Abstract
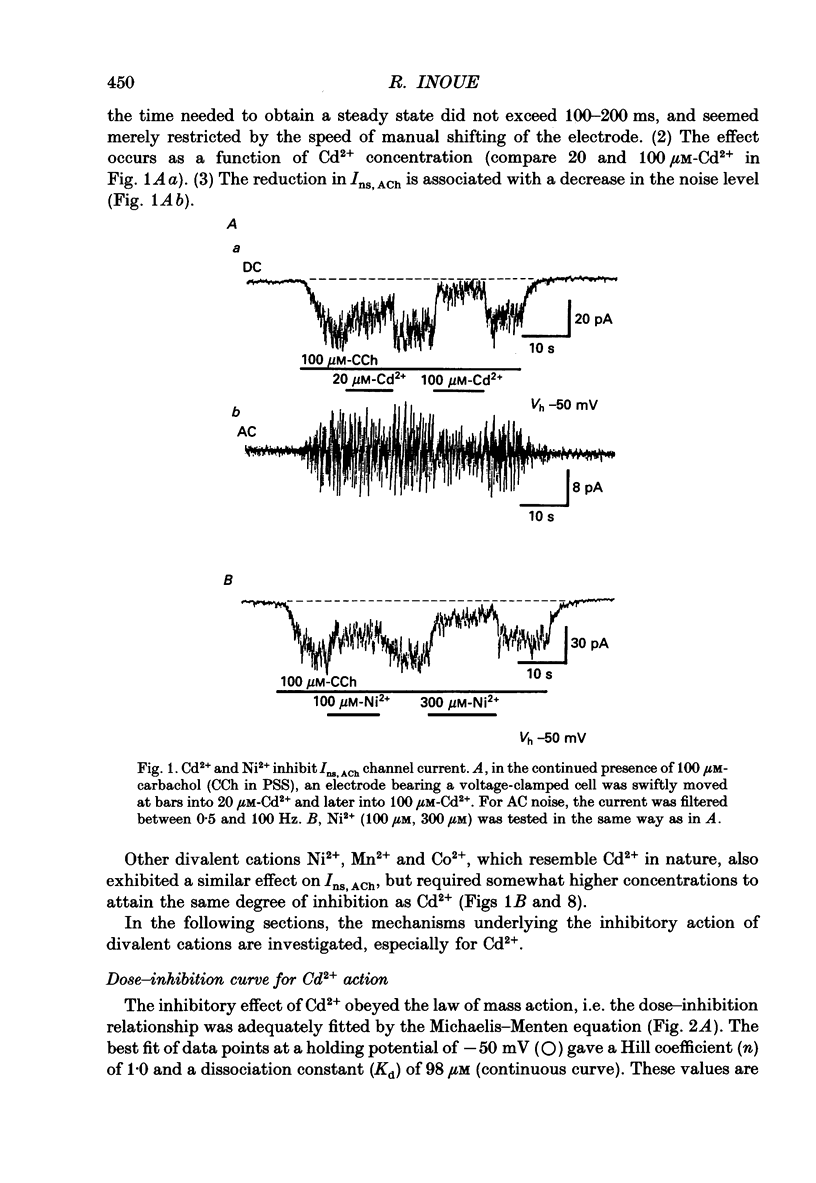
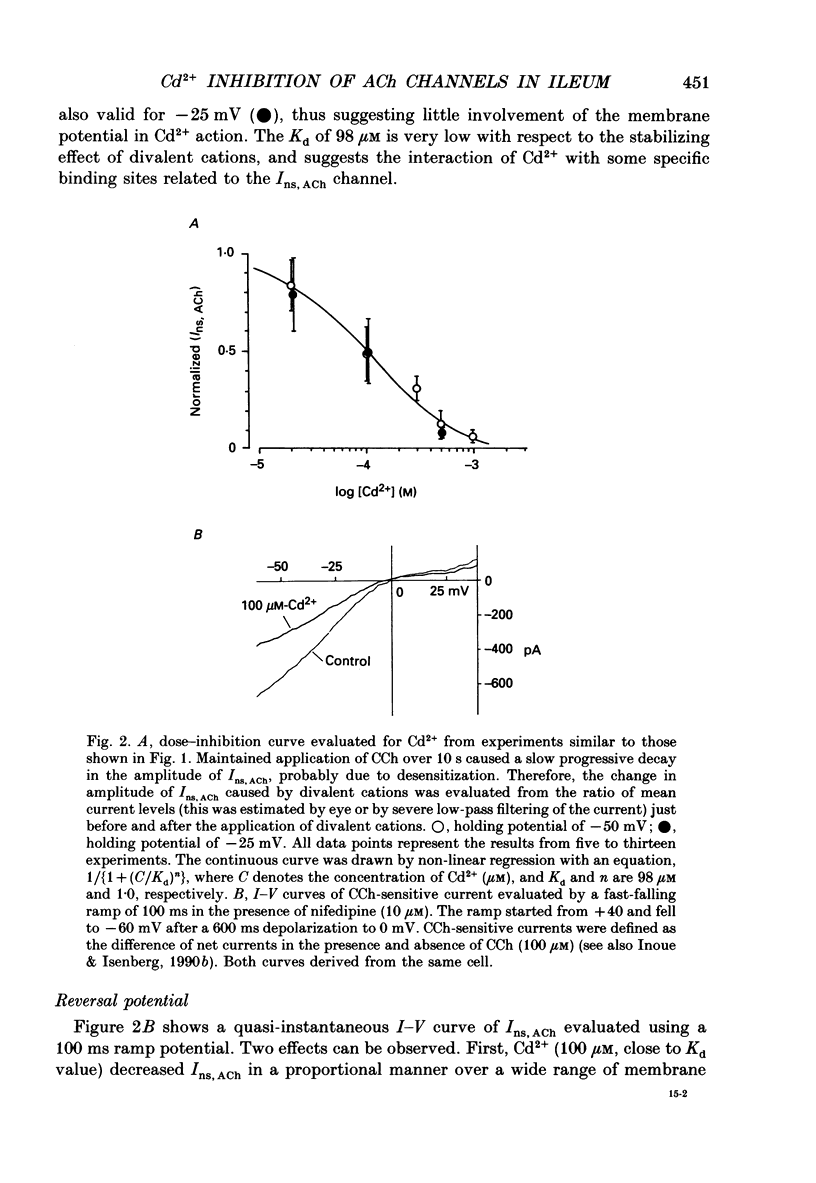
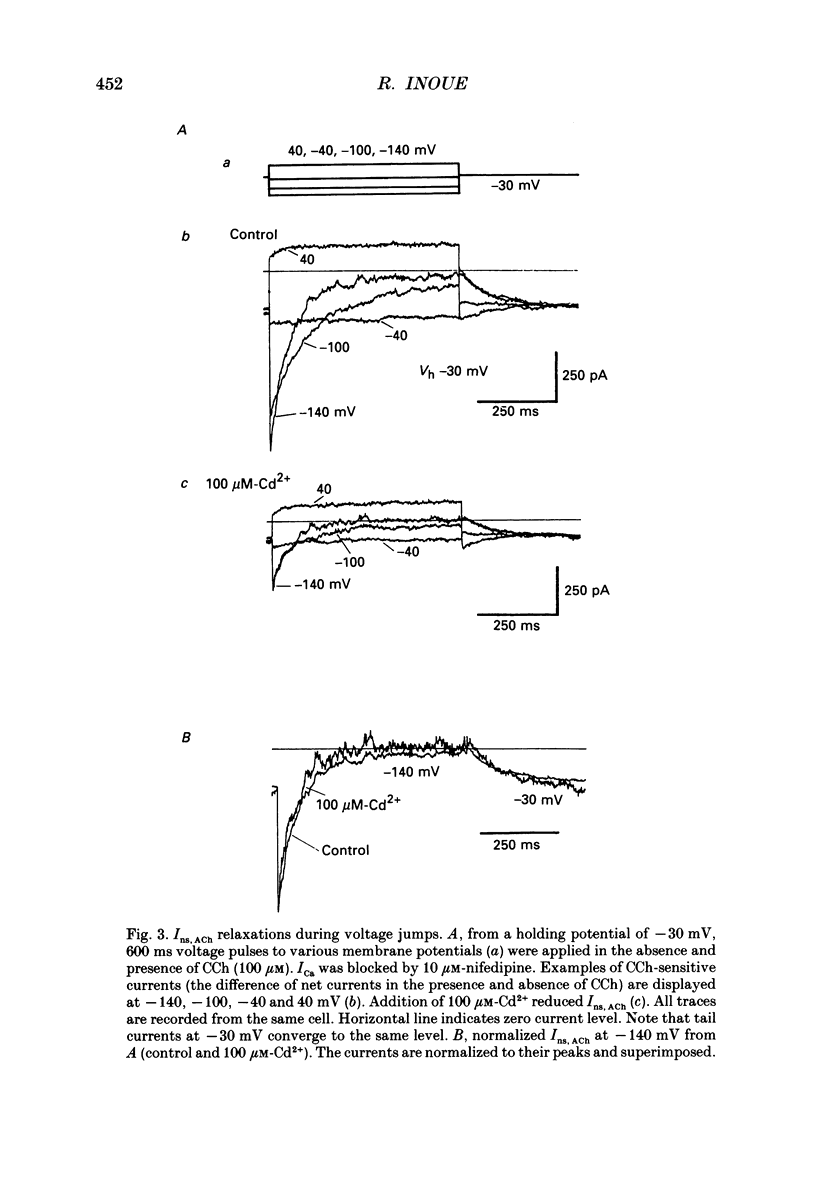
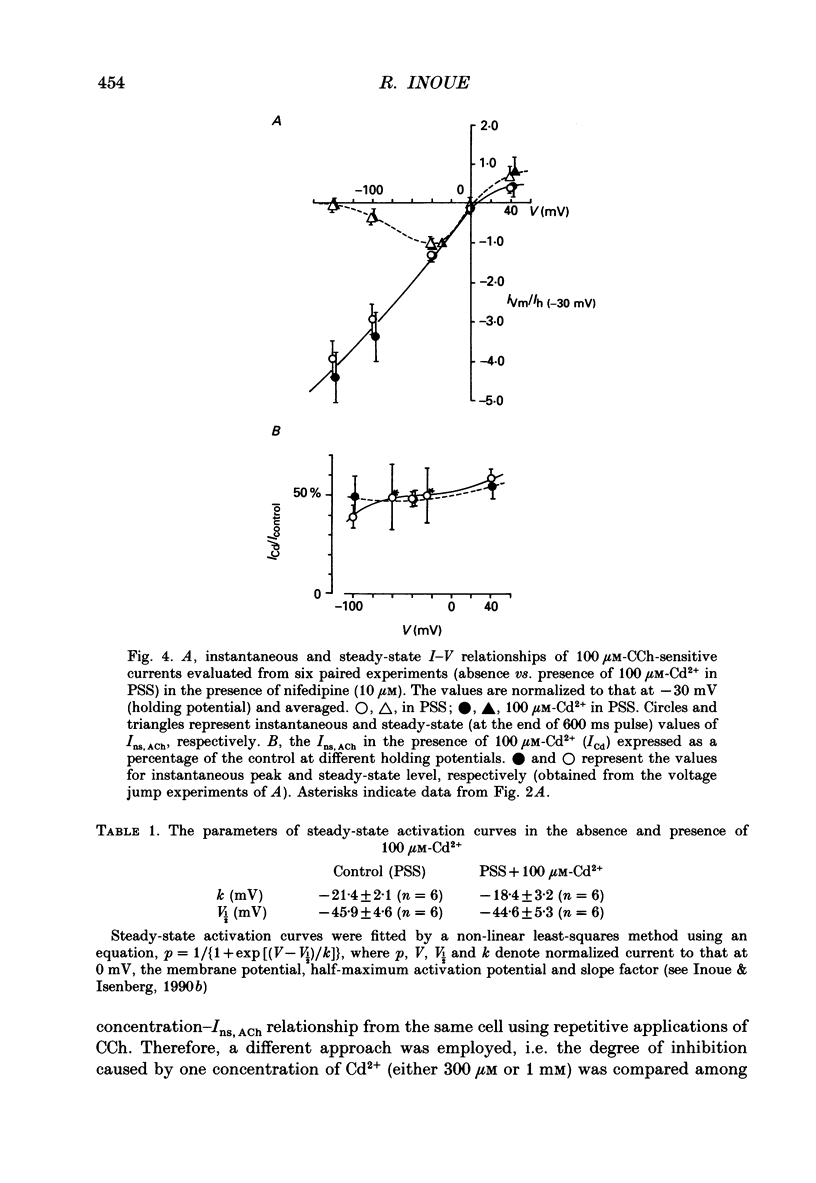
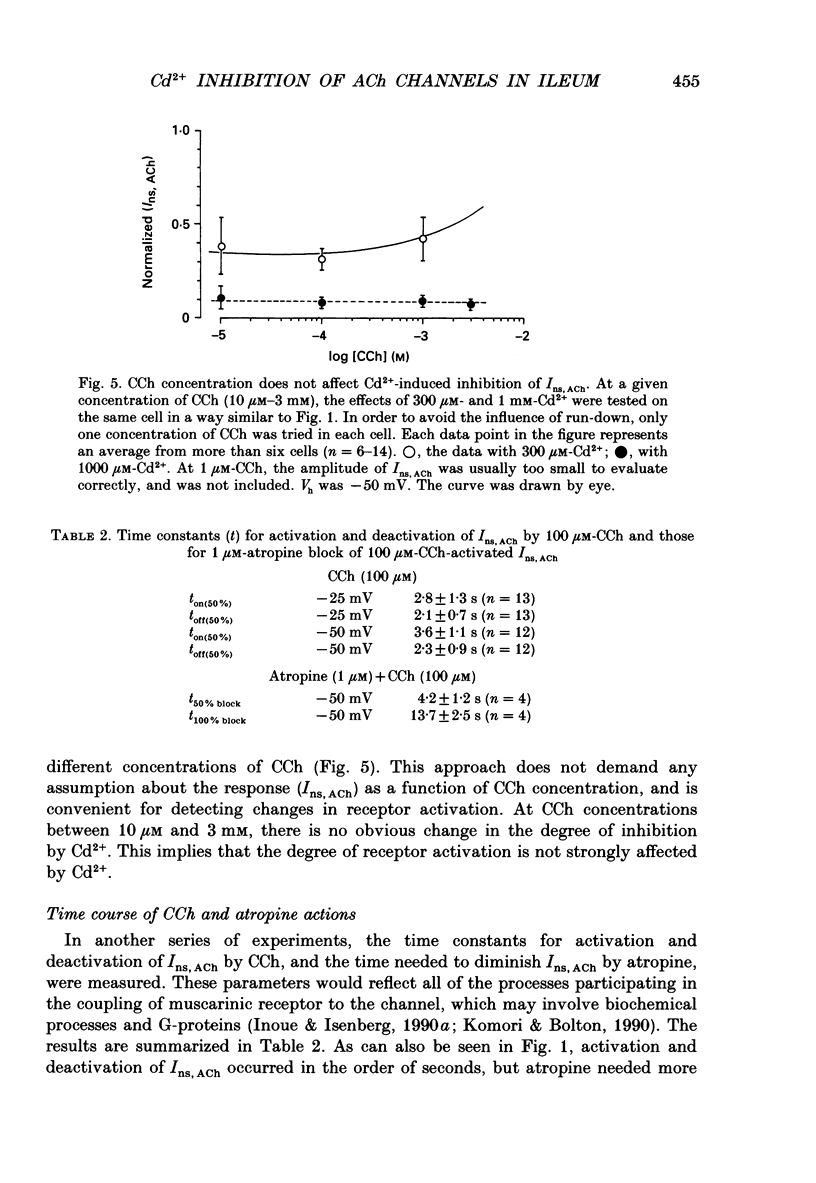
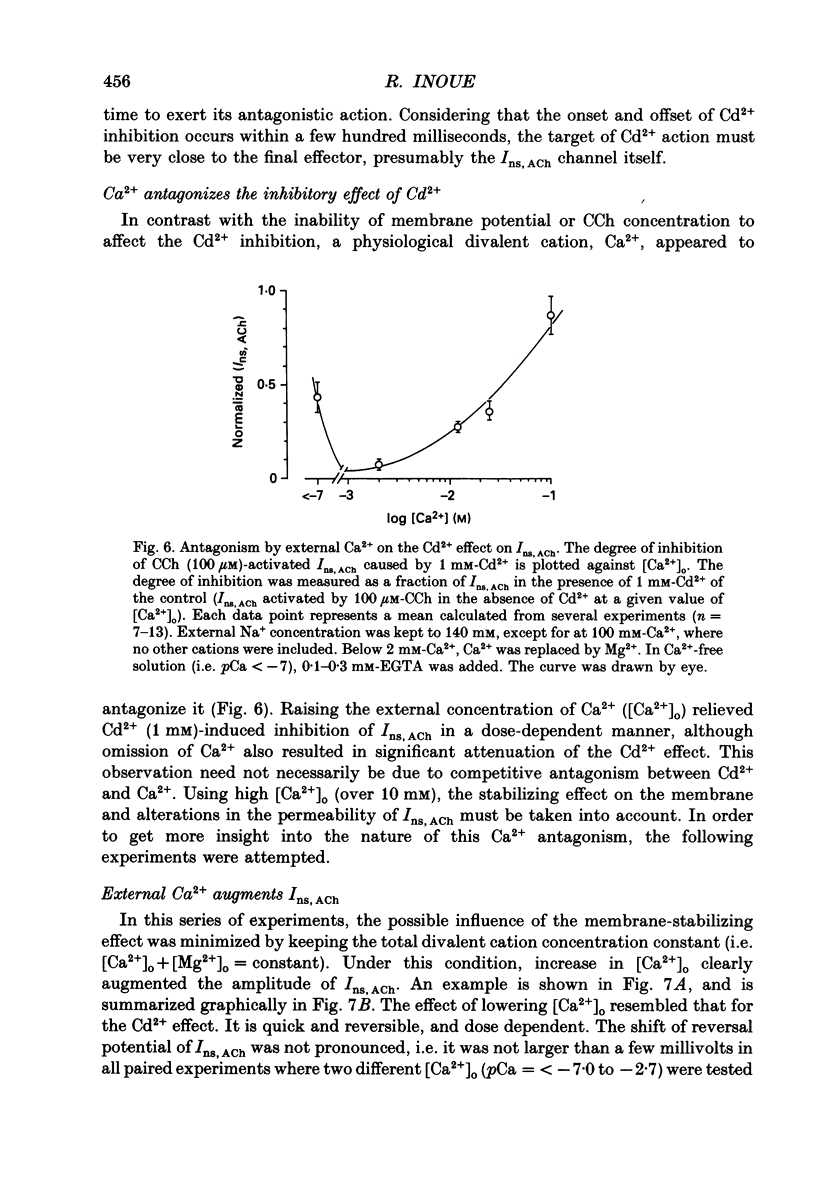
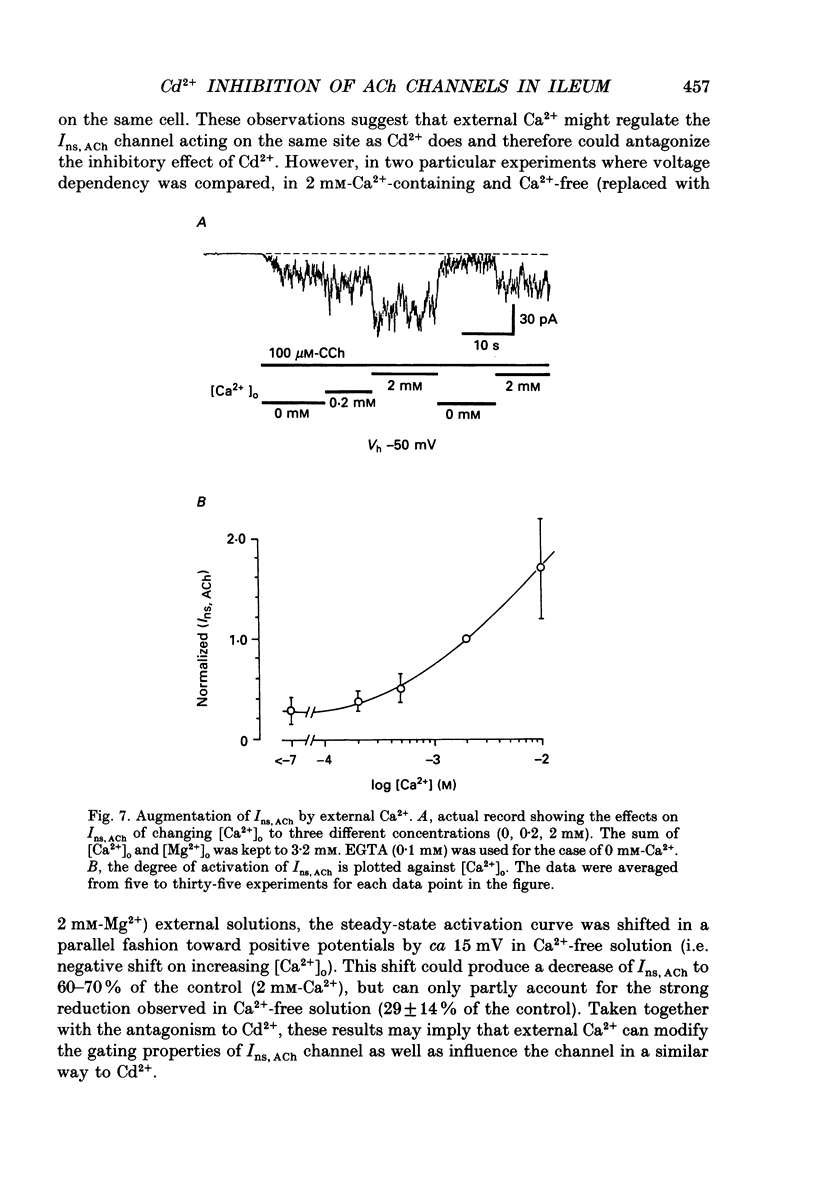
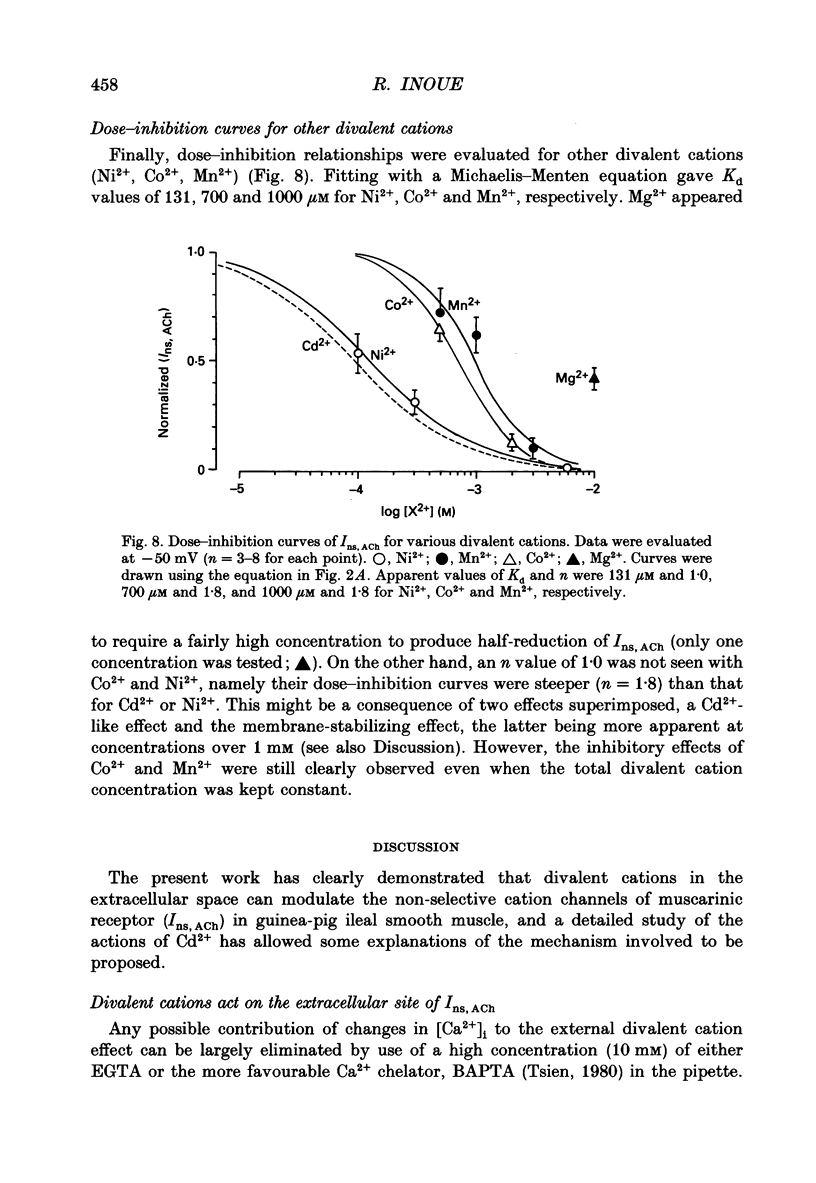
1. Inhibitory actions of the external divalent cations, Cd2+, Ni2+, Mn2+ and Co2+ on carbachol (CCh)-induced inward current (Ins,ACh) were investigated in caesium aspartate-loaded single longitudinal smooth muscle cells of guinea-pig ileum, using a rapid solution-switching device, under voltage-clamped conditions. 2. Cd2+ (20-1000 microM), when added to the external solution, reduced Ins,ACh rapidly and reversibly. This effect occurred dose dependently, the relationship being adequately described by a Michaelis-Menten equation with a Hill coefficient (n) of 1.0 and a dissociation constant (Kd) of 98 microM. 