Abstract
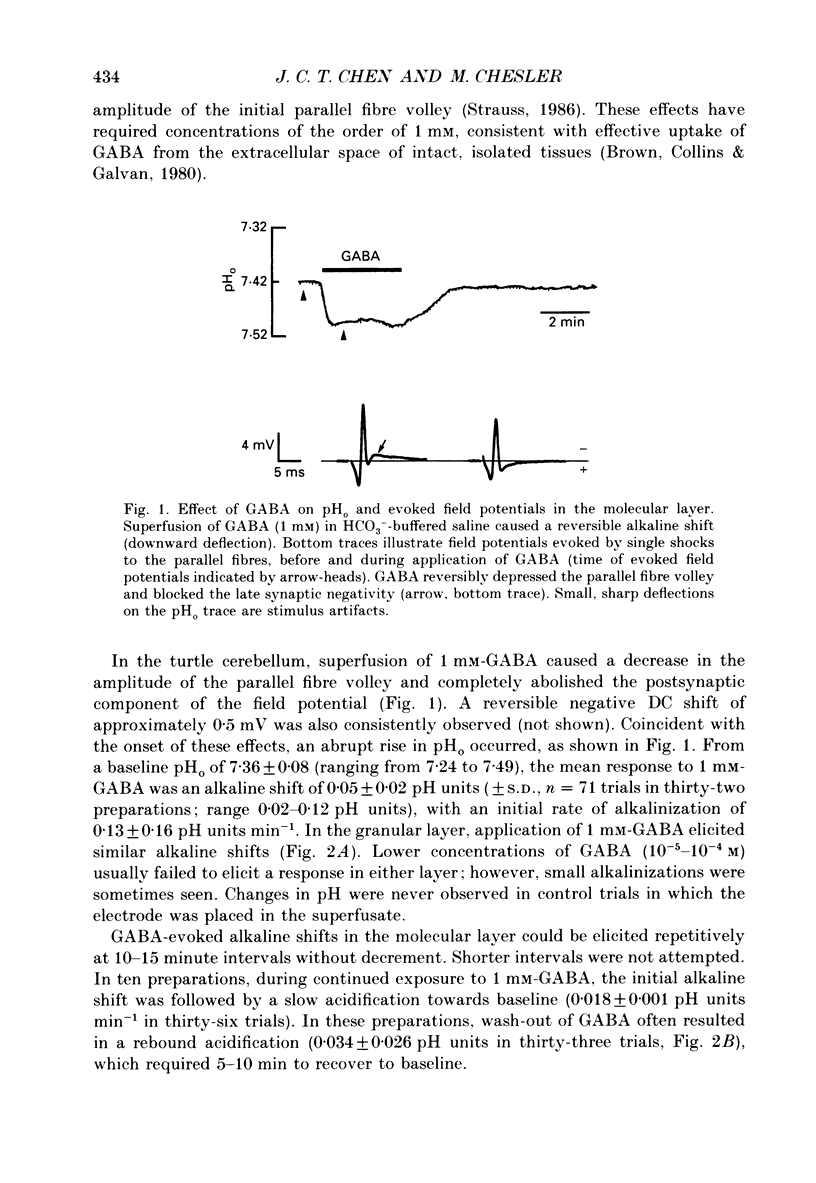
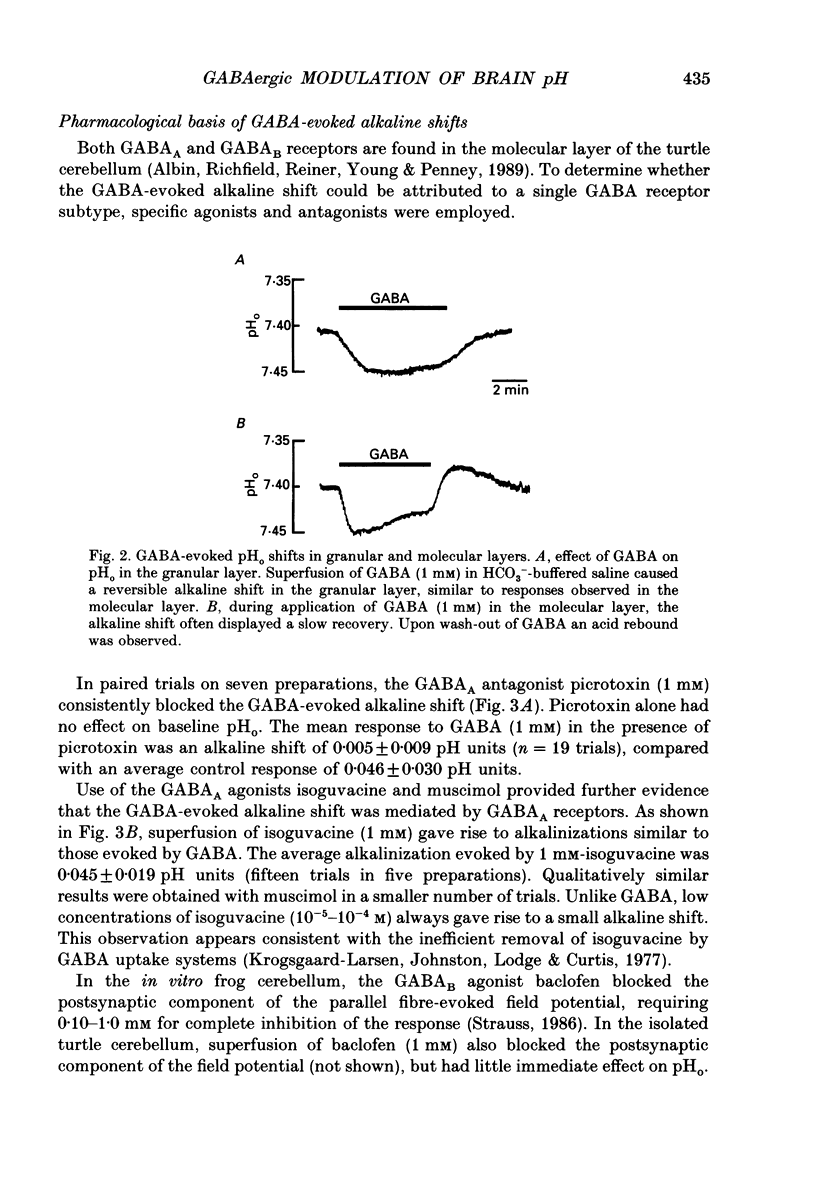
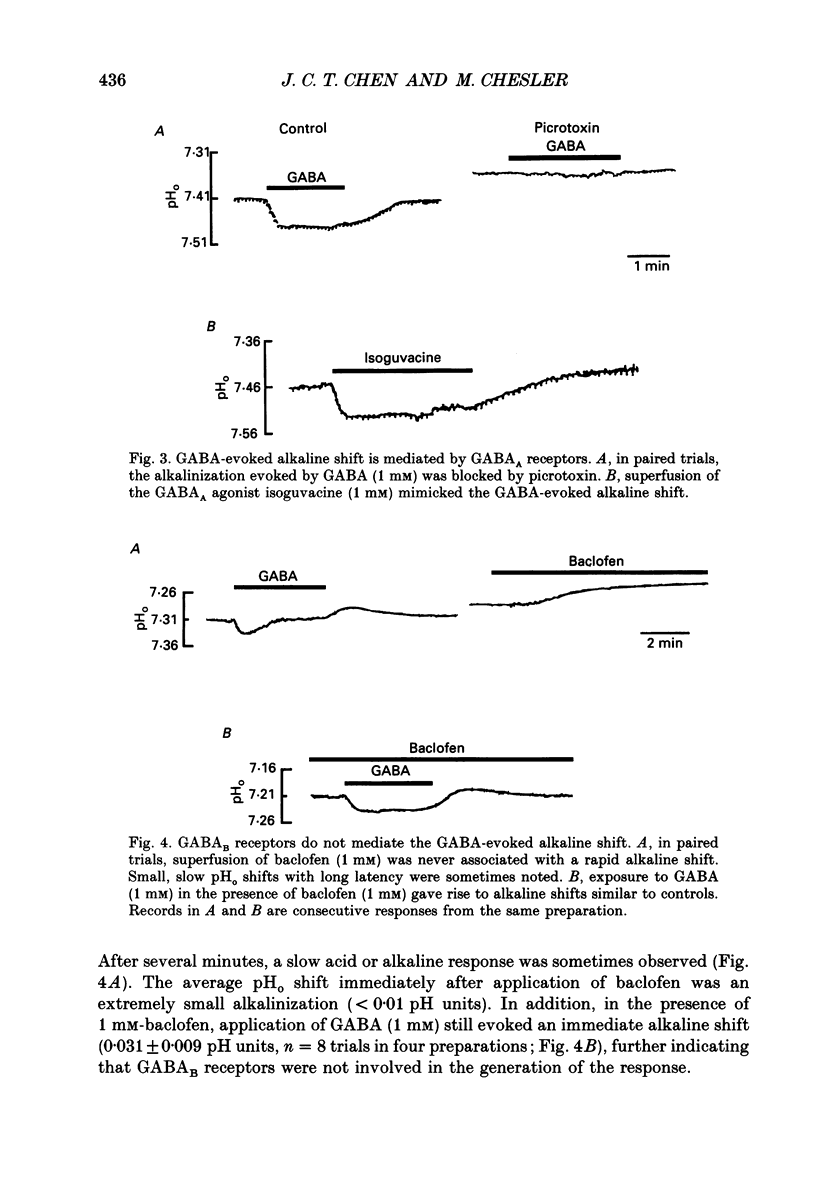
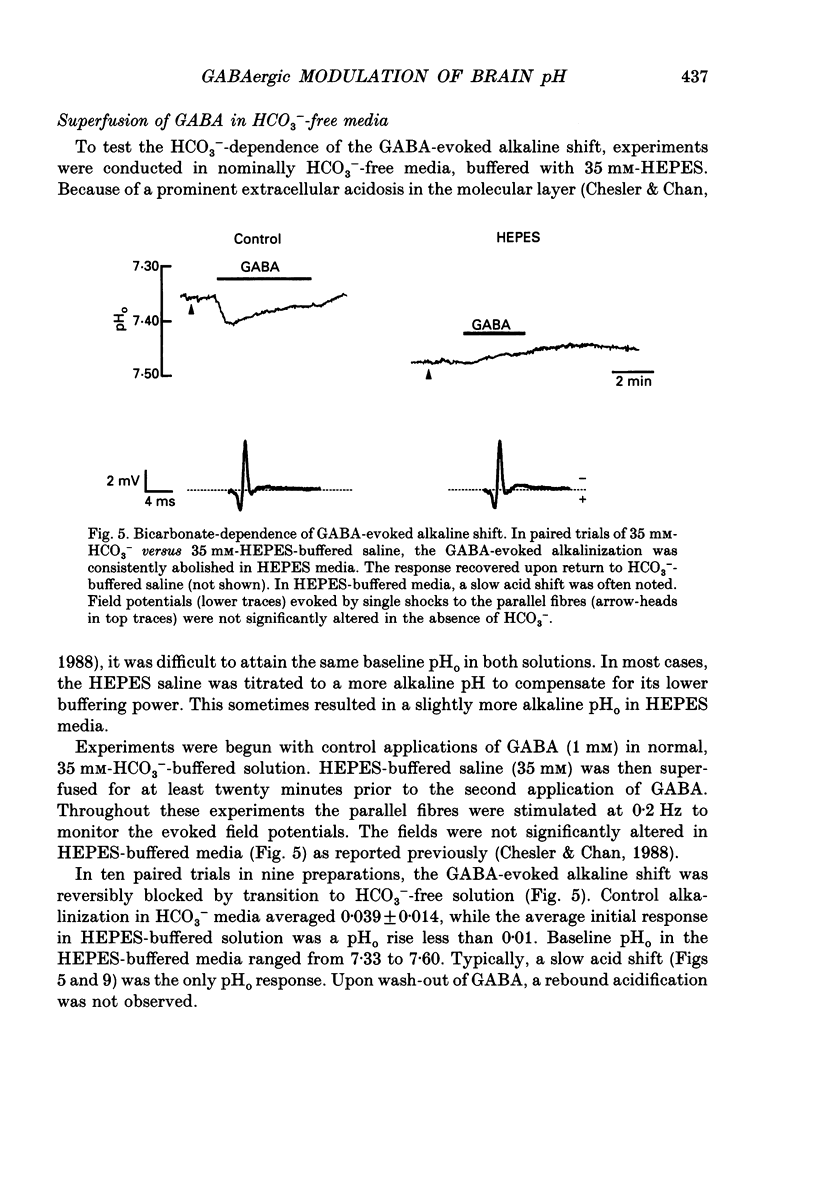
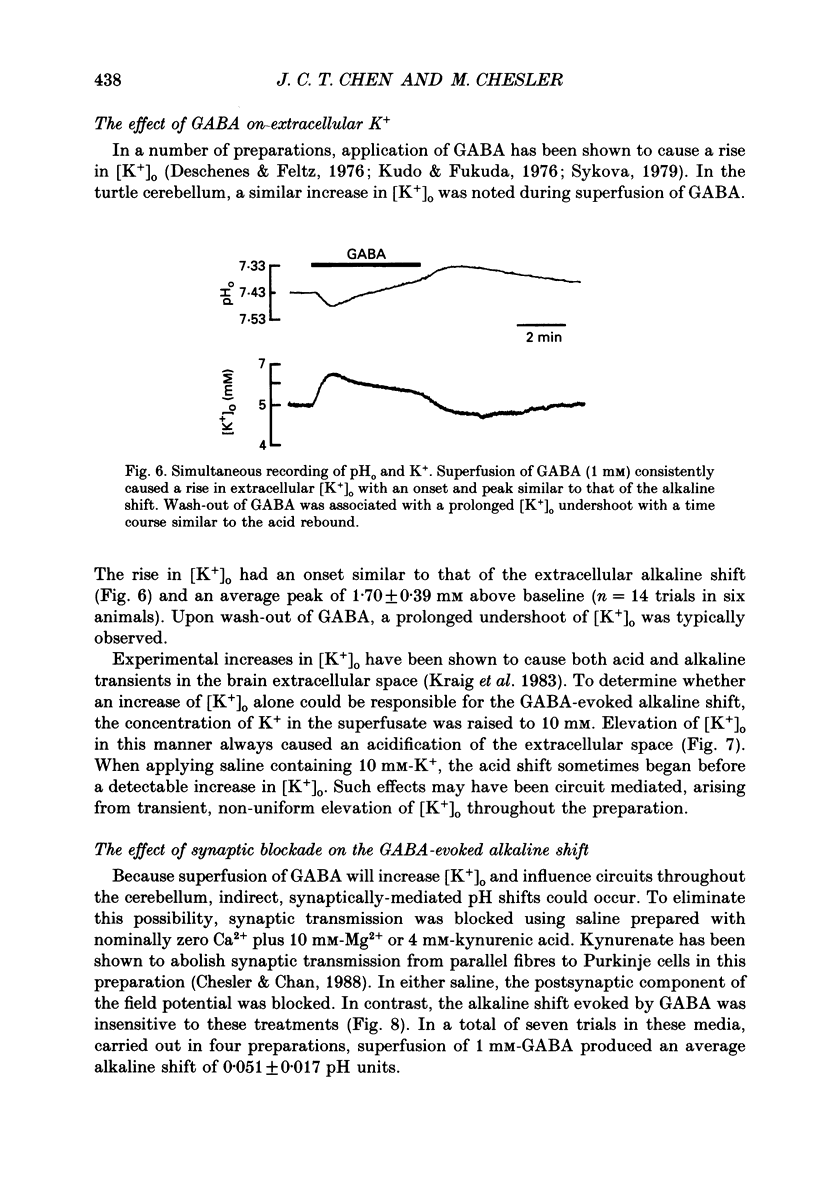
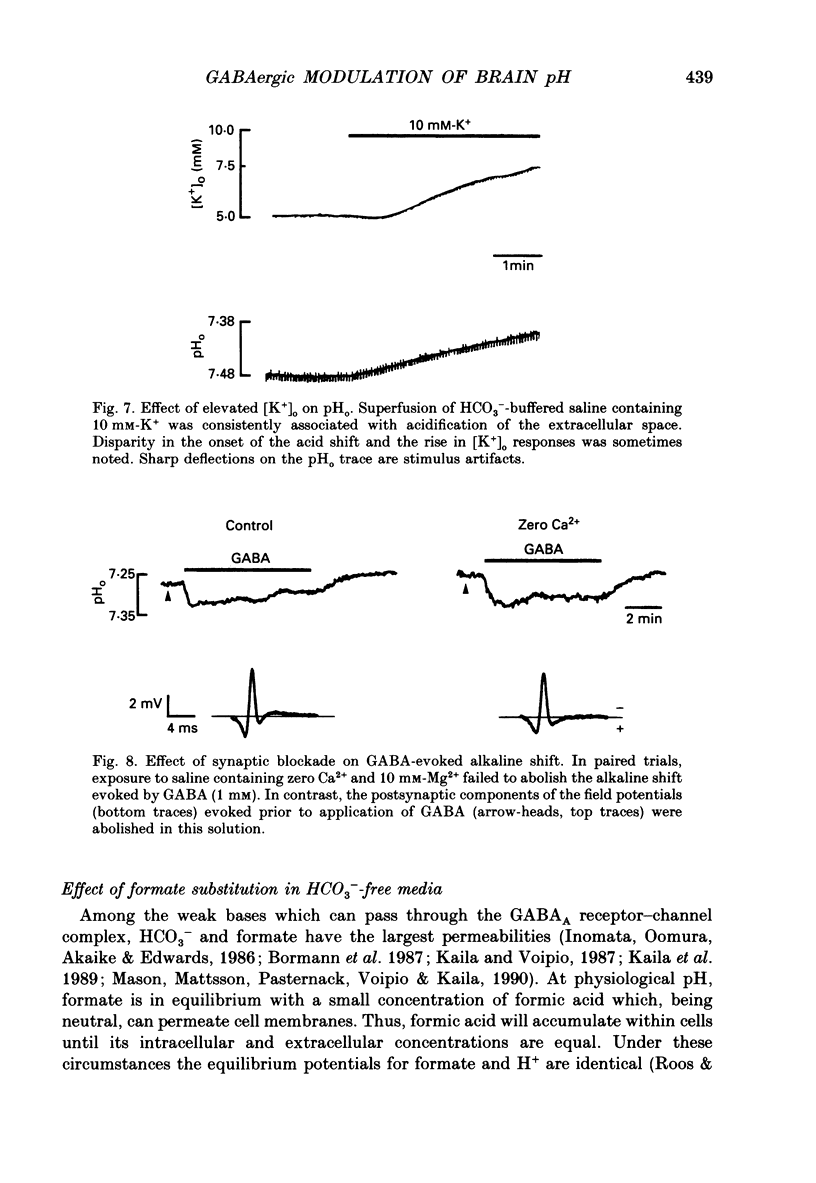
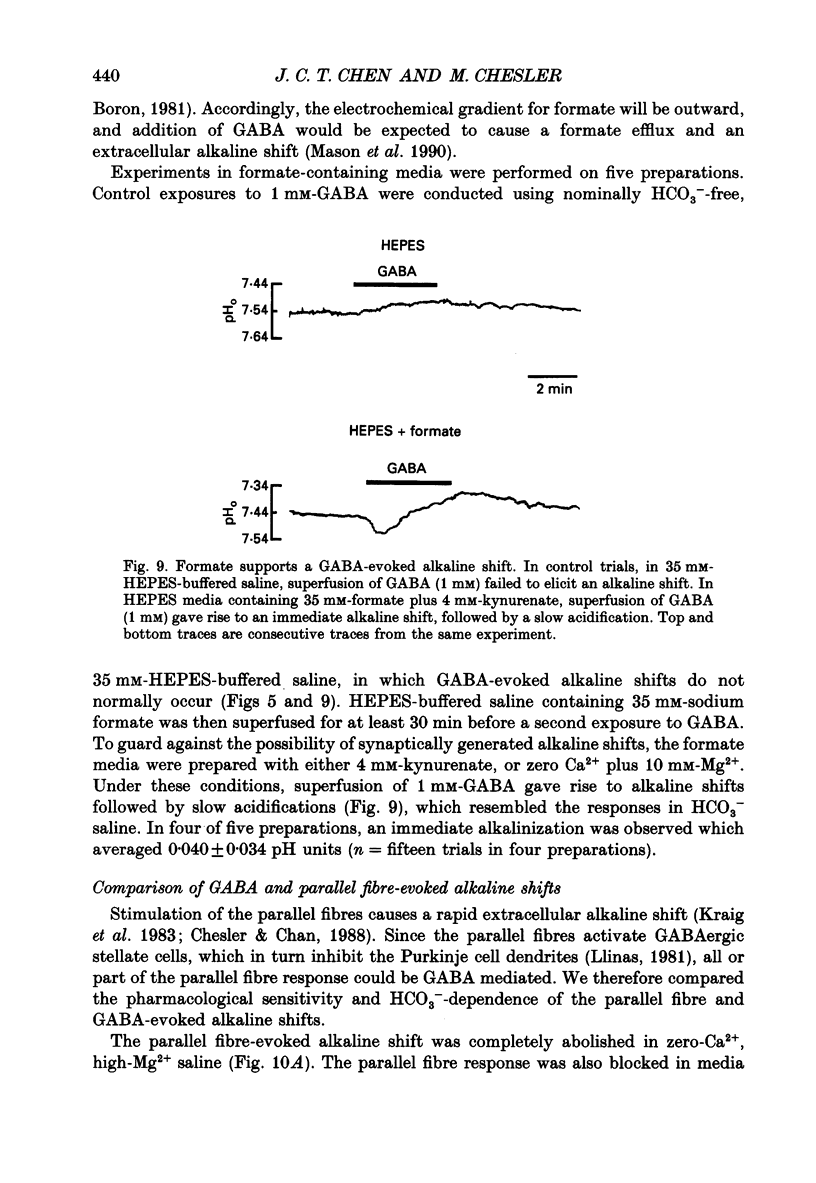
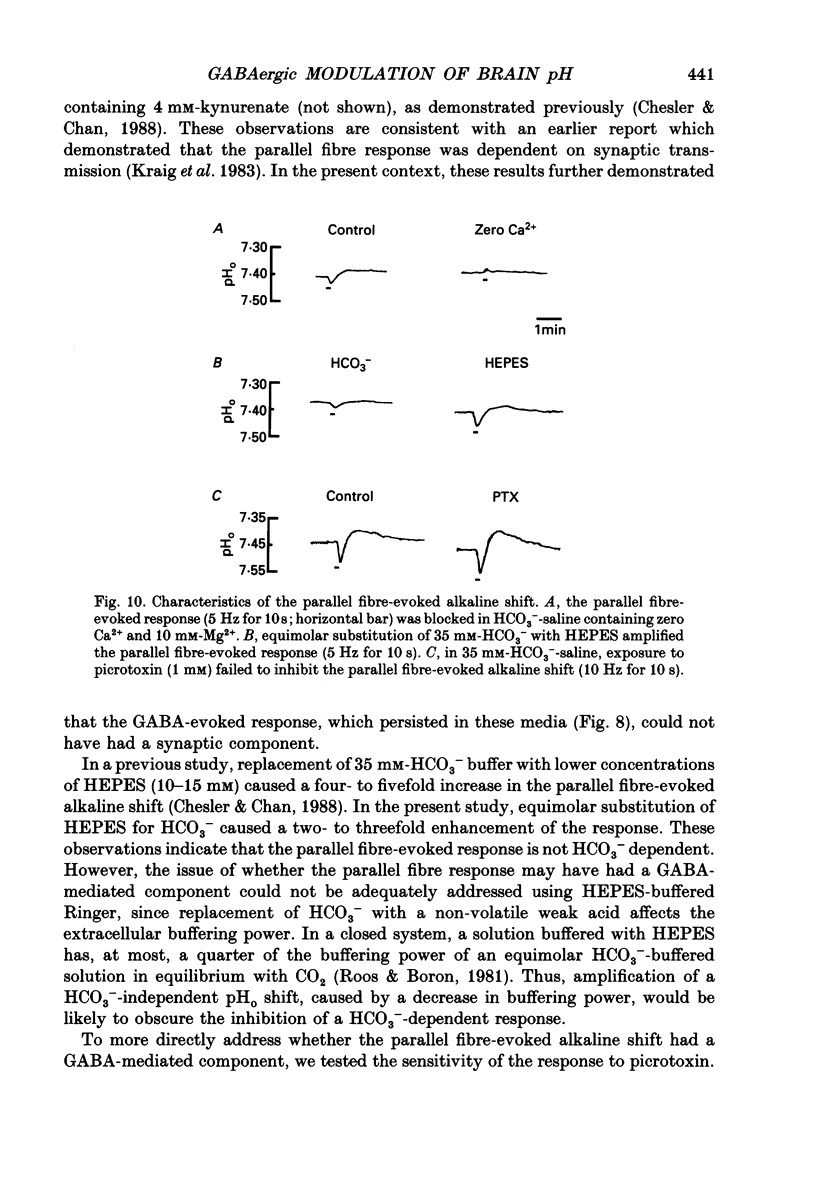
1. The effect of gamma-aminobutyric acid (GABA) on extracellular pH (pHo) was investigated in the turtle cerebellum, in vitro, using double-barrelled, H(+)-selective microelectrodes. Responses evoked by GABA were compared with pHo shifts evoked by repetitive stimulation of the parallel fibres. 2. In media buffered with 35 mM-HCO3- and 5% CO2, superfusion of GABA (1 mM) elicited an abrupt alkaline shift in the molecular layer, which averaged 0.05 +/- 0.02 pH units (+/- S.D., range 0.02-0.12 pH units). pHo often recovered in the continued presence of GABA, and displayed a rebound acidification upon wash-out. 3. The GABA-evoked alkaline shift was blocked by picrotoxin and was mimicked by the GABAA agonists isoguvacine and muscimol. The GABAB agonist baclofen did not elicit an alkaline shift. Alkaline shifts evoked by stimulation of the parallel fibres were unaffected by picrotoxin. 