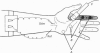
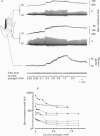
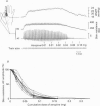
Abstract
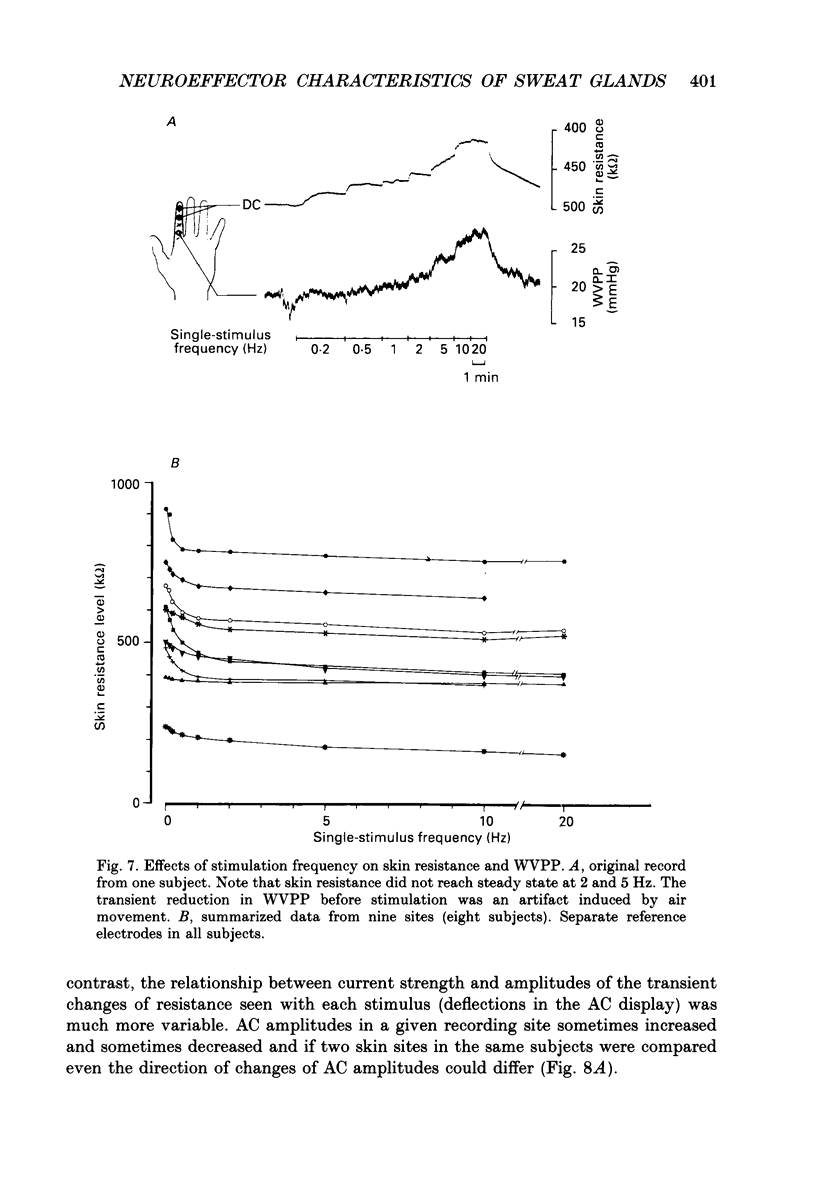
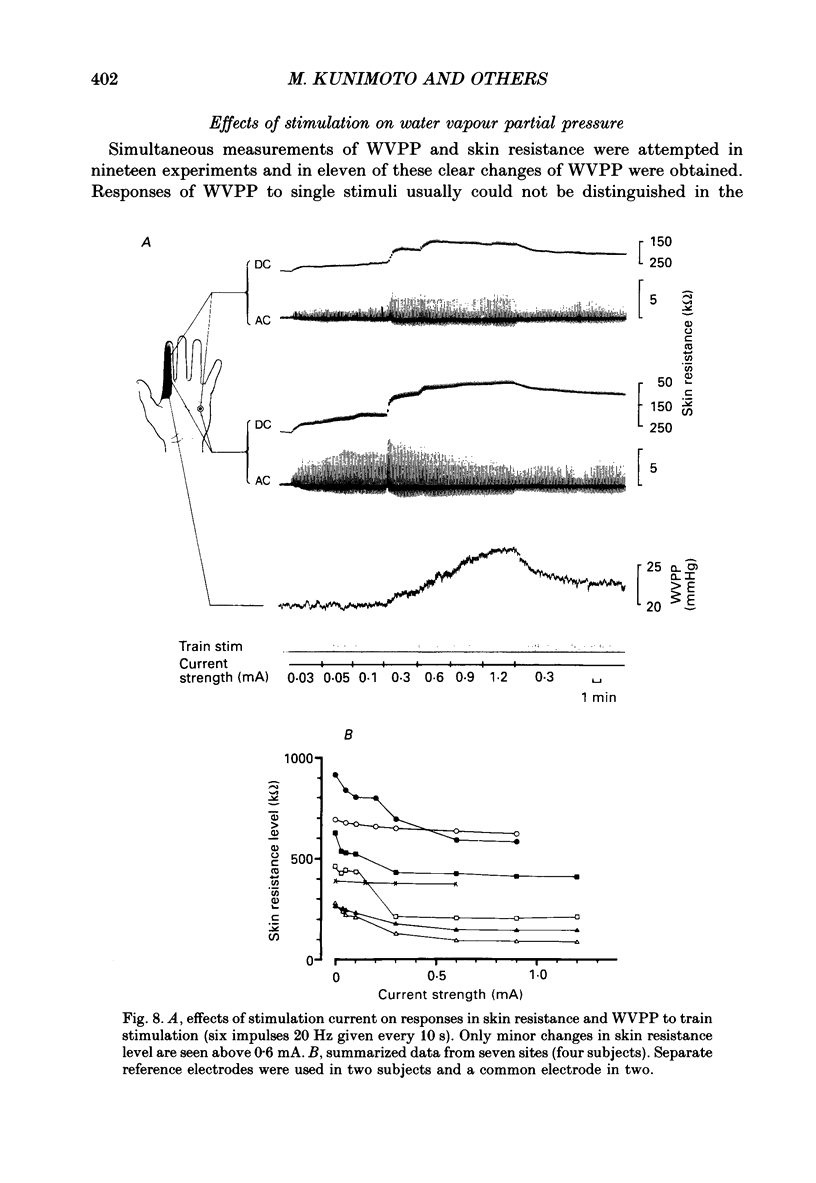
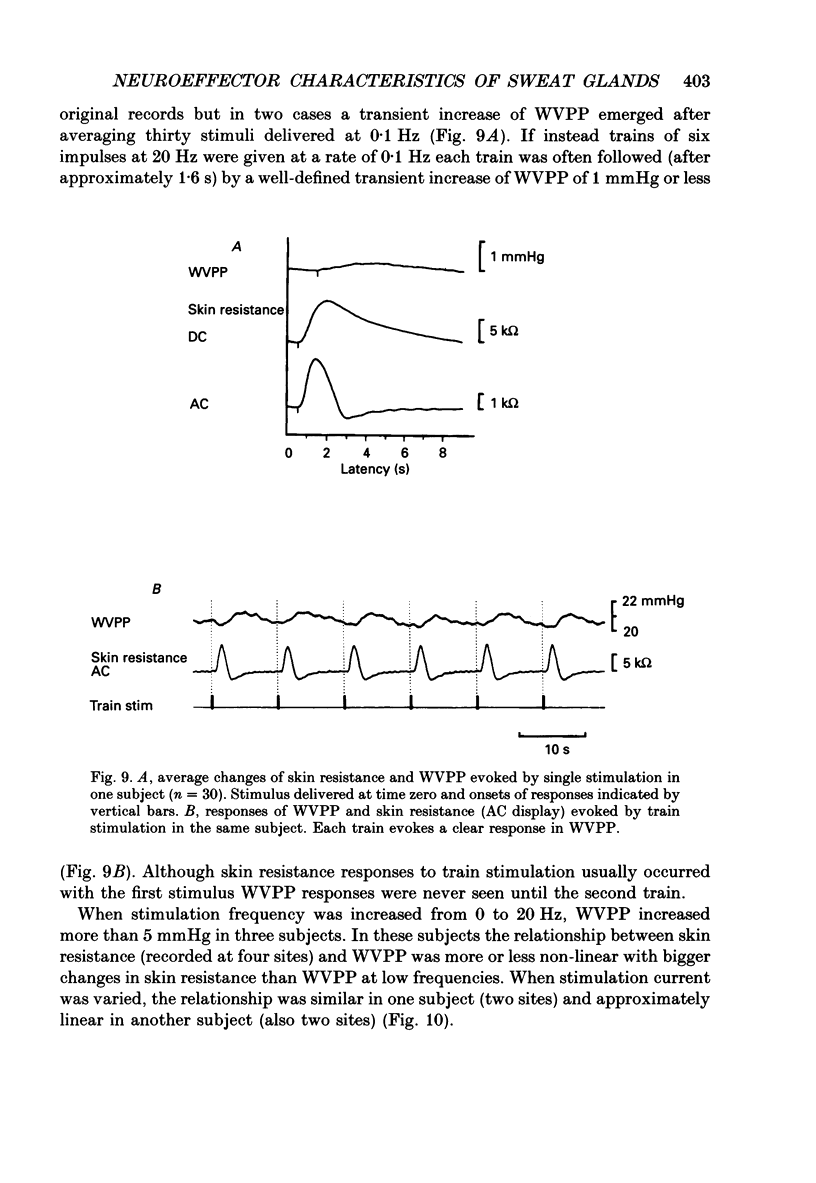
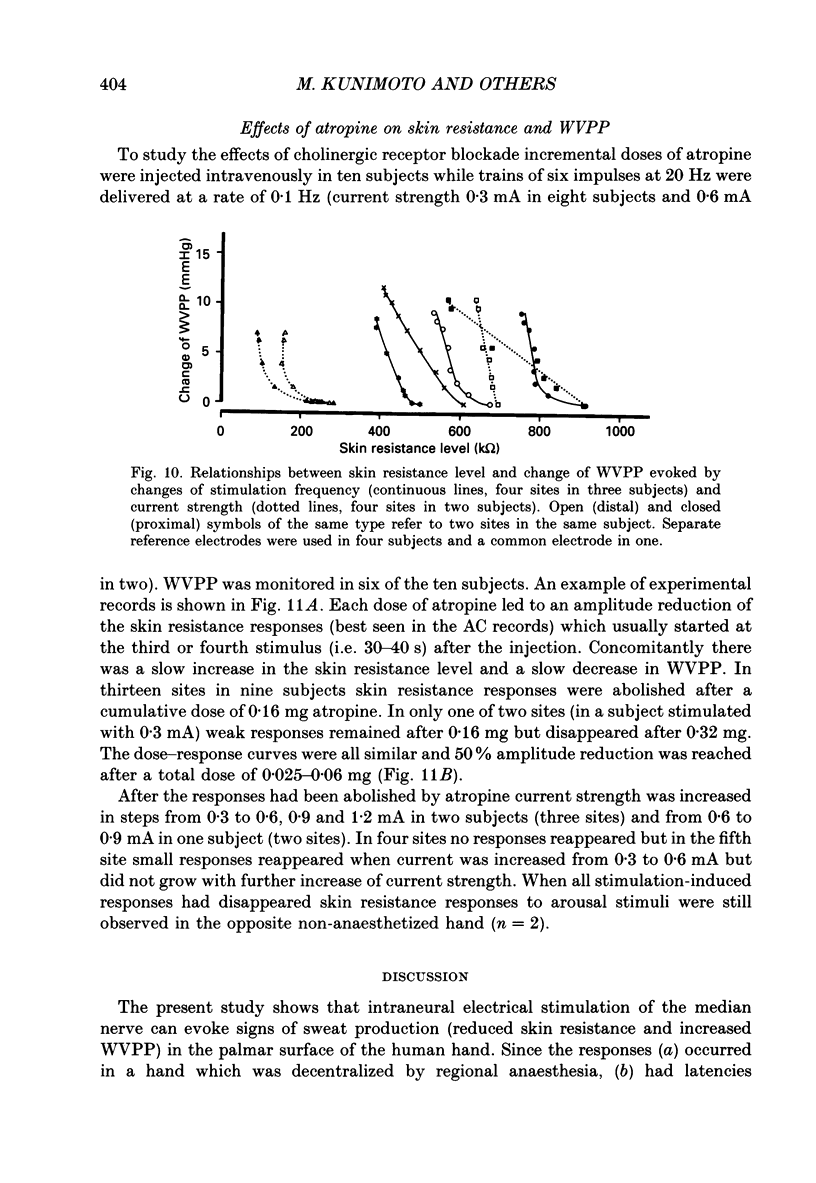
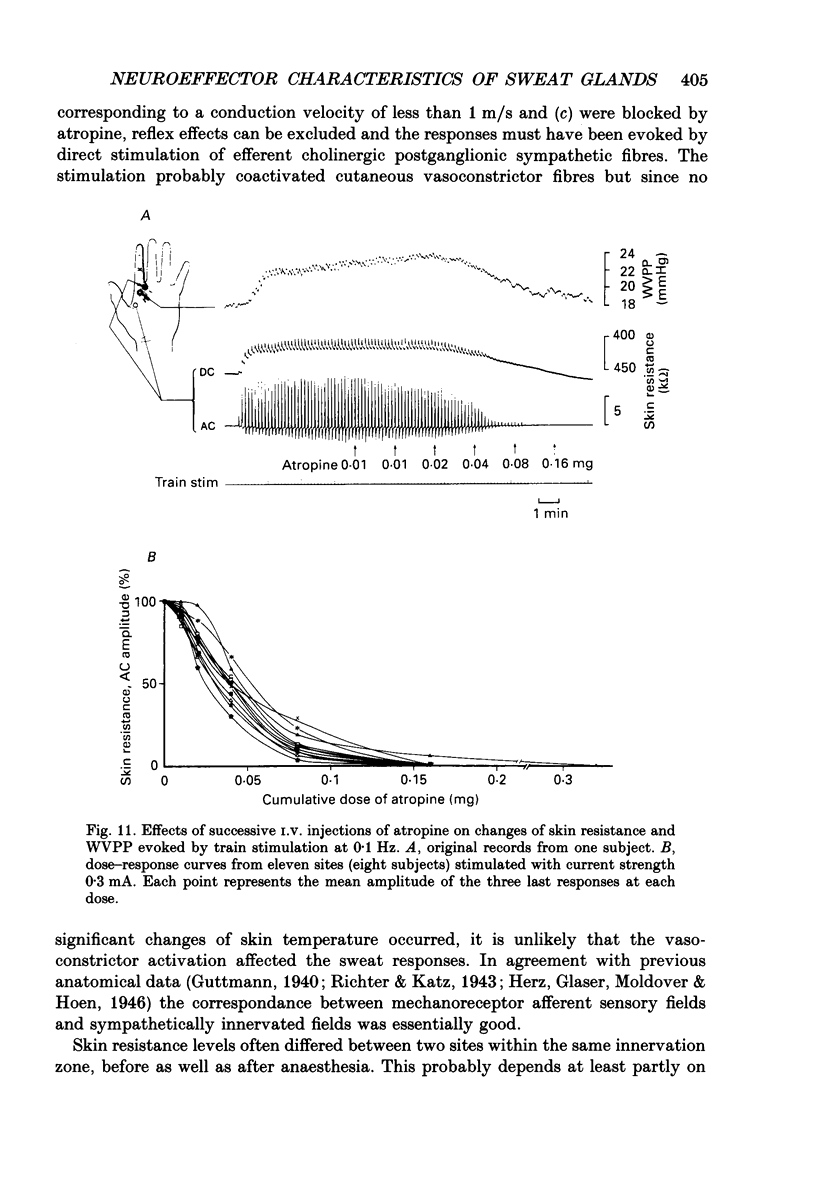
1. Intraneural electrical stimuli (0.3-1.2 mA, 0.2 ms) were delivered via a tungsten microelectrode inserted into a cutaneous fascicle in the median nerve at the wrist in twenty-eight normal subjects. The effects on sweat glands within the innervation zone were monitored as changes of skin resistance and water vapour partial pressure (WVPP). Regional anaesthesia of the brachial plexus in the axilla eliminated spontaneous sympathetic activity and reflex effects. 2. At stimulation frequencies of 0.1 Hz each stimulus evoked a transient skin resistance reduction, the amplitude of which varied initially but reached a steady state of less than 10 k omega