3. The inhibitory action of Cd2+ was associated neither with agonist concentration (CCh) nor with changes in reversal potential, and was voltage independent. 4. The appearance and removal of the effect of Cd2+ were both rapid (a few hundred milliseconds), in sharp contrast with a relatively slow time course of atropine or CCh action (of the order of seconds). 5. Other divalent cations (Ni2+, Mn2+, Co2+, Mg2+) applied externally also suppressed Ins,ACh, but less potently. The sequence of apparent potency was Cd2+ (98 microM) greater than or equal to Ni2+ (131 microM) much greater than Co2+ (700 microM) greater than or equal to Mn2+ (1000 microM) much greater than Mg2+ (approximately 10 mM). 6. External Ca2+ increased Ins,ACh dose dependently and antagonized the inhibitory effect of Cd2+. However, this effect may not be a simple competition with Cd2+. 7. These results show that external divalent cations strongly modulate Ins,ACh channels, possibly through direct interaction with the channel protein.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Kinetics of agonist conductance changes during hyperolarization at frog endplates. Br J Pharmacol. 1975 Feb;53(2):308–310. doi: 10.1111/j.1476-5381.1975.tb07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F., Walmsley D. An investigation of sodium-calcium exchange in the smooth muscle of guinea-pig ureter. J Physiol. 1987 Oct;391:325–346. doi: 10.1113/jphysiol.1987.sp016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amédée T., Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Potassium, chloride and non-selective cation conductances opened by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Apr;423:551–568. doi: 10.1113/jphysiol.1990.sp018039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Sneddon P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol. 1980 Oct;70(2):229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. P., Gallis B., Blumenthal D. K., Pallen C. J., Wang J. H., Krebs