4. In nominally HCO3(-)-free solutions, buffered with 35 mM-HEPES, superfusion of GABA caused either no pHo change or a slow acid shift. In contrast, the alkaline shift evoked by stimulation of the parallel fibres became enhanced in HEPES-buffered media. 5. The alkaline shift evoked by GABA was accompanied by an increase in extracellular K+ ([K+]o) which averaged 1.7 mM above baseline. Experimental elevation of [K+]o to a comparable level always caused a pure acid shift in the extracellular space. 6. The GABA-evoked alkaline shift persisted when synaptic transmission was blocked using 4 mM-kynurenic acid or saline prepared with nominally zero Ca2+ and 10 mM-Mg2+. The alkaline shift evoked by repetitive stimulation of the parallel fibres was completely abolished in these media. 7. Although the GABA-evoked alkaline shift was blocked in nominally HCO3(-)-free media, substitution of 35 mM-formate for HCO3- restored the GABA response. Superfusion of 1 mM-GABA in formate saline produced an alkaline shift of 0.040 +/- 0.034 pH units. 8. These results indicate that gating of GABAA channels in the vertebrate CNS gives rise to an HCO3- efflux which can significantly increase the pH of the brain microenvironment. However, this mechanism cannot account for the extracellular alkalinization caused by parallel fibre stimulation. Extracellular alkaline shifts capable of modulating local synaptic operations may therefore be a consequence of either excitatory or inhibitory synaptic transmission.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aram J. A., Lodge D. Epileptiform activity induced by alkalosis in rat neocortical slices: block by antagonists of N-methyl-D-aspartate. Neurosci Lett. 1987 Dec 29;83(3):345–350. doi: 10.1016/0304-3940(87)90112-1. [DOI] [PubMed] [Google Scholar]
- Balestrino M., Somjen G. G. Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. J Physiol. 1988 Feb;396:247–266. doi: 10.1113/jphysiol.1988.sp016961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Clapham D. E. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Collins G. G., Galvan M. Influence of cellular transport on the interaction of amino acids with gamma-aminobutyric acid (GABA)-receptors in the isolated olfactory cortex of the guinea-pig. Br J Pharmacol. 