et al. (2019) |
| 450 nm | 1500 lux | 24 h | Drug pre protection | 49.3 ± 10.5 | Shimizu et al. (2022) |
| 450 nm | 2.3 mW/cm2 | 12 h | Drug pre protection | — | Wang et al. (2023) |
| 430 nm | 1000 lux | 1 h | A2E pretreatment, drug pretreatment | — | Xie et al. (2021) |
3.2 Summary of animal modeling conditions
Blue light damage animal modeling is the use of specific wavelengths of blue light to cause certain damage to animal eyes, in order to simulate the retinal damage that may occur in humans after prolonged exposure to blue light sources such as electronic screens. Its experimental technology is relatively mature and has a wide range of sources. The dorsal central region of the rat retina is considered a functional analogue of the human macular, which is the sharpest visual area (Gao et al., 2020). It has advantages such as simulation, controllability, repeatability, and safety. Therefore, through this model, researchers can better observe the impact of blue light on the visual system and explore methods for preventing or treating light damage, thereby providing scientific basis for protecting human visual health.
The animal model of blue light damage is generally selected from 5–6 week old SD rats, which need to undergo 7 days of adaptive feeding and dark maintenance before officially starting modeling. Because dark maintenance can eliminate the effects of other light sources on rat eyes and increase the photosensitivity of rat eyes. The blue light wavelength selected for the experiment is generally concentrated around 450 nm, and the blue light irradiation time is mostly 3 h, 6 h, 8 h, 12 h. Among them, the 12 h light exposure/12 h dark cycle is used for the 12 h light exposure, and the duration of irradiation depends on the light intensity of the selected lamp source. It is more appropriate to choose a duration of 2 weeks (14 days) for light exposure modeling. Studies have shown that blue light illumination within the range of 2000–10000 lux can cause damage to the retina of rats (Shang et al., 2014). There is a certain conversion relationship between blue light illumination and blue light irradiance, for example, at 450 nm blue light, 1W/m2 = 28lux, and color temperature is also related to blue light irradiance. During experiments, a thermometer can be placed inside the cage to investigate the temperature inside the cage. If the temperature is too high, certain temperature dispersion measures need to be taken. At present, animal experiments on blue light induced damage in rats are mostly based on blue light intensity. The distance from the light source to the rat’s eyes needs to be determined by considering the actual height of the rat cage and the magnitude of blue light intensity at different distances (Figure 4).
FIGURE 4.
Schematic diagram of animal modeling.
In addition, if a positive drug group is required to evaluate drug efficacy, the blue light irradiation positive drug group generally needs to be administered orally 1 week before modeling and continue until the end of light exposure. The relevant inspection indicators are tested after the end of light exposure, and in vivo intraocular examination can choose slit lamp microscopy, fundus photography, and ERG detection. After the completion of the live examination, blood samples are usually collected and euthanized from the rats 24 h later, and the eyeballs are removed to prepare retinal sections. The examination indicators generally include HE staining, immunohistochemistry, Western blot detection, TUNEL assay, oxidative stress (ROS), and transmission electron microscopy (TEM) (Shang et al., 2014), in order to analyze the protective effect of drugs on the retina of rats with blue light injury. The modeling conditions for the blue light damaged cell model are shown in (Table 2).
TABLE 2.
Summary of modeling conditions for blue light damage animal models (rats, rabbits).
| Light source | Animal type | Wavelength | Duration of illumination | Number of days | Blue light illuminance | Blue light irradiance | Color temperature | Distance | References |
|---|---|---|---|---|---|---|---|---|---|
| OSRAM DULUXL BLUE LED Blue light | SD rat (5–6 weeks old) | 480 nm | 12 h | 14 days | — | — | 8000 k | 10 cm | Chen (2021) |
| JDL Corporation (Hangzhou China)LED Blue light | SD rat | 435–445 nm | 8 h | 10 days | 31,360/44240lux | 11.2 W/cm2 | — | 35 cm | Li et al. (2021b) |
| 450 nm Semiconductor blue laser light source Zhuhai Aike Optoelectronics Technology Co., Ltd. | C57BL/6J mouse | 450 nm | 3 min irradiation/30 min rest-6 cycles,12 times | 10 days | 7.14/10.0725lux | 2.55 mW/cm2 Intermittent low amplitude illumination | — | — | Zhang (2023a) |
| 450 nm | 6 h | 1 days | 7.14/10.0725lux | 2.55 mW/cm2 Continuous low amplitude illumination | — | — | |||
| 450 nm | 3 min irradiation/30 min rest-6 cycles,12 times |
10 days | 35.672/50.323lux | 12.74 mW/cm2 High amplitude illumination interruption | — | — | |||
| 450 nm | 6 h | 1 days | 35.672/50.323lux | 12.74 mW/cm2 Continuous high amplitude illumination | — | — | |||
| BlueDog Technology Corporation Ltd.,Taibei, Taiwan | SD rat | 460 nm | 12 h | 3, 9, 28 days | 750lux | 0.1 W/nm (power) | 6500 k | 20 cm | Zhang (2023b) |
| Blue light board (Zhongshan Gongxuan Optoelectronics Technology Co., Ltd.) | SD rat | 455 nm | 3 h | 14 days | 3,000 ± 50 lx | — | — | 40 cm | Cheng et al. (2024) |
| Single wavelength LED Suzhou Jingzhi Medical Technology Co., Ltd. | SD rat | 460 nm | 10 min | 1 month | 1000 lux | — | — | 15 cm | Zhu (2021) |
| 460 nm | 10 min | 1 month | 2000 lux | — | — | 15 cm | |||
| Light damage tester (self-made) | SD rat | 452 nm | 1.5 h | 1 days | 1,400∼1 500 lux | — | — | — | Yang et al. (2020) |
| Zhongshan Gongxuan Optoelectronics Technology Co., Ltd. | BN rat | 451 nm | 3 h | 1, 3, 7, 14 days | 1,000 ± 100 lux | — | — | — | Yu et al. (2023a) |
| Lighting and Electromagnetic Department of the Center for Building Science and Technology (CSTB, Saint Martin d’here, France) | Wistar rat | 449, 467, 473 nm | 6, 12, 18, 24, 48, 72 h | 7 days | — | 0.0026 J/cm2(Ocean Optics- R2000+) | — | 25 cm | Jaadane et al. (2015) |
| LED light strip (no manufacturer) | Wistar rat (12 weeks old) | 463 ± 10 nm | 12 h | 10 days | 150 lx | 3.8 W/m2 | — | — | Ziolkowska et al. (2023) |
| — | 3-week-old rabbits (pigmented rabbits and albino rabbits) | 450 ± 50 nm | 12 h | 21 days | 420, 560, 1,120, 1820lux/592.5, 790, 1,580, 2567.5 lux | 150, 200, 400 and 650 mW/cm2 | — | — | Iseli et al. (2016) |
4 Research progress on drugs for preventing and treating eye diseases caused by blue light damage
The drug therapy for anti blue light damage can be divided into chemical drugs, natural drugs, and gene drugs. At present, a series of research results have been achieved on how to alleviate the damage of blue light to eye cells, such as RPE cells and photoreceptor cells, through drugs. Based on the pathogenesis of blue light damage, anti blue light damage drugs can include free radical scavengers, antioxidants, anti-inflammatory drugs, and gene therapy (Pan et al., 2023). Oxidative stress plays an important role in the harm caused by blue light. Research has shown that effective antioxidant extracts related to eliminating free radicals reduce oxidative damage caused by blue light, thereby improving clinical symptoms of ocular surface in dry eye mouse models (Zhu et al., 2022). These three types of drugs each have their own advantages and limitations. The research and application of chemical drugs are relatively extensive, but there may be certain side effects. Natural medicines provide a more natural option, but their effectiveness and safety require further research to confirm. The application of gene therapy in eye diseases is an emerging field, but its technical complexity and potential risks also require further research and evaluation.
4.1 Medication routes and obstacles for the treatment of eye diseases
The anterior segment of the eye is composed of the cornea, conjunctiva, iris, ciliary body, crystalline lens, and aqueous humor, while the posterior segment of the eye includes the posterior two-thirds of the eye, including the vitreous, retina, choroid, and optic nerve (Vaneev et al., 2021). The blood-ocular barrier (BOB) includes the blood-aqueous barrier (BAB) located in the anterior segment of the eye and the blood-retinal barrier (BRB) located in the posterior segment of the eye, which can restrict the entry of drugs from the blood into the eye and is the main obstacle in treating eye diseases (Lyu et al., 2024). Drugs enter the body orally, are absorbed through the gastrointestinal tract, enter the liver through the portal vein, and some drugs may be metabolized by the liver or bind with plasma proteins before entering the systemic circulation and ultimately reaching the eyes. Among them, drugs need to overcome the first pass effect of the liver and the blood-ocular barrier to reach the eye, so their bioavailability is relatively low. The routes of drug delivery to the eye include local, periocular, intravitreal, suprachoroidal, and subretinal. Intravitreal, suprachoroidal, and subretinal administration is an ideal choice for treating posterior ocular lesions (Varela-Fernandez et al., 2020), as they can bypass more anterior barriers such as tear film, cornea, and sclera, but may cause injection related complications. External use mainly involves applying medication to the surface of the cornea or conjunctiva through eye drops or ointment. Eye surface administration is the most commonly used route of administration, but drugs are easily washed away by tears and difficult to penetrate the corneal barrier, so their therapeutic effect on posterior ocular diseases is limited. The development of new ocular drug delivery systems, such as nanoparticles, liposomes, and hyper choroidal injection (Singh et al., 2024; Wu et al., 2024), can effectively deliver drugs to the posterior segment of the eye (Puglia et al., 2021), which is expected to improve the ocular bioavailability and therapeutic efficacy of drugs.
4.2 The efficacy and mechanism of chemical drugs
At present, the research and application of chemical drugs for the treatment of BLED have become quite popular. The mechanism of action of these drugs is mainly achieved through antioxidant effects and the clearance of free radicals to achieve therapeutic effects. Therefore, anti blue light damage chemical drugs mainly include free radical scavengers and antioxidants. In addition, enzyme activity protectants, optic nerve protectants, calcium ion antagonists, and hormone drugs have also played a positive role in protecting retinal cells. The summary of the pharmacological mechanisms of chemical drugs for preventing blue light damage is shown in (Table 3).
TABLE 3.