E. G. Characterization of the phosphotyrosyl protein phosphatase activity of calmodulin-dependent protein phosphatase. J Biol Chem. 1986 Jul 25;261(21):9890–9895. [PubMed] [Google Scholar]
- Cohen I., Van der Kloot W. Effects of [Ca2+] and [Mg2+] on the decay of miniature endplate currents. Nature. 1978 Jan 5;271(5640):77–79. doi: 10.1038/271077a0. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Contribution of two types of calcium channels to membrane conductance of single myocytes from guinea-pig coronary artery. J Physiol. 1990 Jul;426:19–42. doi: 10.1113/jphysiol.1990.sp018125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Saturation of calcium channels in single isolated smooth muscle cells of guinea-pig taenia caeci. J Physiol. 1988 May;399:419–436. doi: 10.1113/jphysiol.1988.sp017089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt H., Franke C., Dudel J. Calcium dependent gating of the L-glutamate activated, excitatory synaptic channel on crayfish muscle. Pflugers Arch. 1988 Jan;411(1):17–26. doi: 10.1007/BF00581641. [DOI] [PubMed] [Google Scholar]
- Honoré E., Martin C., Mironneau C., Mironneau J. An ATP-sensitive conductance in cultured smooth muscle cells from pregnant rat myometrium. Am J Physiol. 1989 Aug;257(2 Pt 1):C297–C305. doi: 10.1152/ajpcell.1989.257.2.C297. [DOI] [PubMed] [Google Scholar]
- Inoue R., Brading A. F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990 Jul;100(3):619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990 May;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Intracellular calcium ions modulate acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990 May;424:73–92. doi: 10.1113/jphysiol.1990.sp018056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Acetylcholine activates single sodium channels in smooth muscle cells. Pflugers Arch. 1987 Sep;410(1-2):69–74. doi: 10.1007/BF00581898. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Blocking effects of cobalt and related ions on the gamma-aminobutyric acid-induced current in turtle retinal cones. J Physiol. 1986 Apr;373:463–479. doi: 10.1113/jphysiol.1986.sp016058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea pig). Pflugers Arch. 1985 Dec;405(4):340–348. doi: 10.1007/BF00595686. [DOI] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Role of G-proteins in muscarinic receptor inward and outward currents in rabbit jejunal smooth muscle. J Physiol. 1990 Aug;427:395–419. doi: 10.1113/jphysiol.1990.sp018178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J. B., Hess P., Tsien R. W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986 Sep;88(3):321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P., Westbrook G. L. The inhibition of single N-methyl-D-aspartate-activated channels by zinc ions on cultured rat neurones. J Physiol. 1990 Oct;429:429–449. doi: 10.1113/jphysiol.1990.sp018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Vyklický L., Jr Sites of antagonist action on N-methyl-D-aspartic acid receptors studied using fluctuation analysis and a rapid perfusion technique. J Neurophysiol. 1988 Aug;60(2):645–663. doi: 10.1152/jn.1988.60.2.645. [DOI] [PubMed] [Google Scholar]
- Mironneau C., Mironneau J., Savineau J. P. Maintained contractions of rat uterine smooth muscle incubated in a Ca2+-free solution. Br J Pharmacol. 1984 Jul;82(3):735–743. doi: 10.1111/j.1476-5381.1984.tb10813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasu T., Ishida Y. Effects of cadmium ions, D-600 and chlorpromazine on the skinned smooth muscle of guinea-pig taenia coli. Gen Pharmacol. 1986;17(5):531–536. doi: 10.1016/0306-3623(86)90088-1. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977 May;267(2):429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Terada K., Kitamura K., Kuriyama H. Membrane currents recorded from a fragment of rabbit intestinal smooth muscle cell. Am J Physiol. 1986 Sep;251(3 Pt 1):C335–C346. doi: 10.1152/ajpcell.1986.251.3.C335. [DOI] [PubMed] [Google Scholar]
- PATON W. D., RANG H. P. THE UPTAKE OF ATROPINE AND RELATED DRUGS BY INTESTINAL SMOOTH MUSCLE OF THE GUINEA-PIG IN RELATION TO ACETYLCHOLINE RECEPTORS. Proc R Soc Lond B Biol Sci. 1965 Aug 24;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]
- Sumida M., Hamada M., Takenaka H., Hirata Y., Nishigauchi K., Okuda H. Ca2+,Mg2+-ATPase of microsomal membranes from bovine aortic smooth muscle: effects of Sr2+ and Cd2+ on Ca2+ uptake and formation of the phosphorylated intermediate of the Ca2+,Mg2+-ATPase. J Biochem. 1986 Sep;100(3):765–772. doi: 10.1093/oxfordjournals.jbchem.a121769. [DOI] [PubMed] [Google Scholar]
- Tokushige A., Higashino H., Searle B. M., Tamura H., Kino M., Bogden J. D., Aviv A. Cadmium effect on the Na,K-ATPase system in cultured vascular smooth muscle cells. Hypertension. 1984 Jan-Feb;6(1):20–26. doi: 10.1161/01.hyp.6.1.20. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]