1980 Feb;68(2):251–262. doi: 10.1111/j.1476-5381.1980.tb10414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. C., Chesler M. A bicarbonate-dependent increase in extracellular pH mediated by GABAA receptors in turtle cerebellum. Neurosci Lett. 1990 Aug 14;116(1-2):130–135. doi: 10.1016/0304-3940(90)90398-s. [DOI] [PubMed] [Google Scholar]
- Chesler M., Chan C. Y. Stimulus-induced extracellular pH transients in the in vitro turtle cerebellum. Neuroscience. 1988 Dec;27(3):941–948. doi: 10.1016/0306-4522(88)90197-2. [DOI] [PubMed] [Google Scholar]
- Chesler M., Rice M. E. Extracellular alkaline-acid pH shifts evoked by iontophoresis of glutamate and aspartate in turtle cerebellum. Neuroscience. 1991;41(1):257–267. doi: 10.1016/0306-4522(91)90214-9. [DOI] [PubMed] [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34(5):401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Deschenes M., Feltz P. GABA-induced rise of extracellular potassium in rat dorsal root ganglia: an electrophysiological study in vivo. Brain Res. 1976 Dec 24;118(3):494–499. doi: 10.1016/0006-8993(76)90319-x. [DOI] [PubMed] [Google Scholar]
- Endres W., Ballanyi K., Serve G., Grafe P. Excitatory amino acids and intracellular pH in motoneurons of the isolated frog spinal cord. Neurosci Lett. 1986 Dec 3;72(1):54–58. doi: 10.1016/0304-3940(86)90617-8. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Nakamura J., Shinnick-Gallagher P. The effects of temperature, pH and Cl-pump inhibitors on GABA responses recorded from cat dorsal root ganglia. Brain Res. 1983 May 16;267(2):249–259. doi: 10.1016/0006-8993(83)90877-6. [DOI] [PubMed] [Google Scholar]
- Hackett J. T. GABA selectively blocks parallel fiber-Purkinje cell synaptic transmission in the frog cerebellum in vitro. Brain Res. 1974 Nov 22;80(3):527–531. doi: 10.1016/0006-8993(74)91038-5. [DOI] [PubMed] [Google Scholar]
- Heinemann U., Lux H. D. Undershoots following stimulus-induced rises of extracellular potassium concentration in cerebral cortex of cat. Brain Res. 1975 Jul 25;93(1):63–76. doi: 10.1016/0006-8993(75)90286-3. [DOI] [PubMed] [Google Scholar]
- Inomata N., Oomura Y., Akaike N., Edwards C. The anion selectivity of the gamma-aminobutyric acid controlled chloride channel in the perfused spinal ganglion cell of frog. Neurosci Res. 1986 Jul;3(5):371–383. doi: 10.1016/0168-0102(86)90029-5. [DOI] [PubMed] [Google Scholar]
- Jarolimek W., Misgeld U., Lux H. D. Activity dependent alkaline and acid transients in guinea pig hippocampal slices. Brain Res. 1989 Dec 29;505(2):225–232. doi: 10.1016/0006-8993(89)91447-9. [DOI] [PubMed] [Google Scholar]