Summary of the pharmacological mechanisms of chemical drugs for preventing blue light damage.
| Chemical compound | Type | Protection mechanism | Administration method | Dosage | References |
|---|---|---|---|---|---|
| Dimethyl thiourea (DMTU) | Free radical scavenger | Dimethylthiourea can reduce the content of malondialdehyde (MDA) in the retina of rats after photodamage, and has a protective effect on the retina | Rat Intravenous injection |
50 mg/kg | Jin and Wan (2005) |
| Vitamin C | Free radical scavenger | Vitamin C has an anti lipid peroxidation effect and can significantly reduce the DNA damage of peripheral lymphocytes in healthy individuals caused by hydrogen peroxide. Vitamin C can increase the expression of Bcl-2 in retinal pigment epithelium after exposure to light, thereby inhibiting cell apoptosis | Rat Gavage method |
100 mg/kg | Yin et al. (2011), Du et al. (2022) |
| Vitamin E | Antioxidant | Vitamin E can enhance the activity of intracellular SOD, resist lipid peroxidation, and reduce DNA damage of peripheral lymphocytes in healthy individuals caused by hydrogen peroxide,can protect photoreceptor cells | Human retinal pigment epithelium (hRPE) cells | 10, 50, 100 μmol/L | Ueda et al. (2016), Wang et al. (2022) |
| Hydrogen sulfide | Antioxidant | H2S can clear intracellular ROS, increase SOD activity, and reduce intracellular oxidative stress and damage | Rat Sodium hydrosulfide as donor intraperitoneal injection |
80, 120 μmol/kg | Zhu (2022) |
| Butylated hydroxytoluene (BHT) | Antioxidant | Butyl hydroxytoluene has the effect of inhibiting lipid peroxidation and malondialdehyde (MDA) production | — | — | Jiang (2002) |
| N-acetylcysteine (NAC) | Antioxidant | It can inhibit the production of ROS and the activation of NF - κ B, protect photoreceptor cells from damage caused by blue light, enhance cell viability, suppress cell death and erythropoietin, inhibit blue light induced caspase-3/7 activation and autophagy | Mouse cone photoreceptor derived cells (661 W) | — | Kuse et al. (2014) |
| Astaxanthin (AST) | Antioxidant | Astaxanthin can activate the Nrf2 signaling pathway, promote the expression of antioxidant enzymes and phase II detoxification genes, reduce intracellular ROS levels, alleviate mitochondrial damage, and ultimately protect ARPE-19 cells from oxidative stress damage induced by blue LED. | Mouse photoreceptor cells (661W) | 1, 5, 10, 20 and 50 μM | Liu et al. (2021), Lai et al. (2020) |
| α-lipoic acid (ALA) | Antioxidant、Iron chelator | Alpha lipoic acid can inhibit iron mediated oxidative damage, suppress excessive iron accumulation, and may weaken retinal damage caused by oxidative stress through Nrf2 related endogenous antioxidant stress pathways. Lipoic acid can also counteract the accumulation of lipid peroxides inside the lens by increasing the activity of SOD and GSH Px, which may have a certain protective effect on photooxidative damage to the lens | ARPE-19 cells | 150 μmol L-1 | Yu et al. (2023b), Zhao et al. (2014) |
| Lipoic acid-niacin diad (N2L) | Antioxidant | N2L can increase the expression of various antioxidant proteins and counteract oxidative stress damage caused by blue light, which may be related to its upstream protein Nrf2/ARE pathway | Intraperitoneal injection | 1.0, 2.5, 5.0 mg/kg | Cheng et al. (2024) |
| Fucoxanthin | Preventive antioxidant | Fucoidin has a significant improvement effect on visible light induced RPE cell phagocytic dysfunction by regulating the Nrf2 signaling pathway, and has good light absorption performance and antioxidant activity | - | 0.1, 1, 10 mg/kg/d | Liu et al. (2016), Chen et al. (2021) |
| Lutein | Enzyme activity protectant | Participate in the formation of retinal macular pigment, quench oxygen free radicals, inhibit lipid peroxidation and the expression of c-fos gene to exert its protective effect | Rat Intravitreal injection Gavage method |
0.5, 1.0 and 2.0 mg/mL; 25, 50, 100 mg/kg | Wang et al. (2008), Yang et al. (2020) |
| Zeaxanthin | Enzyme activity protectant | Blue light inhibits the activity of retinal cytochrome oxidase and sodium potassium ATPase, activates the activity of prostaglandin synthase G/H, and thus causes retinal damage | Mouse Gavage method |
10 mg/kg/d | Sahin et al. (2019a), Ren et al. (2017) |
| Methoxyphenylpropionic Acid | Enzyme activity protectant | Non selective prostaglandin synthase G/H inhibitors can prevent a decrease in retinal ERGa and b-wave amplitude, protecting retinal function | — | — | Xu and Wang (2007) |
| Ciclofenaziae | Enzyme activity protectant | Non selective prostaglandin synthase G/H inhibitors can prevent a decrease in retinal ERGa and b-wave amplitude, protecting retinal function | — | — | Xu and Wang (2007) |
| Vitamin B1 | Optic nerve protector | Vitamin B1 can reduce oxidative stress and protect cells from damage caused by free radicals; It can also protect the optic nerve from damage by enhancing the adaptability of nerve cells to energy demands | Oral administration | 10 mg | Lakhan and Vieira (2010), Bai et al. (2023) |
| Methycobal | Optic nerve protector | Methycobal has a protective effect against glutamate induced neurotoxicity in retinal cells; It also has antioxidant and anti-inflammatory effects, helping to reduce oxidative stress and inflammatory reactions caused by photodamage | — | — | Kikuchi et al. (1997) |
| 17-b estradiol | Optic nerve protector | Protect retinal mullium cells from H2O2 mediated oxidative damage, weaken H2O2 mediated cytotoxicity, and prevent light induced retinal degeneration and photoreceptor cell apoptosis | — | — | Shaban and Richter (2002) |
| Glutamate receptor antagonist MK-801 | Optic nerve protector | It can reduce the proliferation of retinal pigment cells, alleviate laser damage to the retina, regulate the repair of retinal damage through glutamate, and has neuroprotective and anti proliferative effects on nerve cells | Rat Intramuscular injection |
2 mg/kg | Yan (2006), Jiang (2002) |
| Flunarizine | Calcium antagonist | Flunarizine can prevent inositol triphosphate from releasing calcium ions into cells, showing sufficient protective effects on retinal pigment epithelial cells and photoreceptors | — | — | Xu (2008) |
| Flunarizine hydrochloride (FNZ) | Calcium antagonist | It can reduce the production of intracellular oxygen free radicals and stabilize the cell membrane | Rat Eye drops administration; Gavage method; Intravenous injection |
Eye drops and gastric lavage group 14 mg/kg, Intravenous injection 5 mg/kg | Gong (2011), Dai (2020) |
| Taurine | Antioxidant、Calcium antagonist | Reduce intracellular calcium ion concentration to weaken calcium dependent Fas mediated apoptosis of neutrophils. And it can effectively eliminate free radicals, but it requires early administration | mouse; Intravenous injection | 200 mg/kg | Gong (2011), Tao et al. (2019) |
| Glucocorticoid | Hormones | It can alleviate inflammatory reactions, maintain cell membrane structure, protect microcirculation, block lipid peroxidation, reduce the production and damage of free radicals, and have a preventive and therapeutic effect on retinal photodamage | Intravitreal injection | — | Feng (2012), Li and Tang (2023) |
| Estrogen | Hormones | Inhibit apoptosis of photoreceptor cells, inhibit the synthesis of alpha receptor nitric oxide synthase (NOS), reduce the production of nitric oxide, and alleviate retinal damage | Rat Hypodermic injection |
50 μg/kg | Chen (2005), Xu (2008) |
In addition, blue light damage is a cause of various eye diseases. Although current treatment methods are limited and mainly focus on relieving symptoms and delaying disease progression, researchers are actively exploring new treatment strategies. Research shows that both blue light damage and diabetes retinopathy (DR) involve the damage and dysfunction of retinal nerve cells. The locally administered NOX4 inhibitor GLX7013114 can effectively protect retinal neurons and amacrine cells, and alleviate oxidative stress and inflammatory reactions (Dionysopoulou et al., 2023). It may become a new drug for treating blue light damage. In addition to GLX7013114, lidocaine pretreatment has also been shown to prolong the lifespan and improve the function of retinal ganglion cells (RGCs) (Chou et al., 2018), which may provide another neuroprotective approach for treating blue light injury.
4.3 The efficacy and mechanism of natural medicine
At present, research on natural medicines for combating blue light damage is mostly focused on antioxidants and anti-inflammatory drugs. For example, caffeine, as an approved drug for clinical use, has anti-inflammatory and neuroprotective effects, and its mechanism of action is oxidative stress and inflammatory response. Currently, studies have shown that caffeine may be a potential candidate for the treatment of retinal degeneration (Conti et al., 2021). The research results indicate that natural medicine has significant therapeutic effects in preventing and treating eye diseases caused by blue light (Li C. et al., 2021). Natural medicine, with its multi-component and multi-target characteristics, can play a role in different stages. It can not only effectively eliminate free radicals and reduce oxidative damage, but also inhibit inflammatory reactions and protect the integrity of eye cells. The following summarizes the individual components of natural medicine for preventing blue light damage and their pharmacological mechanisms, in order to provide reference for research and application in related fields (Figure 5). The summary of the pharmacological mechanisms of natural medicine monomers for preventing blue light damage is shown in (Table 4); The summary of the pharmacological mechanism of natural medicine for preventing blue light damage is shown in (Table 5).
FIGURE 5.
The compound structure of traditional Chinese medicine monomers includes:1 Procyanidin B2; 2 Cyanidin 3-O-glucoside chloride; 3 Delphinidin; 4 Resveratrol; 5 Quercetin; 6 Taxifolin; 7 Silymarin; 8 Catechin; 9 Epigallocatechin Gallate; 10 Ferulic acid; 11 Chlorogenic Acid; 12 Curcumin; 13 Ginsenoside Rg3; 14 Ginsenoside Rb1; 15 Ginsenoside Rd; 16 Puerarin; 17 Naringenin; 18 Manassantin B; 19 Celastrol; 20 Raddeanin A; 21 Tetramethylpyrazine; 22 Oridonin; 23 Hydroxysafflor Yellow A; 24 Matrine; 25 Berberine; 26 Astragaloside IV.
TABLE 4.