after, on average, nine responses. If preceded by stimulation-free intervals of 5 min or more, up to fifteen stimuli were required before the first response occurred. With higher frequencies individual responses started to merge, skin resistance levels decreased successively and levelled off around 10 Hz. The total change of resistance (0-10 Hz) was 101 +/- 46 (n = 9) k omega and the higher the pre-stimulus level, the larger the reduction (r = 0.68, P less than 0.05). 3. Stimulus-response latencies to the onset of a skin resistance reduction (single stimuli or trains of six impulses/20 Hz given at 0.1 Hz) shortened initially but reached steady-state values after on average nine to twelve impulses. Average conduction velocity between stimulating electrode and skin resistance recording site was 0.78 m/s and average time for electrical neuroeffector transfer in sweat glands was estimated to be 348 ms. 4. In addition to direct stimulation-induced resistance responses there were also small spontaneous reductions of resistance. They were seen in all subjects and at all frequencies but were more common in some subjects and occurred predominantly at the beginning of stimulation or at changes of frequency. They occurred independently at two skin sites in the same subject and disappeared during stimulation-free periods and after atropine. 5. With train stimulation (six impulses/20 Hz) at 0.1 Hz, each train evoked transient increases of WVPP of 1 mmHg or less in some subjects (latency around 1.6 s). After averaging weak increases were seen also after single stimuli in two subjects. Increases of stimulation current or frequency led to slowly developing sustained increases of WVPP concomitant with decreases in skin resistance. 6. Responses in skin resistance and WVPP to train stimulation at 0.1 Hz were suppressed in a dose-dependent way by I.V. injections of atropine.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


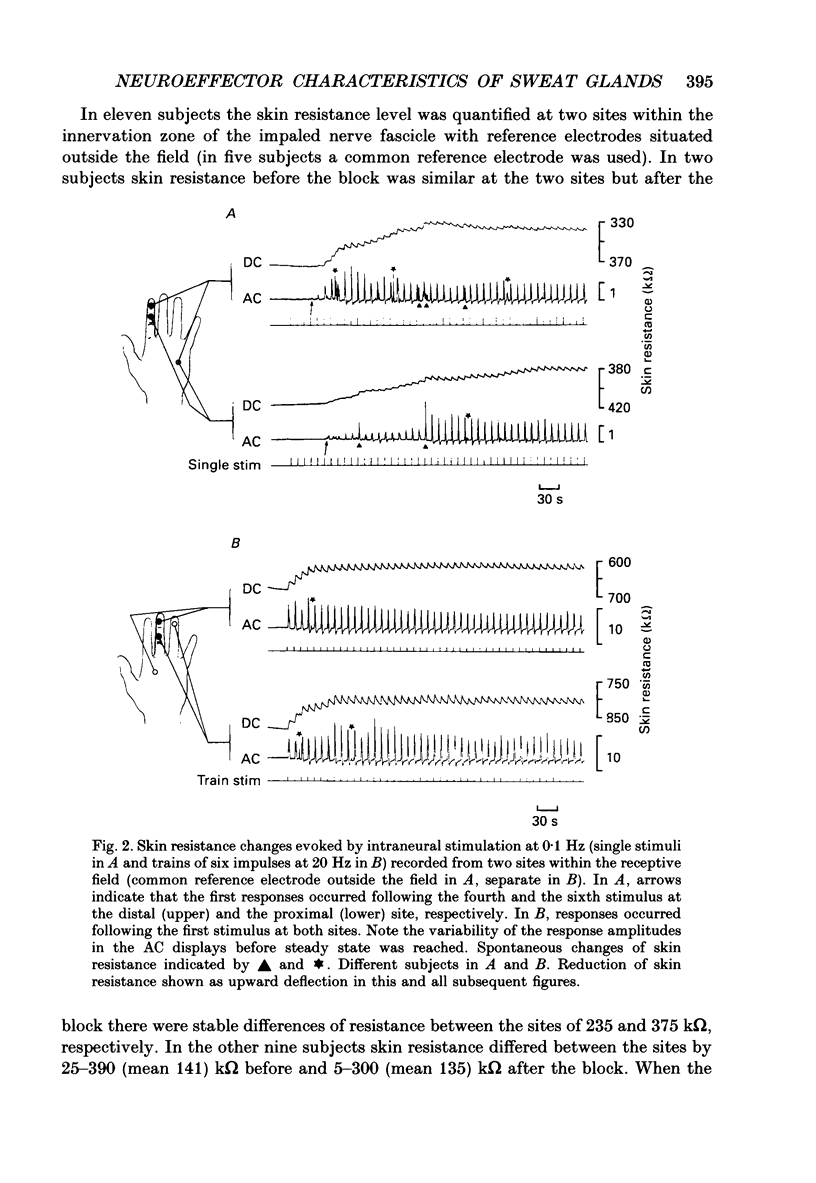

