Abstract
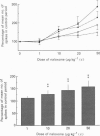
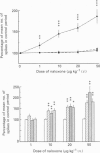
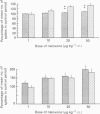
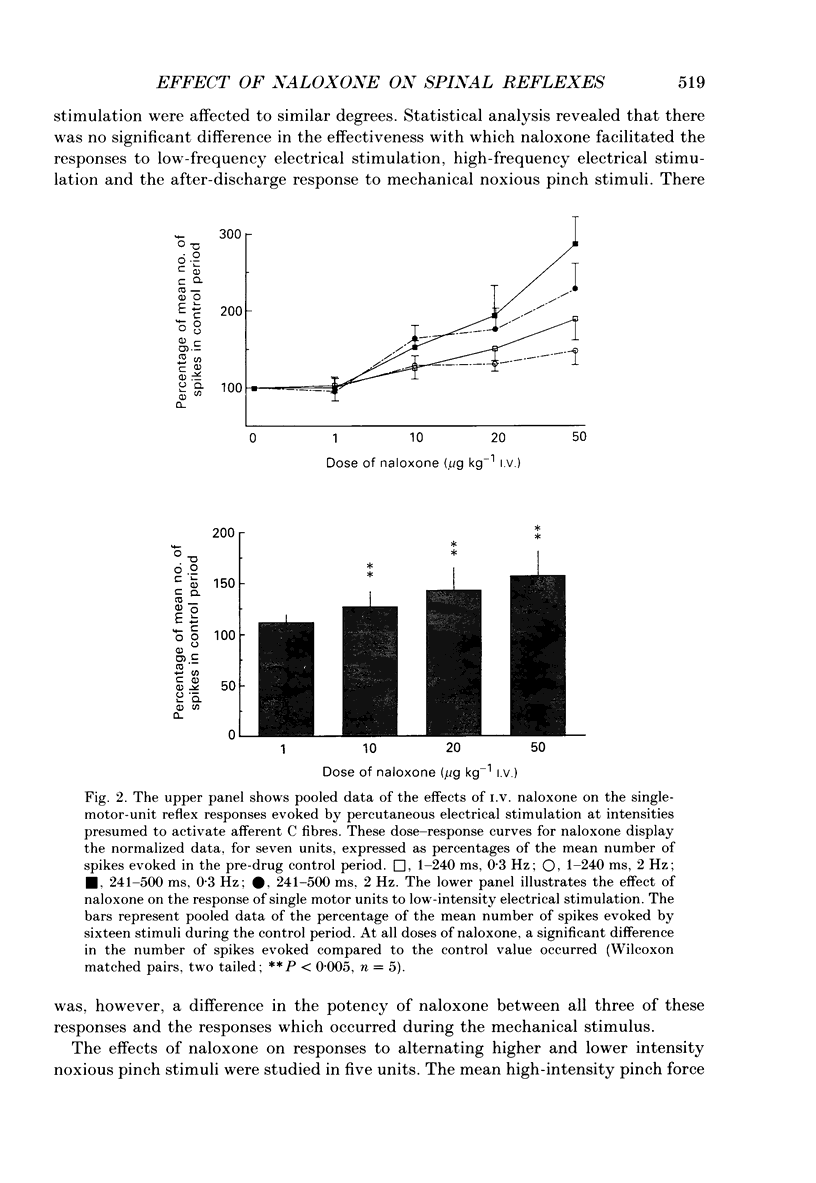
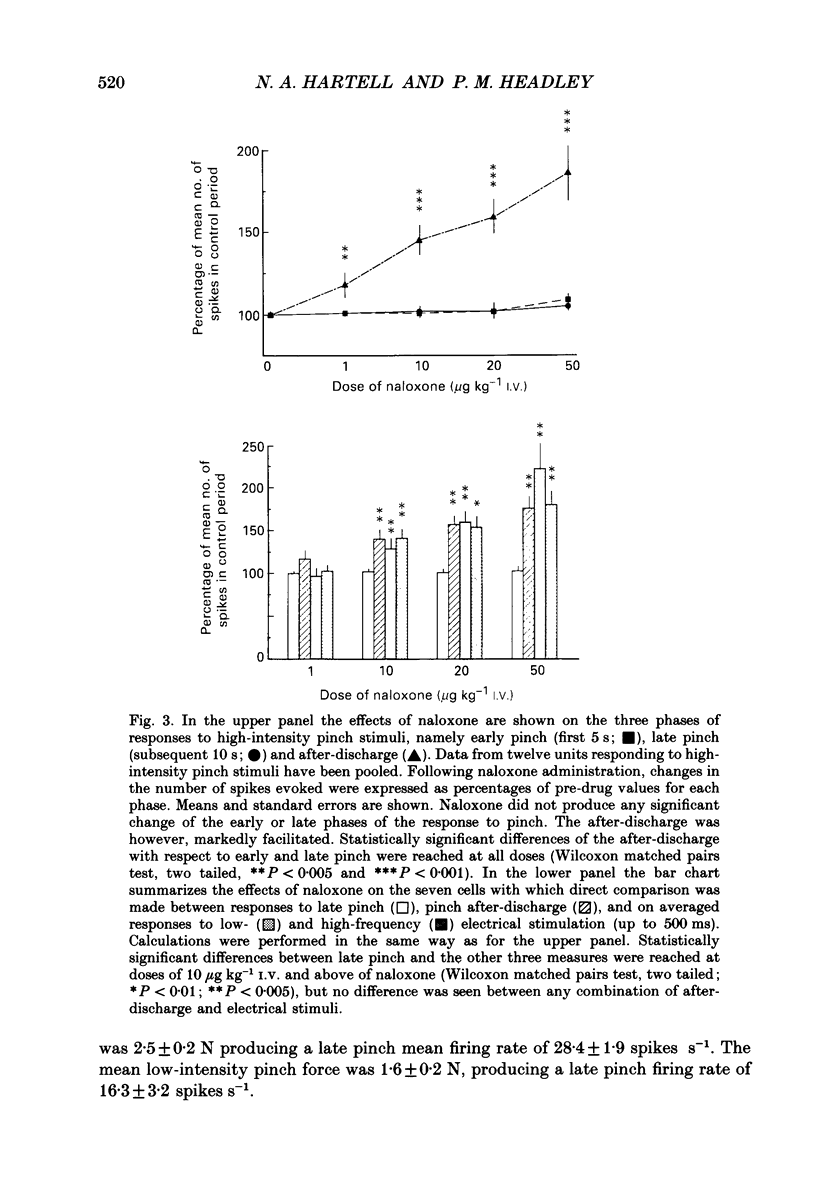
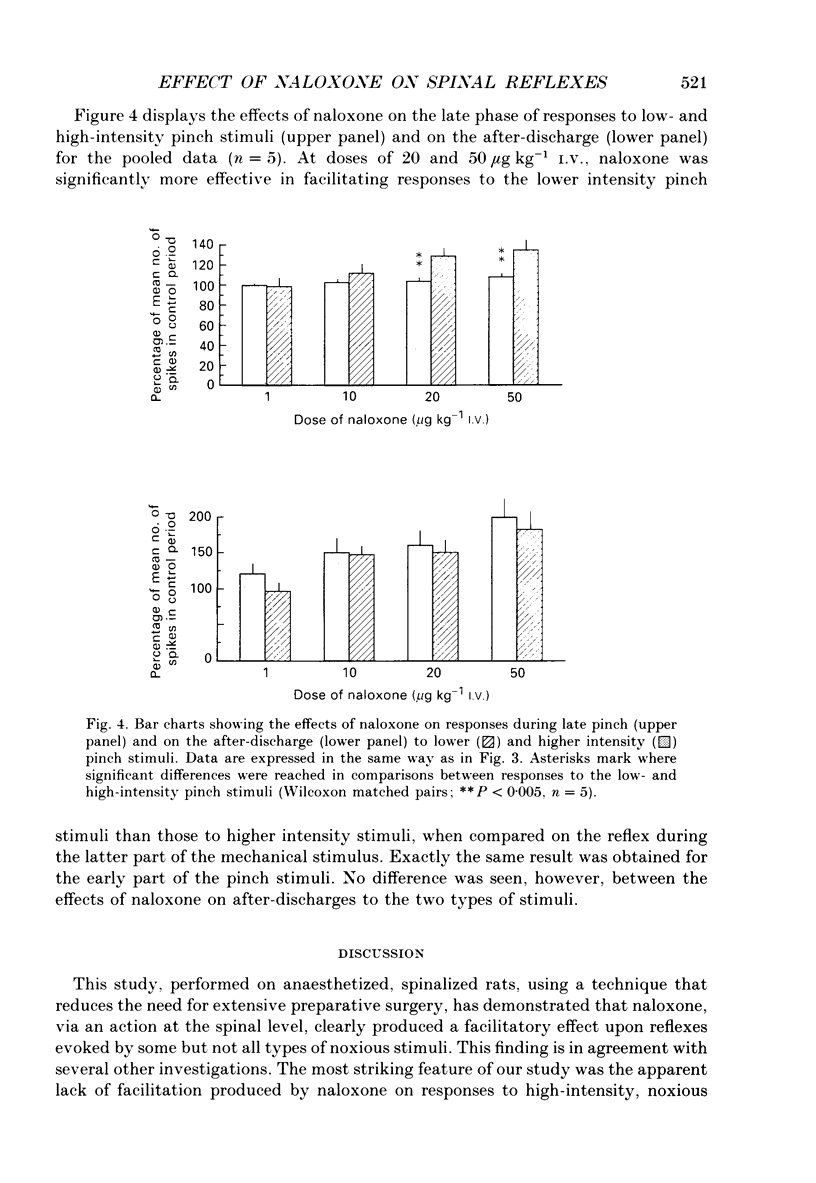
1. Previous studies of the effects of naloxone on spinal neural responses have yielded disparate results. The reasons for this remain unclear but may relate to the diversity of animal preparations used, the route of administration of naloxone, the site and modality of the stimuli and the intensity of afferent input used. 2. A model requiring little preparative surgery compared to most other electrophysiological preparations has now been used to investigate the effects of naloxone (1, 10, 20 and 50 micrograms