Summary of pharmacological mechanisms of monomers in natural medicine for preventing blue light damage.
| Drug ingredients | Protection mechanism | Administration method | Dosage | References |
|---|---|---|---|---|
| Procyanidin B2 | Procyanidin B2 inhibit blue light induced RPE cell damage by alleviating oxidative damage, endoplasmic reticulum stress response, and mitochondrial apoptosis pathways | ARPE-19 human retinal pigment epithelial cells | 0.1, 0.5, 1, 5, 10 μmol/L | Xu et al. (2018), Zhao (2015) |
| Cyanidin 3-O-glucoside chloride (C3G) | C3G and its phenolic acid metabolites attenuate visible light induced retinal degeneration in vivo by activating the Nrf2/HO-1 pathway and inhibiting NF - κ B | Pigmented rabbit Oral administration |
0.11 mmol/kg/d | Wang et al. (2016) |
| Delphinidin | Delphinidin can reduce the content of intracellular peroxide product TBARS after light damage, increase the activity of antioxidant enzyme systems (SOD, GSH Px, GST), and have a protective effect against light induced oxidative stress damage to the retina | 661W photoreceptor cells | 5, 10, 20 μmol/L | Peng et al. (2019) |
| Resveratrol | Upregulation of cell viability, SOD, CAT, GSH; downregulation of cell proliferation, ERK1/2 and MEK expression, as well as caspase-3, caspase-9,ROS,p-p38,p-ERK,p-JNK. | ARPE-19 cells | 50, 100, 200 and 400 μmol/L | King et al. (2005), Ye and Meng (2021) |
| Quercetin | Upregulate cell viability, mitochondrial membrane potential, and phagocytic function; Downregulate the production of lipid hydroperoxides and singlet oxygen | ARPE-19 cells | 25 μM, 50 μM and 100 μM | Olchawa et al. (2020) |
| Taxifolin | Taxifolin may protect the eyes from oxidative stress damage by regulating NRF2 levels and promoting phase II antioxidant enzyme activity | Rat Gavage method |
10 mg/kg, 50 mg/kg | Zhang (2023c) |
| Silymarin | Silymarin can significantly protect RGCs from blue light damage by activating the MEK/ERK/CREB pathway, indicating that inflammatory factors may become targets for treating eye diseases | Retinal ganglion cells (RGCs) | 50 μM and 100 μM | Shen et al. (2019) |
| Catechin | Catechin can significantly reduce the expression of NF - κ B and proinflammatory mediators (IL-1 β, IL-6, TNF - α) in diabetes retinopathy rats, so it can be used as an effective component in the treatment of diabetes retinopathy | Rat Intravitreal injection |
50, 100, 200 mg/kg/d | Wang et al. (2018) |
| Epigallocatechin gallate (EGCG) | Upregulation of cell viability; Downregulate the expression of ROS, H2O2, p-JNK, p-ERK, p-p38, and COX-2; The expression of caspase-3 is not affected | Rat Oral administration |
— | Chan et al. (2008), Zhang et al. (2008) |
| Ferulic Acid | Ferulic acid can alleviate cell damage induced by sodium iodate in retinal degeneration mice, and oral administration of ferulic acid can provide protective effects on the retina | Mouse Oral administration |
10, 30 mg/kg/d | Kohno et al. (2020) |
| Chlorogenic acid | Chlorogenic acid has anti-inflammatory and antioxidant effects. Chlorogenic acid can activate the Nrf2 signaling pathway, promote Nrf2 nuclear entry and binding with ARE, thereby promoting the expression of phase II detoxifying enzymes (HO-1, NQO1, GCLC, and GCLM) and exerting antioxidant effects | ARPE-19 cells | 25, 50, 100 μmol/L | Liu (2021) |
| Curcumin | Curcumin can protect RPE cells from blue light irradiation by reducing ROS levels and increasing VEGF, GSH, and GSH Px levels | ARPE-19 cells | 20 μM | Bardak et al. (2017) |
| Ginsenoside Rg3 | Ginsenoside Rg3 has various biological effects such as antioxidant and scavenging of oxygen free radicals. Inhibiting the expression of vascular endothelial growth factor (VEGF) has an inhibitory effect on the formation of corneal and choroidal neovascularization | Human umbilical vein endothelial cells HUVEC |
100.0 μmol/L | Lu et al. (2017) |
| Ginsenoside Rb1 Ginsenoside Rd |
The combined action of ginsenoside Rb1 and Rd (a saponin compound of Panax notoginseng saponins) can promote changes in the expression of miR-155 and SHIP1 in the retina and maintain them at normal levels, inhibiting light induced retinal degeneration | Mouse Intraperitoneal injection |
Rb1 (65 mg/kg) and Rd (22.5 mg/kg) | Bian et al. (2017) |
| Puerarin | Puerarin has antioxidant and scavenging effects on oxygen free radicals in the body, which may reduce the production of advanced glycation end products (AGEs) and alleviate tissue damage caused by AGEs through antioxidant and lipid-lowering effects. It reduces the expression of hypoxia inducible factor (HIF-1 α) and the production of VEGF, thereby inhibiting the formation of new blood vessels in ischemic tissues | RPE cells | 0.01, 0.1, 1 g/L | Nguyen et al. (2019), Li et al. (2006) |
| Naringenin | Naringin has various pharmacological activities such as anti-inflammatory and antioxidant effects, which can protect the retinal pigment epithelium and inhibit the formation of new blood vessels | Rat Eye drops administration |
10 g/L 3 times |
Shen et al. (2010a) |
| Manassantin B | Manassantin B has a protective effect against free radical damage, which can inhibit the generation of O2, antagonize the effects of OH and H2O2 on cell membrane lipid peroxidation, and enhance the activity of self antioxidant enzymes in the body | — | — | Jiang (2002) |
| Celastrol | Celastrol has anti-inflammatory, anti-tumor and immunosuppressive activities. Studies have shown that Triptolide can inhibit photoreceptor cell death, alleviate oxidative stress response of retinal pigment epithelium and photoreceptors, reduce the expression of pro-inflammatory genes in the retina, and inhibit the activation and gliosis of retinal microglia | Mouse Intraperitoneal injection |
5 mg/kg | Yang et al. (2011), Bian et al. (2016) |
| Raddeanin A | Raddeanin A can inhibit oxygen induced retinal neovascularization, and its mechanism may be related to the inhibition of VEGFR2 mediated AKT and ERK1/2 signaling pathways | Mouse Intraperitoneal injection |
180 mg/mL | Huang (2013) |
| Tetramethylpyrazine | Tetramethylpyrazine can inhibit the proliferation of endothelial cells and reverse hypoxia damage in human retinal pigment epithelial cells (ARPE-19). By inhibiting the expression of HIF-1 α in RPE cells induced by AGEs, the production of new blood vessels is suppressed | Rat Eye drops administration |
25 μg 1%TMP eye drops | Shen et al. (2010b), Zou et al. (2007) |
| Oridonin | Research has shown that oridonin can effectively inhibit the proliferation, migration, and angiogenesis of monkey retinal endothelial cells, and suppress hypoxia induced retinal neovascularization in mice by blocking the focal adhesion kinase FAK/Paxillin signaling pathway | Mouse Intraperitoneal injection |
— | Dong (2011) |
| Hydroxysafflor Yellow A | Hydroxysafflor Yellow A has pharmacological activities such as anti-inflammatory and antioxidant effects. Studies have shown that hydroxysafflower yellow pigment A can inhibit the proliferation of high glucose induced choroidal endothelial cells in rhesus monkeys, possibly by downregulating the expression of VEGF mRNA induced by high glucose | Rat Intraperitoneal injection |
5.0 mg/kg | Fu et al. (2023) |
| Matrine | Matrine can effectively inhibit the proliferation of retinal microvascular endothelial cells (RRMECs) in rats and induce apoptosis of RRMECs, mainly by downregulating VEGF expression | Rat Gavage method |
30, 60 and 90 mg/(kg·d) | Zhang (2019) |
| Berberine | The activation of the PI3K/AKT/ERK pathway can protect the retina from light induced degeneration, which may be related to reducing retinal oxidative stress | Mouse Gavage method |
200 mg/kg/d | Song et al. (2016) |
| Astragaloside IV | Astragaloside IV upregulates the PINK1/Parkin signaling pathway in cells, inhibits the production of large amounts of ROS, avoids mitochondrial damage and apoptosis, and maintains cell viability and autophagy process | ARPE-19 cells | 50 mg/L | Li et al. (2021c) |
TABLE 5.
Summary of the pharmacological mechanisms of traditional Chinese medicine for preventing blue light damage.
| Drug ingredients | Protection mechanism | Administration method | Dosage | References |
|---|---|---|---|---|
| Wolfberry Extract | The polysaccharides in goji berries can significantly enhance the enzymatic antioxidant system enzyme activity, inhibit the production and accumulation of peroxidation product malondialdehyde, significantly reduce the body’s oxidation rate and degree, and alleviate oxidative stress damage to the eyes | Human retinal pigment epithelial cells (HRPE) | 0.01, 0.1, 1 mg/mL | Dou (2011) |
| Dendrobium Officinale Extract | The polysaccharides in Dendrobium officinale can downregulate the levels of inflammatory factors and VEGF through the NF - κ B signaling pathway, inhibiting the inflammatory response and neovascularization in the retina of rats | Rat; Gavage method | 100, 200, 300 mg/kg | Qin et al. (2021) |
| Blackcurrant Extract | Anthocyanins in blackcurrant can bind with free radicals under dehydrogenation, terminating the chain reaction of free radicals; It can also inhibit peroxidation through chelation reaction with metal catalysts | ARPE-19 cells | 0.01, 0.10, 10.00 μg/mL | Wang et al. (2024a) |
| Blueberry Extract | Anthocyanins in blueberries can significantly reduce the levels of blood lactate and malondialdehyde, as well as significantly enhance the activity of superoxide dismutase and glutathione peroxidase, inhibit the large-scale production of downstream product NF kB, and achieve the effect of preventing oxidative damage | ARPE-19 cells | 0.01, 0.10, 10.00 μg/mL | Wang et al. (2024b) |
| Cassia Seed Extract | Anthraquinones in Cassia seed can inhibit the activity of aldose reductase in ocular lens cells; Inhibit the generation of end products of oxidative glycosylation; Inhibiting oxidative DNA modification and nitroso tyrosine accumulation in retinal cells to achieve protective effects against oxidative stress damage in the eye | ARPE-19 cells | 0.01, 0.10, 10.00 μg/mL | Wang et al. (2024a) |
| Chrysanthemum Extract | Osmanthus glycosides in chrysanthemums have the ability to scavenge DPPH free radicals in vitro, effectively alleviate oxidative stress in myocardial cells, improve amblyopia, dry eye syndrome, and macular function without altering retinal structure | HRPE-19 cells | 25, 50, 100 μM | Yu (2019), Kan et al. (2020) |
| Bilberry Extract | Anthocyanins in bilberry can upregulate cell viability; Downregulate ROS generation, p38 MAPK phosphorylation, JNK phosphorylation | 661W cells | 1–30 μg/mL | Ogawa et al. (2013), Ogawa et al. (2014) |
| Palmleaf raspberry fruit | It has antioxidant effects on singlet oxygen by regulating the activation of NF - κ B, p38 MAPK, autophagy, and caspase-3/7 signaling pathways, inhibiting the production and activation of pro apoptotic proteins, and protecting retinal photoreceptors | Retinal ganglion cell (RGCs) | 0.1, 1, 10, 25 μg/mL | Li (2017) |
| Marigold Extract | Lutein and zeaxanthin in marigold can upregulate antioxidant capacity, retinal Rho, Sag,Gnat1,NCAM,GAP43,BDNF,NGF,IGF1,Nrf2,HO-1; Downregulate the expression of NF kB and GFAP. | Rat; Gavage method | 100 mg/kg | Sahin et al. (2019b), Wang et al. (2024a) |
4.4 Efficacy mechanism of gene drug therapy
At present, some gene drugs can effectively treat BLED by enhancing cellular antioxidant capacity, regulating endoplasmic reticulum stress, and fundamentally reducing the damage of blue light to eye cells, thereby achieving good therapeutic effects. The mechanism of action of gene drugs is clear, and they can target eye cells with precise drug delivery and action, reducing side effects on other tissues and organs. The summary of the pharmacological mechanisms of gene therapy for preventing blue light damage is shown in (Table 6).