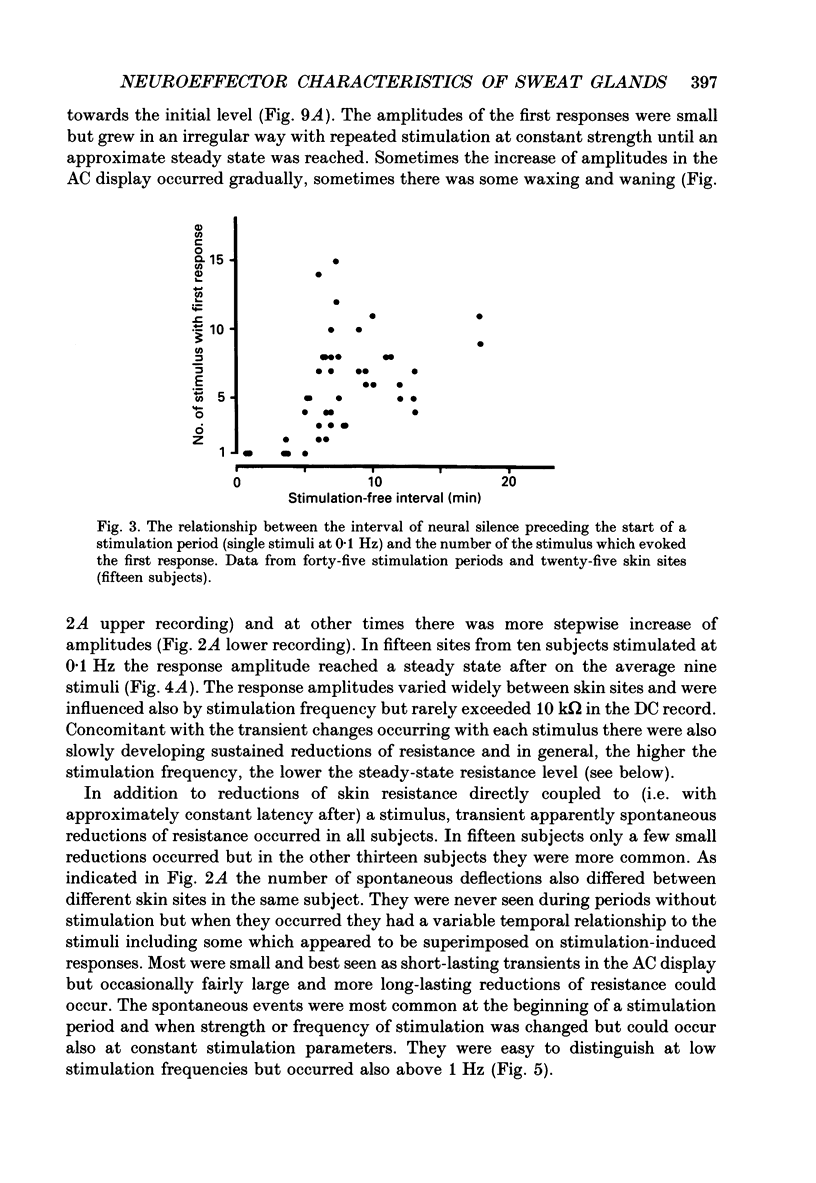
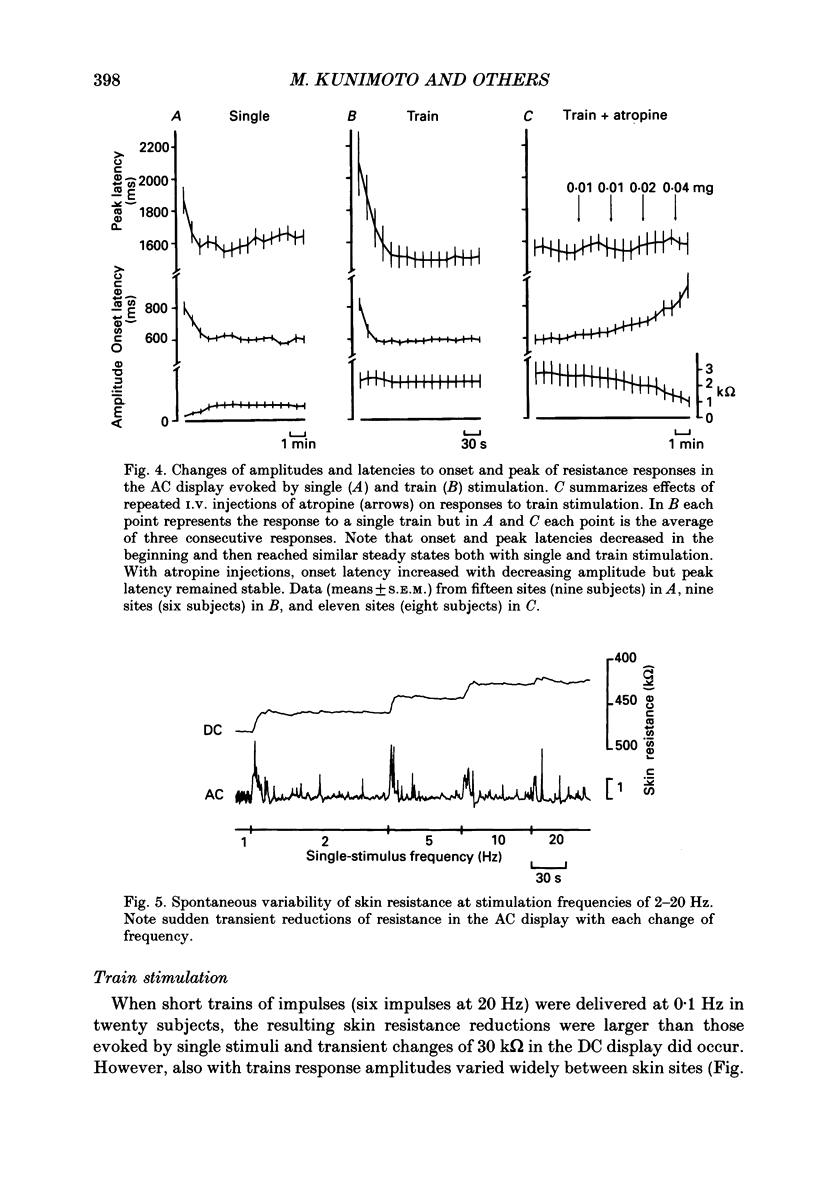
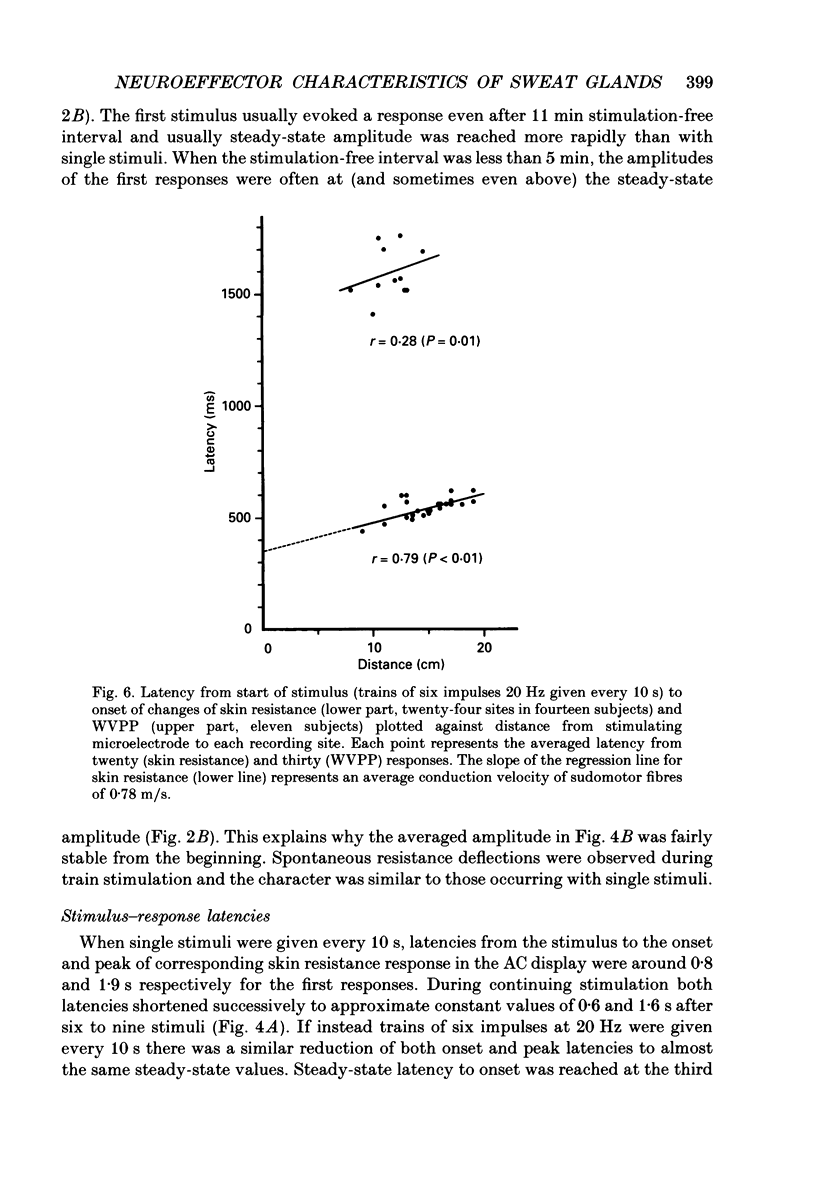







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams T., Vaughan J. A. Human eccrine sweat gland activity and palmar electrical skin resistance. J Appl Physiol. 1965 Sep;20(5):980–983. doi: 10.1152/jappl.1965.20.5.980. [DOI] [PubMed] [Google Scholar]
- Bini G., Hagbarth K. E., Hynninen P., Wallin B. G. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol. 1980 Sep;306:537–552. doi: 10.1113/jphysiol.1980.sp013413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael E. A., Honeyman W. M., Kolb L. C., Stewart W. K. Peripheral conduction rate in the sympathetic nervous system of man. J Physiol. 1941 Mar 25;99(3):338–343. doi: 10.1113/jphysiol.1941.sp003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLKOW B. Nervous control of the blood vessels. Physiol Rev. 1955 Jul;35(3):629–663. doi: 10.1152/physrev.1955.35.3.629. [DOI] [PubMed] [Google Scholar]