- Kaila K., Pasternack M., Saarikoski J., Voipio J. Influence of GABA-gated bicarbonate conductance on potential, current and intracellular chloride in crayfish muscle fibres. J Physiol. 1989 Sep;416:161–181. doi: 10.1113/jphysiol.1989.sp017755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K., Saarikoski J., Voipio J. Mechanism of action of GABA on intracellular pH and on surface pH in crayfish muscle fibres. J Physiol. 1990 Aug;427:241–260. doi: 10.1113/jphysiol.1990.sp018170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K., Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987 Nov 12;330(6144):163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Lux H. D., Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987 May;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig R. P., Ferreira-Filho C. R., Nicholson C. Alkaline and acid transients in cerebellar microenvironment. J Neurophysiol. 1983 Mar;49(3):831–850. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Johnston G. A., Lodge D., Curtis D. R. A new class of GABA agonist. Nature. 1977 Jul 7;268(5615):53–55. doi: 10.1038/268053a0. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Fukuda H. Alteration of extracellular K+-activity induced by amino acids in the frog spinal cord. Jpn J Pharmacol. 1976 Jun;26(3):385–387. doi: 10.1254/jjp.26.385. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Johnson J. W., Ascher P., Gähwiler B. H. Patch-clamp recording of amino acid-activated responses in "organotypic" slice cultures. Proc Natl Acad Sci U S A. 1988 May;85(9):3221–3225. doi: 10.1073/pnas.85.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C., Kocsis J. D. Effects of GABA on stimulus-evoked changes in [K+]o and parallel fiber excitability. J Neurophysiol. 1982 Sep;48(3):608–621. doi: 10.1152/jn.1982.48.3.608. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Mattsson K., Pasternack M., Voipio J., Kaila K. Postsynaptic fall in intracellular pH and increase in surface pH caused by efflux of formate and acetate anions through GABA-gated channels in crayfish muscle fibres. Neuroscience. 1990;34(2):359–368. doi: 10.1016/0306-4522(90)90145-t. [DOI] [PubMed] [Google Scholar]
- Numann R. E., Wong R. K. Voltage-clamp study on GABA response desensitization in single pyramidal cells dissociated from the hippocampus of adult guinea pigs. Neurosci Lett. 1984 Jun 29;47(3):289–294. doi: 10.1016/0304-3940(84)90528-7. [DOI] [PubMed] [Google Scholar]
- Rice M. E., Nicholson C. Glutamate- and aspartate-induced extracellular potassium and calcium shifts and their relation to those of kainate, quisqualate and N-methyl-D-aspartate in the isolated turtle cerebellum. Neuroscience. 1990;38(2):295–310. doi: 10.1016/0306-4522(90)90029-4. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Acidification of interstitial fluid in hippocampal formation caused by seizures and by spreading depression. Brain Res. 1984 Oct 8;311(1):186–188. doi: 10.1016/0006-8993(84)91416-1. [DOI] [PubMed] [Google Scholar]
- Strauss P. Comparison of the effect of gamma-aminobutyric acid and its derivative baclofen on the activity of Purkinje cells of frog cerebellum in vitro. Physiol Bohemoslov. 1986;35(4):289–298. [PubMed] [Google Scholar]
- Syková E. GABA-induced changes of extracellular K+-activity in the frog spinal cord. Physiol Bohemoslov. 1979;28(2):189–192. [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis S. F., Cull-Candy S. G. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990 May 24;345(6273):347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]