TABLE 6.
Summary of pharmacological mechanisms of gene therapy for anti blue light damage.
| Gene therapy | Protection mechanism | Administration method | Dosage | References |
|---|---|---|---|---|
| Nrf2 stress response transcription factor | Research has found that Nrf2 can to some extent protect photoreceptor cells from oxidative stress damage | 661W cells Nrf2 knockdown |
— | Chen et al. (2017) |
| Knocking out the cell surface chemokine receptor 2 (CCR2) gene | Research has found that knocking out the cell surface chemokine receptor 2 (CCR2) gene can significantly alleviate the death of mouse retinal photoreceptor cells caused by chronic blue light irradiation, while also inhibiting the activation and proliferation of microglia during light induced retinal degeneration | 661W cells; Knock out CCR2 | — | Hu et al. (2016) |
| Leukemia inhibitory factor (LIF) | Leukemia inhibitory factor (LIF) has an inhibitory effect on photodamage in retinal photoreceptor cells, possibly by activating the JAK3/STAT3 signaling channel to suppress downstream Bax/Bcl-2 apoptotic channels | Mouse; Light pre adaptation | — | Dong et al. (2018) |
| Extracellular vesicles derived from bone marrow mesenchymal stem cells (MSC-Exos) | By downregulating vascular endothelial growth factor-A (VEGF-A), we aim to improve the blue light stimulation and laser-induced retinal damage in retinal pigment epithelial cells (RPE cells) | Rat; Transplant | 1.0, 2.0, 3.0 µL (50 μg/mL) | He et al. (2018) |
| Tissue factor targeting peptide (TF-TP) | Pre treatment of RPE cells with tissue factor targeting peptide (TF-TP) can reduce blue light damage and increase cell survival rate. Its mechanism of action is related to the inhibition of the Bax/Bcl-2 apoptosis pathway by TF-TP. | RPE cells | 150 μmol/L | Li et al. (2017) |
| Heat shock protein 5(HSPA5) | HSPA5 has the ability to regulate endoplasmic reticulum stress, alleviate A2E and blue light damage, and promote RPE cell survival | RPE cells Transfection of HSPA5 interference series |
— | Feng et al. (2019) |
| Estrogen related receptor α(ERRα) | Under blue light induction conditions, ERR α may exert anti apoptotic effects by promoting Bcl-2 expression in ARPE-19 cells | ARPE-19 cells Overexpression of ERR α |
— | Lin, 2020; An (2022) |
| Glutaredoxin 2 (Grx2) | Grx2 may exert antioxidant stress effects by inhibiting the JNK signaling pathway, reducing cell apoptosis, and thereby alleviating the damage of blue light to the retina | Mouse; Knockout and Knockin of Grx2 Gene | — | Bin (2021) |
| Ceramide like protein (CERKL) | CERKL can alleviate blue light induced oxidative stress damage in ARPE-19 cells by activating SIRT1 protein expression and promoting deacetylation of E2F1 | SiRNA CERKL and pcDNA3.1-CERKL transfection into ARPE-19 cells | — | Zhuang et al. (2022) |
| miRNA (miR-22-3p) | Overexpression of miR-22-3p can inhibit RGC apoptosis by suppressing PTEN, activating the PI3K/Akt/Nrf2 pathway, and thereby suppressing RGC apoptosis | Rat; Intravitreal injection | 1 μL | Zhang et al. (2022) |
| Methyltransferase like protein (METTL7B) | Under blue light induction, METTL7B may exert a protective effect on RPE cells after light injury by promoting HO-1 expression in ARPE-19 cells; METTL7B may control ARPE-19 cell apoptosis through the BCL-2/BAX pathway by promoting BCL-2 and reducing BAX protein accumulation | ARPE-19 cells; Overexpression of METTL7B | — | Wang (2023) |
| Poly (ADP ribose) polymerase (PARP-1) | PARP-1 inhibitors can significantly alleviate the decrease in Rhodopsin expression induced by blue light irradiation in the retina. Inhibiting PARP-1 can inhibit mitochondrial autophagy and have a synergistic protective effect on retinal photodamage | 661W cells; PARP-1 knockdown cell line | — | Zhang (2023a), Zhang (2023b) |
5 Future perspectives
With the popularization of electronic equipment, the incidence rate of eye blue light injury is expected to continue to rise, especially among young and elderly people. It is predicted that by 2050, the incidence rate of eye diseases such as myopia, diabetes retinopathy, macular degeneration and glaucoma will increase significantly (Antemie et al., 2023; Landreneau et al., 2021). Therefore, future research directions may focus more on the role of blue light damage in ophthalmic diseases in young and elderly people. In the field of ocular pathophysiology, research will utilize more advanced imaging techniques to achieve real-time monitoring of the damage process of blue light to ocular tissues. The study may reveal new associations between blue light damage and genetics, environment, and lifestyle, and explore how these factors collectively affect eye health. Experimental modeling will focus more on simulating the actual human exposure to blue light, including factors such as light intensity, wavelength, and duration. It is also possible to develop models that are closer to human pathophysiological characteristics, such as using gene editing techniques to create animal models with specific genetic backgrounds.
Although some drugs and supplements have been used to treat blue light damage, their efficacy and safety still need further validation. Natural medicine has unique advantages in treating BLED due to its multi-component synergistic effect, overall regulation, and mild and safe characteristics. Therefore, it is necessary to explore the mechanism of action of natural medicine components in preventing and treating blue light damage, as well as how to integrate them with modern medicine. Meanwhile, delving deeper into the molecular mechanisms of blue light damage can help discover new therapeutic targets. At present, RNA nanomedicine has been used to achieve specific targets in the clinical treatment of eye diseases (Zhang et al., 2024). In order to improve the therapeutic effect, it is necessary to develop new drugs and formulations to enhance the permeability and bioavailability of drugs in the eye. It is pointed out that hydrogels can prolong the residence time of drugs in the eye and control drug release, thus improving the bioavailability of drugs (Han et al., 2024). These research findings provide new directions for future drug development.
In addition, the ocular microbiome is a dynamic ecosystem that plays a crucial but yet to be fully explored role in human health. The immune pardon mechanism of the eye includes the blood aqueous barrier, blood retinal barrier, and anti-inflammatory cytokines, all of which are important components. Recent studies have begun to explore the role of the ocular surface in the lung eye axis, emphasizing the bidirectional relationship between the respiratory system and eye health, suggesting that respiratory diseases may have an impact on eye diseases (Allam et al., 2024). Other studies have shown that gut microbiota is not only involved in the occurrence and development of various extraintestinal diseases, but also closely related to ophthalmic diseases such as uveitis, age-related macular degeneration, and glaucoma. The concept of gut eye axis can be used to explain the impact of gut microbiota imbalance on eye health (Zhang H. et al., 2023; Napolitano et al., 2021). Although research on the gut eye axis is currently insufficient and controversial, and cannot fully explain all existing issues, elucidating the pathological mechanisms related to gut microbiota and common eye diseases may provide new ideas for disease diagnosis and treatment.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work is supported by the Science and Technology Project of Haihe Laboratory of Modern Chinese Medicine (Grant number: 24HHZYSS00007).