- Fagius J., Wallin B. G. Sympathetic reflex latencies and conduction velocities in normal man. J Neurol Sci. 1980 Sep;47(3):433–448. doi: 10.1016/0022-510x(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Hallin R. G., Hongell A., Torebjörk H. E., Wallin B. G. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972 Feb;84(2):164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Hongell A., Hallin R. G., Torebjörk H. E. Afferent impulses in median nerve fascicles evoked by tactile stimuli of the human hand. Brain Res. 1970 Dec 18;24(3):423–442. doi: 10.1016/0006-8993(70)90183-6. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Kagayama M., Nishiyama A. Membrane potential and input resistance in acinar cells from cat and rabbit submaxillary glands in vivo: effects of autonomic nerve stimulation. J Physiol. 1974 Oct;242(1):157–172. doi: 10.1113/jphysiol.1974.sp010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy W. R., Sakuta M., Quick D. C. Rodent eccrine sweat glands: a case of multiple efferent innervation. Neuroscience. 1984 Mar;11(3):741–749. doi: 10.1016/0306-4522(84)90057-5. [DOI] [PubMed] [Google Scholar]
- Lidberg L., Wallin B. G. Sympathetic skin nerve discharges in relation to amplitude of skin resistance responses. Psychophysiology. 1981 May;18(3):268–270. doi: 10.1111/j.1469-8986.1981.tb03033.x. [DOI] [PubMed] [Google Scholar]
- Lloyd D. P. SECRETION AND REABSORPTION IN SWEAT GLANDS. Proc Natl Acad Sci U S A. 1959 Mar;45(3):405–409. doi: 10.1073/pnas.45.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G. E. Measurement of water exchange through skin. Med Biol Eng Comput. 1977 May;15(3):209–218. doi: 10.1007/BF02441040. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Bullard R. W. Characteristics of subthreshold sudomotor neural impulses. J Appl Physiol. 1972 Sep;33(3):300–305. doi: 10.1152/jappl.1972.33.3.300. [DOI] [PubMed] [Google Scholar]