Author contributions
YuY: Writing–original draft, Writing–review and editing, Visualization. YW: Writing–original draft, Writing–review and editing, Visualization. YZ: Writing–review and editing. YaY: Writing–review and editing. GA: Writing–review and editing. ZL: Supervision, Writing–review and editing. DQ: Conceptualization, Supervision, Writing–original draft, Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Alaimo A., Linares G. G., Bujjamer J. M., Gorojod R. M., Alcon S. P., Martinez J. H., et al. (2019). Toxicity of blue led light and A2E is associated to mitochondrial dynamics impairment in ARPE-19 cells: implications for age-related macular degeneration. Arch. Toxicol. 93 (5), 1401–1415. 10.1007/s00204-019-02409-6 [DOI] [PubMed] [Google Scholar]
- Allam V., Patel V. K., De Rubis G., Paudel K. R., Gupta G., Chellappan D. K., et al. (2024). Exploring the role of the ocular surface in the lung-eye axis: insights into respiratory disease pathogenesis. Life Sci. 349, 122730. 10.1016/j.lfs.2024.122730 [DOI] [PubMed] [Google Scholar]
- An N. (2022). Effect of lentivirus mediated ERRα gene overexpression on blue light induced apoptosis of retinal pigment epithelial cells. Master’s thesis. Guizhou Medical University. [Google Scholar]
- Antemie R. G., Samoila O. C., Clichici S. V. (2023). Blue light-ocular and systemic damaging effects: a narrative review. Int. J. Mol. Sci. 24 (6), 5998. 10.3390/ijms24065998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M., Miao D., Li Y., Lyu J., Liu Z., Li Y., et al. (2023). Ginsenoside Rg1 injection combined with inosine tablets and vitamin B1 for the treatment of primary retinitis pigmentosa. Int. Eye Sci. 23 (12), 2035–2039. 10.3980/j.issn.1672-5123.2023.12.19 [DOI] [Google Scholar]
- Bardak H., Uguz A. C., Bardak Y. (2017). Curcumin regulates intracellular calcium release and inhibits oxidative stress parameters, VEGF, and caspase-3/-9 levels in human retinal pigment epithelium cells. Physiol. Int. 104 (4), 301–315. 10.1556/2060.104.2017.4.3 [DOI] [PubMed] [Google Scholar]
- Bian M., Du X., Cui J., Wang P., Wang W., Zhu W., et al. (2016). Celastrol protects mouse retinas from bright light-induced degeneration through inhibition of oxidative stress and inflammation. J. Neuroinflamm. 13, 50. 10.1186/s12974-016-0516-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian M., Du X., Wang P., Cui J., Xu J., Gu J., et al. (2017). Combination of ginsenoside Rb1 and Rd protects the retina against bright light-induced degeneration. Sci. Rep. 7 (1), 6015. 10.1038/s41598-017-06471-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin Y. (2021). The protective effect and mechanism of Grx2 in blue light induced retinal light damage. Dissertation. Chongqing Medical University. [Google Scholar]
- Bonfiglio V., Rejdak R., Nowomiejska K., Zweifel S. A., Justus W. M., Romano G. L., et al. (2022). Efficacy and safety of subthreshold micropulse yellow laser for persistent diabetic macular edema after vitrectomy: a pilot study. Front. Pharmacol. 13, 832448. 10.3389/fphar.2022.832448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. M., Huang J. H., Lin H. H., Chiang H. S., Chen B. H., Hong J. Y., et al. (2008). Protective effects of (-)-epigallocatechin gallate on UVA-induced damage in ARPE19 cells. Mol. Vis. 14, 2528–2534. 10.3390/ijms16035789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. J., Lin T. B., Peng H. Y., Liu H. J., Lee A. S., Lin C. H., et al. (2021). Cytoprotective potential of fucoxanthin in oxidative stress-induced age-related macular degeneration and retinal pigment epithelial cell senescence in vivo and in vitro . Mar. Drugs. 19 (2), 114. 10.3390/md19020114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Wu C., Xu Z., Kuse Y., Hara H., Duh E. J. (2017). Nrf2 protects photoreceptor cells from photo-oxidative stress induced by blue light. Exp. Eye Res. 154, 151–158. 10.1016/j.exer.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. (2005). The protective effect of estrogen on retinal photodamage. Master’s thesis. Sichuan University. [Google Scholar]
- Chen Y. (2021). Study on the protective effect of Buyang Huanwu Decoction on retinal blue light damage in rats. Master’s thesis. Liaoning University of Traditional Chinese Medicine. [Google Scholar]
- Cheng T., Zou Y., Jian L., Zhang M., Dou Y. (2024). Prevention and treatment of lipoic acid-niacin on blue-light-induced retinal damage in rats. Int. Eye Sci. 24 (02), 196–202. 10.3980/j.issn.1672-5123.2024.2.04 [DOI] [Google Scholar]
- Cho H. M., Lee S. J., Choung S. Y. (2023). Protective effects of Panax ginseng berry extract on blue light-induced retinal damage in ARPE-19 cells and mouse retina. J. Ginseng Res. 47 (1), 65–73. 10.1016/j.jgr.2022.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W., Lee J. B., Cui L., Li Y., Li Z., Choi J. S., et al. (2016). Therapeutic efficacy of topically applied antioxidant medicinal plant extracts in a mouse model of experimental dry eye. Cell. Longev. 2016, 4727415. 10.1155/2016/4727415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J. (2022). Research progress of pathogenesis and surgical treatment of dry eye. Chin. Foreign Med. Res. 20 (33), 181–184. 10.14033/j.cnki.cfmr.2022.33.045 [DOI] [Google Scholar]
- Chou T. H., Musada G. R., Romano G. L., Bolton E., Porciatti V. (2018). Anesthetic preconditioning as endogenous neuroprotection in glaucoma. Int. J. Mol. Sci. 19 (1), 237. 10.3390/ijms19010237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Lazzara F., Romano G. L., Platania C., Drago F., Bucolo C. (2021). Caffeine protects against retinal inflammation. Front. Pharmacol. 12, 824885. 10.3389/fphar.2021.824885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougnard-Gregoire A., Merle B., Aslam T., Seddon J. M., Aknin I., Klaver C., et al. (2023). Blue light exposure: ocular hazards and prevention-A narrative review. Ophthalmol. Ther. 12 (2), 755–788. 10.1007/s40123-023-00675-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M. (2020). Preparation and evaluation of flunarizine hydrochloride ophtihalmic in situ organogel for systemic delivery. Master’s thesis. Anhui University of Chinese Medicine. [Google Scholar]
- Davies S., Elliott M. H., Floor E., Truscott T. G., Zareba M., Sarna T., et al. (2001). Photocytotoxicity of lipofuscin in human retinal pigment epithelial cells. Free Radic. Biol. Med. 31 (2), 256–265. 10.1016/s0891-5849(01)00582-2 [DOI] [PubMed] [Google Scholar]
- Dionysopoulou S., Wikstrom P., Bucolo C., Romano G. L., Micale V., Svensson R., et al. (2023). Topically administered NOX4 inhibitor, GLX7013114, is efficacious in treating the early pathological events of diabetic retinopathy. Diabetes 72 (5), 638–652. 10.2337/db22-0515 [DOI] [PubMed] [Google Scholar]
- Dong S., Liu S., Li Q. (2018). Protective effects of light preconditioning on retinal photoreceptor cells aganist light damage. Recent Adv. Ophthalmol. 38 (05), 412–415+420. 10.13389/j.cnki.rao.2018.0096 [DOI] [Google Scholar]
- Dong Y. (2011). Research on inhibitory activity of small molecular compounds on tumor growth & metastasis via inhibiting angiogenesis and mechanism. Dissertation. East China Normal University. [Google Scholar]
- Dou R. (2011). Experimental Study of the lycium barbarum polysaccharide on the role of retinal pigment epithelial cells. Master’s thesis. Shandong University of Traditional Chinese Medicine. [Google Scholar]
- Du B., Xu Y., Wei Z. (2022). Vitamin C alleviates macular degeneration in rats by regulating function of macrophages. J. Clin. Med. Pract. 26 (17), 130–134. 10.7619/jcmp.20220296 [DOI] [Google Scholar]
- Fan B., Zhang C., Chi J., Liang Y., Bao X., Cong Y., et al. (2022). The molecular mechanism of retina light injury focusing on damage from short wavelength light. Oxidative Med. Cell. Longev. 2022, 8482149. 10.1155/2022/8482149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Lu B., Zhu H., Sun X. J., Sun X. D. (2019). HSPA5 regulates endoplasmic reticulum stress for cell survival in retinal pigment epithelial cells. Recent Adv. Ophthalmol. 39 (12), 1111–1115. 10.13389/j.cnki.rao.2019.0255 [DOI] [Google Scholar]
- Feng J. H., Dong X. W., Yu H. L., Shen W., Lv X. Y., Wang R., et al. (2021). Cynaroside protects the blue light-induced retinal degeneration through alleviating apoptosis and inducing autophagy in vitro and in vivo . Phytomedicine 88, 153604. 10.1016/j.phymed.2021.153604 [DOI] [PubMed] [Google Scholar]
- Feng M. (2012). Protective effect of saturated hydrogen saline against blue light-induced retinal damage in rats. Dissertation. Huazhong University of Science and Technology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Wang T., Cao J., Li G., Zheng L., Xu W., et al. (2023). The protective effect of hydroxysafflor yellow A on light-induced retinal injury in rats. J. Otolaryngology Ophthalmol. Shandong Univ. 37 (05), 128–134. 10.6040/j.issn.1673-3770.0.2022.455 [DOI] [Google Scholar]
- Gao Y., Huang J., Yu Y. (2020). Research progress on animal models of retinal photodamage. J. Mudanjiang Med. Univ. 41 (04), 133–135. 10.13799/j.cnki.mdjyxyxb.2020.04.036 [DOI] [Google Scholar]
- Golconda P., Andrade-Medina M., Oberstein A. (2023). Subconfluent ARPE-19 cells display mesenchymal cell-state characteristics and behave like fibroblasts, rather than epithelial cells, in experimental HCMV infection studies. Viruses-Basel. 16 (1), 49. 10.3390/v16010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X. (2011). Blue-light induced changes of L-type calcium channel subunit mRNA expression and free Calcium ion of Human Retinal Pigment epithelium Cells in vitro . Master’s thesis. Zunyi Medical University. [Google Scholar]
- Han J., Shu H., Zhang L., Huang S. (2024). Latest advances in hydrogel therapy for ocular diseases. Polymer 306, 127207. 10.1016/j.polymer.2024.127207 [DOI] [Google Scholar]
- Hazim R. A., Volland S., Yen A., Burgess B. L., Williams D. S. (2019). Rapid differentiation of the human RPE cell line, ARPE-19, induced by nicotinamide. Exp. Eye Res. 179, 18–24. 10.1016/j.exer.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G. H., Zhang W., Ma Y. X., Yang J., Chen L., Song J., et al. (2018). Mesenchymal stem cells-derived exosomes ameliorate blue light stimulation in retinal pigment epithelium cells and retinal laser injury by VEGF-dependent mechanism. Int. J. Ophthalmol. 11 (4), 559–566. 10.18240/ijo.2018.04.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellinen L., Hagstrom M., Knuutila H., Ruponen M., Urtti A., Reinisalo M. (2019). Characterization of artificially re-pigmented ARPE-19 retinal pigment epithelial cell model. Sci. Rep. 9 (1), 13761. 10.1038/s41598-019-50324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W. H., Sangkhathat C., Lu M. K., Lin W. Y., Liu H. P., Lin Y. L. (2024). Dendrobium nobile polysaccharide attenuates blue light-induced injury in retinal cells and in vivo in Drosophila. Antioxidants 13 (5), 603. 10.3390/antiox13050603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Zhang Y., Wang J., Mao P., Lv X., Yuan S., et al. (2016). Knockout of Ccr2 alleviates photoreceptor cell death in rodent retina exposed to chronic blue light. Cell Death Dis. 7 (11), e2468. 10.1038/cddis.2016.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. (2013). Research on function and molecular mechanism of RaddeaninA in inhibition of ocular neovascularization. Master’s thesis. East China Normal University. [Google Scholar]
- Huang Y., Chen X., Jiang Z., Luo Q., Wan L., Hou X., et al. (2022). Transcriptome sequencing reveals tgf-β-mediated noncoding RNA regulatory mechanisms involved in DNA damage in the 661W photoreceptor cell line. Genes 13 (11), 2140. 10.3390/genes13112140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseli H. P., Korber N., Koch C., Karl A., Penk A., Huster D., et al. (2016). Scleral cross-linking by riboflavin and blue light application in young rabbits: damage threshold and eye growth inhibition. Graefes Arch. Clin. Exp. Ophthalmol. 254 (1), 109–122. 10.1007/s00417-015-3213-x [DOI] [PubMed] [Google Scholar]
- Jaadane I., Boulenguez P., Chahory S., Carre S., Savoldelli M., Jonet L., et al. (2015). Retinal damage induced by commercial light emitting diodes (LEDs). Free Radic. Biol. Med. 84, 373–384. 10.1016/j.freeradbiomed.2015.03.034 [DOI] [PubMed] [Google Scholar]
- Jiang Y. (2002). Research progress on prevention and treatment of retinal photodamage. Chin. J. Pract. Ophthalmology 03, 166–168. 10.3760/cma.j.issn.1006-4443.2002.03.002 [DOI] [Google Scholar]
- Jin H. L., Jeong K. W. (2022). Transcriptome analysis of long-term exposure to blue light in retinal pigment epithelial cells. Biomol. Ther. 30 (3), 291–297. 10.4062/biomolther.2021.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Wan Q. (2005). The effect of dimethylthiourea(DMTU) on MDA levels in rats with retinal photodamage. Chin. J. Ophthalmol. Otorhinolaryngology 5 (02), 79–80. 10.14166/j.issn.1671-2420.2005.02.008 [DOI] [Google Scholar]
- Kan J., Wang M., Liu Y., Liu H., Chen L., Zhang X., et al. (2020). A novel botanical formula improves eye fatigue and dry eye: a randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 112 (2), 334–342. 10.1093/ajcn/nqaa139 [DOI] [PubMed] [Google Scholar]
- Kara-Junior N., Espindola R. F., Gomes B. A., Ventura B., Smadja D., Santhiago M. R. (2011). Effects of blue light-filtering intraocular lenses on the macula, contrast sensitivity, and color vision after a long-term follow-up. J. Cataract. Refract. Surg. 37 (12), 2115–2119. 10.1016/j.jcrs.2011.06.024 [DOI] [PubMed] [Google Scholar]
- Keenan T., Cukras C. A., Chew E. Y. (2021). Age-related macular degeneration: epidemiology and clinical aspects. Adv. Exp. Med. Biol. 1256, 1–31. 10.1007/978-3-030-66014-7_1 [DOI] [PubMed] [Google Scholar]
- Kikuchi M., Kashii S., Honda Y., Tamura Y., Kaneda K., Akaike A. (1997). Protective effects of methylcobalamin, a vitamin B12 analog, against glutamate-induced neurotoxicity in retinal cell culture. Invest. Ophthalmol. Vis. Sci. 38 (5), 848–854. [PubMed] [Google Scholar]
- King R. E., Kent K. D., Bomser J. A. (2005). Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem.-Biol. Interact. 151 (2), 143–149. 10.1016/j.cbi.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Kohno M., Musashi K., Ikeda H. O., Horibe T., Matsumoto A., Kawakami K. (2020). Oral administration of ferulic acid or ethyl ferulate attenuates retinal damage in sodium iodate-induced retinal degeneration mice. Sci. Rep. 10 (1), 8688. 10.1038/s41598-020-65673-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimi H., Lee D., Ibuki M., Katada Y., Negishi K., Tsubota K., et al. (2021). Inhibition of the HIF-1α/BNIP3 pathway has a retinal neuroprotective effect. Faseb J. 35 (8), e21829. 10.1096/fj.202100572R [DOI] [PubMed] [Google Scholar]
- Kuse Y., Ogawa K., Tsuruma K., Shimazawa M., Hara H. (2014). Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci. Rep. 4, 5223. 10.1038/srep05223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T. T., Yang C. M., Yang C. H. (2020). Astaxanthin protects retinal photoreceptor cells against high glucose-induced oxidative stress by induction of antioxidant enzymes via the PI3K/Akt/Nrf2 pathway. Antioxidants 9 (8), 729. 10.3390/antiox9080729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan S. E., Vieira K. F. (2010). Nutritional and herbal supplements for anxiety and anxiety-related disorders: systematic review. Nutr. J. 9, 42. 10.1186/1475-2891-9-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreneau J. R., Hesemann N. P., Cardonell M. A. (2021). Review on the myopia pandemic: epidemiology, risk factors, and prevention. Mo Med. 118 (2), 156–163. [PMC free article] [PubMed] [Google Scholar]
- Lee H. S., Cui L., Li Y., Choi J. S., Choi J. H., Li Z., et al. (2016). Correction: influence of light emitting diode-derived blue light overexposure on mouse ocular surface. PLoS One 11 (11), e0167671. 10.1371/journal.pone.0167671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Li Q., Hong J., Chen F., Guo C. (2021a). Protective effect of Astragaloside IV on the blue light-induced damage of retinal pigment epithelium and its mechanism. Recent Adv. Ophthalmol. 41 (11), 1006–1011. 10.13389/j.cnki.rao.2021.0212 [DOI] [Google Scholar]
- Li D., Zou X., Chen J., Xu Z., Yu Y., Zhou W., et al. (2017). Protective effects of tissue factor targeting peptide on human retinal pigment epithelial cell damage induced by blue light. Chin. J. Exp. Ophthalmol. 35 (7), 7. 10.3760/cma.j.issn.2095-0160.2017.07.006 [DOI] [Google Scholar]
- Li G. (2017). Study on the protective effect of traditional Chinese medicine raspberry on cultured retinal ganglion cells in vitro . Master’s thesis. Liaoning University of Traditional Chinese Medicine. [Google Scholar]
- Li H., Zhang M., Wang D., Dong G., Chen Z., Li S., et al. (2021b). Blue light from cell phones can cause chronic retinal light injury: the evidence from a clinical observational study and a SD rat model. Biomed. Res. Int. 2021, 3236892. 10.1155/2021/3236892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Tang A. (2023). Advances in the application of intravitreal injection of glucocorticoids. J. Gannan Med. Univ. 43 (04), 429–434. 10.3969/j.issn.1001-5779.2023.04.020 [DOI] [Google Scholar]
- Li W., Jiang D., Guo L., Wang P., Zhang L. (2006). Puerarin inhibits the proliferation of human retinal pigment epithelial cells and the expression of hypoxia-inducible factor-1α in human RPE cells induced by advanced glycation end products. Int. Eye Sci. (03), 580–583. 10.3969/j.issn.1672-5123.2006.03.020 [DOI] [Google Scholar]
- Li X., Jing N., Liu X. (2021c). Research progress of traditional Chinese medicine in the treatment of ocular diseases. Int. Med. Health Guid. News 27 (16), 4. 10.1016/j.lfs.2024.123045 [DOI] [Google Scholar]
- Li Y., Zhang P., Huang C., Wang W. (2020). Dual effect of blue light on Fusariumsolani clinical corneal isolates in vitro . Lasers Med. Sci. 35 (6), 1299–1305. 10.1007/s10103-019-02911-4 [DOI] [PubMed] [Google Scholar]
- Lin C. W., Yang C. M., Yang C. H. (2020). Protective effect of astaxanthin on blue light light-emitting diode-induced retinal cell damage via free radical scavenging and activation of PI3K/Akt/Nrf2 pathway in 661W cell model. Mar. Drugs. 18 (8), 387. 10.3390/md18080387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X. (2020). Role of estrogen-related receptor α in blue light-induced apoptosis of human retinal pigment epithelial cells. Master’s thesis. Jinan University. [Google Scholar]
- Liu T. (2019). Preliminary study of the effect of different wavelengths LED on lens. Dissertation. Tianjin Medical University. [Google Scholar]
- Liu Y. (2021). Protective role of chlorogenic acid against oxidative damage of ARPE-19 induced by blue light LED. Master’s thesis. Tianjin University of Science and Technology. [Google Scholar]
- Liu Y., Liu M., Zhang X., Chen Q., Chen H., Sun L., et al. (2016). Protective effect of fucoxanthin isolated from laminaria japonica against visible light-induced retinal damage both in vitro and in vivo . J. Agric. Food Chem. 64 (2), 416–424. 10.1021/acs.jafc.5b05436 [DOI] [PubMed] [Google Scholar]
- Liu Y., Ma N., Liu Y., Guo Y., Li H., Shan Y., et al. (2021). Astaxanthin protects retinal pigment epithelial cells from oxidative stress induced by blue light emitting diodes. Food Sci. 42 (21), 128–136. 10.7506/spkx1002-6630-20201017-154 [DOI] [Google Scholar]
- Lu Y., Cai L., Zhu C., Liu G., Zhang Y., Huang J., et al. (2017). Inhibitory effect of ginsenoside RG3 on choroidal neovascularization. Recent Adv. Ophthalmol. 37 (10), 922–925. 10.13389/j.cnki.rao.2017.0234 [DOI] [Google Scholar]
- Lyu J., Chen F., Yu Y. (2024). Advances in novel ocular drug delivery systems. Chin. J. Mod. Appl. Pharm. 41 (03), 398–407. 10.13748/j.cnki.issn1007-7693.20221054 [DOI] [Google Scholar]
- Marek V., Melik-Parsadaniantz S., Villette T., Montoya F., Baudouin C., Brignole-Baudouin F., et al. (2018). Blue light phototoxicity toward human corneal and conjunctival epithelial cells in basal and hyperosmolar conditions. Free Radic. Biol. Med. 126, 27–40. 10.1016/j.freeradbiomed.2018.07.012 [DOI] [PubMed] [Google Scholar]