- Ohman A. Electrodermal activity and vulnerability to schizophrenia: a review. Biol Psychol. 1981 Mar-May;12(2-3):87–145. doi: 10.1016/0301-0511(81)90008-9. [DOI] [PubMed] [Google Scholar]
- Podlesny J. A., Raskin D. C. Physiological measures and the detection of deception. Psychol Bull. 1977 Jul;84(4):782–799. [PubMed] [Google Scholar]
- Roberts D. F., Salzano F. M., Willson J. O. Active sweat gland distribution in Caingang Indians. Am J Phys Anthropol. 1970 May;32(3):395–400. doi: 10.1002/ajpa.1330320309. [DOI] [PubMed] [Google Scholar]
- Sato K., Sato F. Effect of VIP on sweat secretion and cAMP accumulation in isolated simian eccrine glands. Am J Physiol. 1987 Dec;253(6 Pt 2):R935–R941. doi: 10.1152/ajpregu.1987.253.6.R935. [DOI] [PubMed] [Google Scholar]
- Sato K., Sato F. Individual variations in structure and function of human eccrine sweat gland. Am J Physiol. 1983 Aug;245(2):R203–R208. doi: 10.1152/ajpregu.1983.245.2.R203. [DOI] [PubMed] [Google Scholar]
- Sato K. The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev Physiol Biochem Pharmacol. 1977;79:51–131. doi: 10.1007/BFb0037089. [DOI] [PubMed] [Google Scholar]
- Tanaka E., Uchiyama S., Nakano S. Effects of calcitonin gene-related peptide and vasoactive intestinal peptide on nicotine-induced sweating in man. J Auton Nerv Syst. 1990 Jul;30(3):265–268. doi: 10.1016/0165-1838(90)90258-k. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E., Hallin R. G. Responses in human A and C fibres to repeated electrical intradermal stimulation. J Neurol Neurosurg Psychiatry. 1974 Jun;37(6):653–664. doi: 10.1136/jnnp.37.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E., Torebjörk H. E., Wallin B. G. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979 Oct;59(4):919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Wallin B. G., Blumberg H., Hynninen P. Intraneural stimulation as a method to study sympathetic function in the human skin. Neurosci Lett. 1983 Apr 11;36(2):189–194. doi: 10.1016/0304-3940(83)90263-x. [DOI] [PubMed] [Google Scholar]