- Marie M., Bigot K., Angebault C., Barrau C., Gondouin P., Pagan D., et al. (2018). Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 9 (3), 287. 10.1038/s41419-018-0331-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira P. I., Oliveira C. R. (2011). Mitochondria as potential targets in antidiabetic therapy. Handb. Exp. Pharmacol. 203, 331–356. 10.1007/978-3-642-17214-4_14 [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Shimazawa M., Sugitani S., Kudo T., Imai S., Inokuchi Y., et al. (2013). Role of endoplasmic reticulum stress in light-induced photoreceptor degeneration in mice. J. Neurochem. 125 (1), 111–124. 10.1111/jnc.12116 [DOI] [PubMed] [Google Scholar]
- Nan L., Zhang Y., Song H., Ye Y., Jiang Z., Zhao S. (2023). Influence of light-EmittingDiode-derived blue light overexposure on rat ocular surface. J. Ophthalmol. 2023, 1097704. 10.1155/2023/1097704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano P., Filippelli M., Davinelli S., Bartollino S., Dell'Omo R., Costagliola C. (2021). Influence of gut microbiota on eye diseases: an overview. Ann. Med. 53 (1), 750–761. 10.1080/07853890.2021.1925150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N. L. M., Wen Y. T., Ho Y. C., Kapupara K., Tsai R. K. (2019). Therapeutic effects of Puerarin against anterior ischemic optic neuropathy through antiapoptotic and anti-inflammatory actions. Invest. Ophthalmol. Vis. Sci. 60 (10), 3481–3491. 10.1167/iovs.19-27129 [DOI] [PubMed] [Google Scholar]
- Niwano Y., Kanno T., Iwasawa A., Ayaki M., Tsubota K. (2014). Blue light injures corneal epithelial cells in the mitotic phase in vitro . Br. J. Ophthalmol. 98 (7), 990–992. 10.1136/bjophthalmol-2014-305205 [DOI] [PubMed] [Google Scholar]
- Ogawa K., Kuse Y., Tsuruma K., Kobayashi S., Shimazawa M., Hara H. (2014). Protective effects of bilberry and lingonberry extracts against blue light-emitting diode light-induced retinal photoreceptor cell damage in vitro . BMC Complement. Altern. Med. 14, 120. 10.1186/1472-6882-14-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Tsuruma K., Tanaka J., Kakino M., Kobayashi S., Shimazawa M., et al. (2013). The protective effects of bilberry and lingonberry extracts against UV light-induced retinal photoreceptor cell damage in vitro . J. Agric. Food Chem. 61 (43), 10345–10353. 10.1021/jf402772h [DOI] [PubMed] [Google Scholar]
- Olchawa M. M., Krzysztynska-Kuleta O. I., Mokrzynski K. T., Sarna P. M., Sarna T. J. (2020). Quercetin protects ARPE-19 cells against photic stress mediated by the products of rhodopsin photobleaching. Photochem. Photobiol. Sci. 19 (8), 1022–1034. 10.1039/d0pp00165a [DOI] [PubMed] [Google Scholar]
- Osborne N. N., Nunez-Alvarez C., Del O. S., Merrayo-Lloves J. (2017). Visual light effects on mitochondria: the potential implications in relation to glaucoma. Mitochondrion 36, 29–35. 10.1016/j.mito.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Ouyang W., Wang S., Yan D., Wu J., Zhang Y., Li W., et al. (2023). The cGAS-STING pathway-dependent sensing of mitochondrial DNA mediates ocular surface inflammation. Signal Transduct. Target. Ther. 8 (1), 371. 10.1038/s41392-023-01624-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X., Yang J., Hong Z., Wu Y., Xie Y., Wang G. (2020). Mechanisms of blue light-induced eye hazard and protective measures: a review. Biomed. Pharmacother. 130, 110577. 10.1016/j.biopha.2020.110577 [DOI] [PubMed] [Google Scholar]
- Ozkaya E. K., Anderson G., Dhillon B., Bagnaninchi P. O. (2019). Blue-light induced breakdown of barrier function on human retinal epithelial cells is mediated by PKC-zeta over-activation and oxidative stress. Exp. Eye Res. 189, 107817. 10.1016/j.exer.2019.107817 [DOI] [PubMed] [Google Scholar]
- Pan Y., Mou Z., Shao Y. (2023). Research progress on the mechanism of ocular damage caused by blue light. Int. Eye Sci. 23 (02), 208–211. 10.3980/j.issn.1672-5123.2023.2.05 [DOI] [Google Scholar]
- Peng J., Du J., Zhong Q., Liu T., Yang L., Wu A., et al. (2019). Protect effect of Delphinidin on light induced oxidative damage of retina. Int. Eye Sci. 19 (10), 1657–1662. 10.3980/j.issn.1672-5123.2019.10.05 [DOI] [Google Scholar]
- Puglia C., Santonocito D., Romeo G., Intagliata S., Romano G. L., Strettoi E., et al. (2021). Lipid nanoparticles traverse non-corneal path to reach the posterior eye segment: in vivo evidence. Molecules 26 (15), 4673. 10.3390/molecules26154673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Lu Y., Qin B. (2019). Mechanism and protection of retina injured by blue light. Int. Eye Sci. 19 (10), 1696–1699. 10.3980/j.issn.1672-5123.2019.10.14 [DOI] [Google Scholar]
- Qin W., Guo M., Li X., Li Y., Sun X., Jin J., et al. (2021). Research progress of Chinese herb monomers in the treatment of diabetic retinopathy. Int. Eye Sci. 21 (08), 1373–1377. 10.3980/j.issn.1672-5123.2021.8.12 [DOI] [Google Scholar]
- Ren D., Zhang H., Wang X., Xue L., Wu W. (2017). Study on the synergistic antioxidant activity of lutein and zeaxanthin. Sci. Technol. Food Industry 38 (17), 296–299+304. 10.13386/j.issn1002-0306.2017.17.058 [DOI] [Google Scholar]
- Sahin K., Akdemir F., Orhan C., Tuzcu M., Gencoglu H., Sahin N., et al. (2019a). (3R, 3'R)-zeaxanthin protects the retina from photo-oxidative damage via modulating the inflammation and visual health molecular markers. Cutan. Ocul. Toxicol. 38 (2), 161–168. 10.1080/15569527.2018.1554667 [DOI] [PubMed] [Google Scholar]
- Sahin K., Gencoglu H., Akdemir F., Orhan C., Tuzcu M., Sahin N., et al. (2019b). Lutein and zeaxanthin isomers may attenuate photo-oxidative retinal damage via modulation of G protein-coupled receptors and growth factors in rats. Biochem. Biophys. Res. Commun. 516 (1), 163–170. 10.1016/j.bbrc.2019.06.032 [DOI] [PubMed] [Google Scholar]
- Sayyad Z., Sirohi K., Radha V., Swarup G. (2017). 661W is a retinal ganglion precursor-like cell line in which glaucoma-associated optineurin mutants induce cell death selectively. Sci. Rep. 7 (1), 16855. 10.1038/s41598-017-17241-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaban H., Richter C. (2002). A2E and blue light in the retina: the paradigm of age-related macular degeneration. Biol. Chem. 383 (3-4), 537–545. 10.1515/BC.2002.054 [DOI] [PubMed] [Google Scholar]
- Shang Y. M., Wang G. S., Sliney D., Yang C. H., Lee L. L. (2014). White light-emitting diodes (LEDs) at domestic lighting levels and retinal injury in a rat model. Environ. Health Perspect. 122 (3), 269–276. 10.1289/ehp.1307294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Zhang W. Y., Chiou G. C. (2010a). Effect of naringenin on NaIO(3)-induced retinal pigment epithelium degeneration and laser-induced choroidal neovascularization in rats. Int. J. Ophthalmol. 3 (1), 5–8. 10.3980/j.issn.2222-3959.2010.01.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Zhao H., Wang Z., Guan W., Kang X., Tai X., et al. (2019). Silibinin declines blue light-induced apoptosis and inflammation through MEK/ERK/CREB of retinal ganglion cells. Artif. Cell. Nanomed. Biotechnol. 47 (1), 4059–4065. 10.1080/21691401.2019.1671430 [DOI] [PubMed] [Google Scholar]
- Shen Y., Zhuang P., Lin B. Q., Zhang W. Y., Cy C. G. (2010b). Effect of Tetramethylpyrazine on RPE degeneration, choroidal blood flow and oxidative stress of RPE cells. Int. J. Ophthalmol. 3 (3), 205–210. 10.3980/j.issn.2222-3959.2010.03.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H., Takayama K., Yamada K., Suzumura A., Sato T., Nishio Y., et al. (2022). Dimethyl fumarate protects retinal pigment epithelium from blue light-induced oxidative damage via the Nrf2 pathway. Antioxidants 12 (1), 45. 10.3390/antiox12010045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Negi R., Vinayagam R., Kang S. G., Shukla P. (2024). Age-related macular degeneration (AMD): pathophysiology, drug targeting approaches, and recent developments in nanotherapeutics. Med. Lith. 60 (10), 1647. 10.3390/medicina60101647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Song J., Wang C., Li Y., Dunaief J. L. (2016). Berberine protects against light-induced photoreceptor degeneration in the mouse retina. Exp. Eye Res. 145, 1–9. 10.1016/j.exer.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S., Mandai M., Kamao H., Takahashi M. (2021). Immunological aspects of RPE cell transplantation. Prog. Retin. Eye Res. 84, 100950. 10.1016/j.preteyeres.2021.100950 [DOI] [PubMed] [Google Scholar]
- Tao Y., He M., Yang Q., Ma Z., Qu Y., Chen W., et al. (2019). Systemic taurine treatment provides neuroprotection against retinal photoreceptor degeneration and visual function impairments. Drug Des. Devel Ther. 13, 2689–2702. 10.2147/DDDT.S194169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. J., Mirza R. G., Gill M. K. (2021). Age-related macular degeneration. Med. Clin. N. Am. 105 (3), 473–491. 10.1016/j.mcna.2021.01.003 [DOI] [PubMed] [Google Scholar]
- Tosini G., Ferguson I., Tsubota K. (2016). Effects of blue light on the circadian system and eye physiology. Mol. Vis. 22, 61–72. [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Zhao J., Kim H. J., Sparrow J. R. (2016). Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 113 (25), 6904–6909. 10.1073/pnas.1524774113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaneev A., Tikhomirova V., Chesnokova N., Popova E., Beznos O., Kost O., et al. (2021). Nanotechnology for topical drug delivery to the anterior segment of the eye. Int. J. Mol. Sci. 22 (22), 12368. 10.3390/ijms222212368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Norren D., Vos J. J. (2016). Light damage to the retina: an historical approach. Eye 30 (2), 169–172. 10.1038/eye.2015.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Fernandez R., Diaz-Tome V., Luaces-Rodriguez A., Conde-Penedo A., Garcia-Otero X., Luzardo-Alvarez A., et al. (2020). Drug delivery to the posterior segment of the eye: biopharmaceutic and pharmacokinetic considerations. Pharmaceutics 12 (3), 269. 10.3390/pharmaceutics12030269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Yu F., Tao Z., Deng S. (2024a). Research progresses on xanthophyll of marigold. J. Trop. Subtropical Bot., 1–10. 10.11926/jtsb.4893 [DOI] [Google Scholar]
- Wang H., Wang M., Liu Z., He Y., Shi J., Zou S. (2024b). Protective effect of a complex of blueberry, blackcurrant, medlar and Cassia obtusifolia against oxidative stress injury in retinal epithelial cells. Mod. Food Sci. Technol. 40 (01), 47–53. 10.13982/j.mfst.1673-9078.2024.1.1613 [DOI] [Google Scholar]
- Wang J. (2023). The role and mechanism of METTL7B in blue light induced apoptosis in retinal RPE cells. Master’s thesis. Guizhou Medical University. [Google Scholar]
- Wang L., Yu X., Zhang D., Wen Y., Zhang L., Xia Y., et al. (2023). Long-term blue light exposure impairs mitochondrial dynamics in the retina in light-induced retinal degeneration in vivo and in vitro . J. Photochem. Photobiol. B-Biol. 240, 112654. 10.1016/j.jphotobiol.2023.112654 [DOI] [PubMed] [Google Scholar]
- Wang M., Zhang C., Lin X. (2008). Protective effect of lutein against blue light-induced retinal damage in rat. J. Hyg. Res. 37 (04), 409–412. 10.3969/j.issn.1000-8020.2008.04.006 [DOI] [PubMed] [Google Scholar]
- Wang S., Li Y., Zhang Y., Chen H., Zhang W. (2022). Effect of vitamin E on retinal pigment epithelial cells injured by high dose blue light. Int. Eye Sci. 22 (02), 189–193. 10.3980/j.issn.1672-5123.2022.2.03 [DOI] [Google Scholar]
- Wang W., Zhang Y., Jin W., Xing Y., Yang A. (2018). Catechin weakens diabetic retinopathy by inhibiting the expression of NF-κB signaling pathway-mediated inflammatory factors. Ann. Clin. Lab. Sci. 48 (5), 594–600. [PubMed] [Google Scholar]
- Wang X., Li Z. (2021). Research progress of eye damage caused by short wave blue light. Med. Recapitulate 27 (01), 116–120. 10.3969/j.issn.1006-2084.2021.01.022 [DOI] [Google Scholar]
- Wang Y., Huo Y., Zhao L., Lu F., Wang O., Yang X., et al. (2016). Cyanidin-3-glucoside and its phenolic acid metabolites attenuate visible light-induced retinal degeneration in vivo via activation of Nrf2/HO-1 pathway and NF-κB suppression. Mol. Nutr. Food Res. 60 (7), 1564–1577. 10.1002/mnfr.201501048 [DOI] [PubMed] [Google Scholar]
- Wheway G., Nazlamova L., Turner D., Cross S. (2019). 661W photoreceptor cell line as a cell model for studying retinal ciliopathies. Front. Genet. 10, 308. 10.3389/fgene.2019.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. Y., Gao A., Giunta M., Tran S. D. (2024). What's new in ocular drug delivery: advances in suprachoroidal injection since 2023. Pharmaceuticals 17 (8), 1007. 10.3390/ph17081007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Hu Q., Li L., Tang X., Zou J., Huang L., et al. (2019). Protective effects of autophagy against blue light-induced retinal degeneration in aged mice. Sci. China-Life Sci. 62 (2), 244–256. 10.1007/s11427-018-9357-y [DOI] [PubMed] [Google Scholar]
- Xiang Y., Zou M., Zhang Y., Jin R., Nie Y. (2020). Drug-loaded and blue-ray filtered hydrogel as a potential intraocular lens for cataract treatment. Pharm. Nanotechnol. 8 (4), 302–312. 10.2174/2211738508666200313144112 [DOI] [PubMed] [Google Scholar]
- Xie T., Cai J., Yao Y., Sun C., Yang Q., Wu M., et al. (2021). LXA4 protects against blue-light induced retinal degeneration in human A2E-laden RPE cells and Balb-c mice. Ann. Transl. Med. 9 (15), 1249. 10.21037/atm-21-3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. (2008). The effect of artificial light on the eye and biothythm and traditional Chinese medicine intervention function. Dissertation. Nanjing University of Chinese Medicine. [Google Scholar]
- Xu J., Wang Y. (2007). Progress in the study of blue light injury of the retina. Int. Eye Sci. (04), 1107–1109. 10.3969/j.issn.1672-5123.2007.04.065 [DOI] [Google Scholar]
- Xu Z., Jiang Y., Zhao Z., Li W., Sun X. (2018). Photooxidation-induced damage in retinal pigment epithelium and protective effect of procyanidins B2. J. Shanghai Jiaot. Univ. Sci. 36 (01), 36–43. 10.3969/j.issn.1671-9964.2018.01.007 [DOI] [Google Scholar]
- Yamazaki K., Ishida K., Otsu W., Muramatsu A., Nakamura S., Yamada W., et al. (2024). Delphinidins from Maqui Berry (Aristotelia chilensis) ameliorate the subcellular organelle damage induced by blue light exposure in murine photoreceptor-derived cells. BMC Complement. Med. Ther. 24 (1), 3. 10.1186/s12906-023-04322-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W. (2006). Injurious effect of low level laser irradiation and therapeutic effect of MK-801 in retinal laser injury. Master’s thesis. Academy of Military Science. [Google Scholar]
- Yang J., Peng H., Chen Q., Jiang Y. (2011). Effect of tripterine on expression of VEGF and PEDF of retinal endothelial cells in mouse with high glucose. Recent Adv. Ophthalmol. 31 (04), 321–323+331. 10.13389/j.cnki.rao.2011.04.006 [DOI] [Google Scholar]
- Yang J., Zhang Y., Zhang L., Liao Z., Hou Y., Zhan Z., et al. (2020). The protective effect of lutein against retinal damage induced by blue light emitting diodes(LEDs) in rats. Food Nutr. China. 26 (06), 54–58. 10.19870/j.cnki.11-3716/ts.2020.06.013 [DOI] [Google Scholar]
- Yang S., Wang C., Qi S. (2023). Research progress on the mechanism of retinal light injury. Int. Eye Sci. 23 (06), 938–942. 10.3980/j.issn.1672-5123.2023.6.11 [DOI] [Google Scholar]
- Ye M. J., Meng N. (2021). Resveratrol acts via the mitogen-activated protein kinase (MAPK) pathway to protect retinal ganglion cells from apoptosis induced by hydrogen peroxide. Bioengineered 12 (1), 4878–4886. 10.1080/21655979.2021.1954742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Thomas F., Lang J. C., Chaum E. (2011). Modulation of oxidative stress responses in the human retinal pigment epithelium following treatment with vitamin C. J. Cell. Physiol. 226 (8), 2025–2032. 10.1002/jcp.22532 [DOI] [PubMed] [Google Scholar]
- Yu H. (2019). Effect of drugs on retinal pigment epithelial cell damage injury Models. Master’s thesis. Southeast University. [Google Scholar]
- Yu Y., Cheng T., Zou X., Zhang M., Yu Y., Zou Y., et al. (2023a). Experimental study of chronic retinal damage induced by blue light exposure in Brown Norway rats. Tianjin Med. J. 51 (11), 1193–1198. 10.11958/20230030 [DOI] [Google Scholar]
- Yu Y., Zhou W., Yu Z., Zhang M., Cheng T., Zou X., et al. (2023b). Protective effects of α-lipoic acid on blue light-induced damage in ARPE-19 cells. Recent Adv. Ophthalmol. 43 (10), 770–774. 10.13389/j.cnki.rao.2023.0155 [DOI] [Google Scholar]
- Zhang B., Rusciano D., Osborne N. N. (2008). Orally administered epigallocatechin gallate attenuates retinal neuronal death in vivo and light-induced apoptosis in vitro . Brain Res. 1198, 141–152. 10.1016/j.brainres.2007.12.015 [DOI] [PubMed] [Google Scholar]
- Zhang C. (2023a). Comparative study of retinal damage induced by laser irradiation at wavelengths of 650 nm and 450 nm and the role of PARP-1 in blue light damage. Dissertation. Jilin University. [Google Scholar]
- Zhang C., Chen Q., Zhou W., Li Z., Yu Y., Deng Q., et al. (2022). Protective effect and mechanism of miR-22-3p on rat retinal ganglion cells exposed to blue light. Recent Adv. Ophthalmol. 42 (10), 780–785. 10.13389/j.cnki.rao.2022.0160 [DOI] [Google Scholar]
- Zhang H., Song T., Kang R., Ren F., Liu J., Wang J. (2023a). Plant bioactive compounds alleviate photoinduced retinal damage and asthenopia: mechanisms, synergies, and bioavailability. Nutr. Res. 120, 115–134. 10.1016/j.nutres.2023.10.003 [DOI] [PubMed] [Google Scholar]
- Zhang P. (2023b). Exploring the efficacy and mechanism of taxifolin on blue light induced dry age-related macular degeneration. Master’s thesis. Jiangxi University of Chinese Medicine. [Google Scholar]
- Zhang S. (2023c). Inhibiting PARP-1 can suppress mitophagy and synergistically protect the retina from light damage. Dissertation. Jilin University. [Google Scholar]
- Zhang Y., Shi Y., Khan M. M., Xiao F., Chen W., Tao W., et al. (2024). Ocular RNA nanomedicine: engineered delivery nanoplatforms in treating eye diseases. Trends Biotechnol. 42, 1439–1452. 10.1016/j.tibtech.2024.05.002 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang T., Wan Z., Bai J., Xue Y., Dai R., et al. (2023b). Alterations of the intestinal microbiota in age-related macular degeneration. Front. Microbiol. 14, 1069325. 10.3389/fmicb.2023.1069325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. (2019). Effect of matrine on proliferation of retinal microvascular endothelial cell and expression of vascular endothelial growth factor in rats with diabetic retinopathy. Eval. Analysis Drug-Use Hosp. China 19 (04), 422–425+428. 10.14009/j.issn.1672-2124.2019.04.011 [DOI] [Google Scholar]
- Zhao L., Wang C., Song D., Li Y., Song Y., Su G., et al. (2014). Systemic administration of the antioxidant/iron chelator α-lipoic acid protects against light-induced photoreceptor degeneration in the mouse retina. Invest. Ophthalmol. Vis. Sci. 55 (9), 5979–5988. 10.1167/iovs.14-15025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. (2015). Grape procyanidins B2 protect RPE cells against photooxidative damage. Master’s thesis. Shanghai Jiao Tong University. [Google Scholar]
- Zhao Z. C., Zhou Y., Tan G., Li J. (2018). Research progress about the effect and prevention of blue light on eyes. Int. J. Ophthalmol. 11 (12), 1999–2003. 10.18240/ijo.2018.12.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Inomata T., Shih K. C., Okumura Y., Fujio K., Huang T., et al. (2022). Application of animal models in interpreting dry eye disease. Front. Med. 9, 830592. 10.3389/fmed.2022.830592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S. (2022). Protective effects of hydrogen sulfide/lycium barbarum polysaccharide on blue light/PM2.5-induced oxidative damage. Master’s thesis. Lanzhou University. [Google Scholar]
- Zhu Y. (2021). Study on the light damage of SD rat's retina by different wavelength of LED lights. Master’s thesis. Tianjin Medical University. [Google Scholar]
- Zhuang H., Wu Z., Chen X., Li C. (2022). CERKL attenuates blue light-induced oxidative stress in human retinal pigment epithelial cells by activating the SIRT1/E2F1 axis. Int. Eye Sci. 22 (08), 1245–1251. 10.3980/j.issn.1672-5123.2022.8.02 [DOI] [Google Scholar]
- Ziolkowska N., Lewczuk B., Szyrynska N., Rawicka A., Vyniarska A. (2023). Low-intensity blue light exposure reduces melanopsin expression in intrinsically photosensitive retinal ganglion cells and damages mitochondria in retinal ganglion cells in wistar rats. Cells 12 (7), 1014. 10.3390/cells12071014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Jiang W., Chiou G. C. (2007). Effect of tetramethylpyrazine on rat experimental choroidal neovascularization in vivo and endothelial cell cultures in vitro . Curr. Eye Res. 32 (1), 71–75. 10.1080/02713680601088787 [DOI] [PubMed] [Google Scholar]