kg-1 I.V.) on single-motor-unit flexion reflex responses to alternating mechanical and electrical stimuli in spinalized rats, anaesthetized with alpha-chloralose. 3. Naloxone caused a dose-dependent facilitation of reflex responses to electrical stimuli delivered at intensities sufficient to activate either A fibres alone or A and C fibre afferents together. The component of the responses presumed to be due primarily to activation of C fibres was enhanced relatively more than the A fibre component. 4. Responses evoked during high-intensity mechanical pinch stimuli were not facilitated by equivalent doses of naloxone. The post-stimulus after-discharge was, however, enhanced by a similar percentage to the response to high-intensity electrical stimuli. 5. Lowering the intensity of the mechanical stimulus led to a diminished firing rate of the units during the stimulus itself. The stimulus was, nevertheless, still noxious. Naloxone was found to have a facilitatory effect on this smaller evoked response both during the pinch stimulus and during the period of after-discharge. The apparent lack of effect of naloxone during the higher intensity mechanical stimulus may be due to neurones in the polysynaptic pathway being activated at near-maximal firing rates. 6. We conclude that the ability of naloxone to facilitate spinal reflexes is not dependent on the nature of the stimulus, at least between electrical and mechanical stimuli, but is more a function of the intensity of the applied stimulus.
Full text
PDF




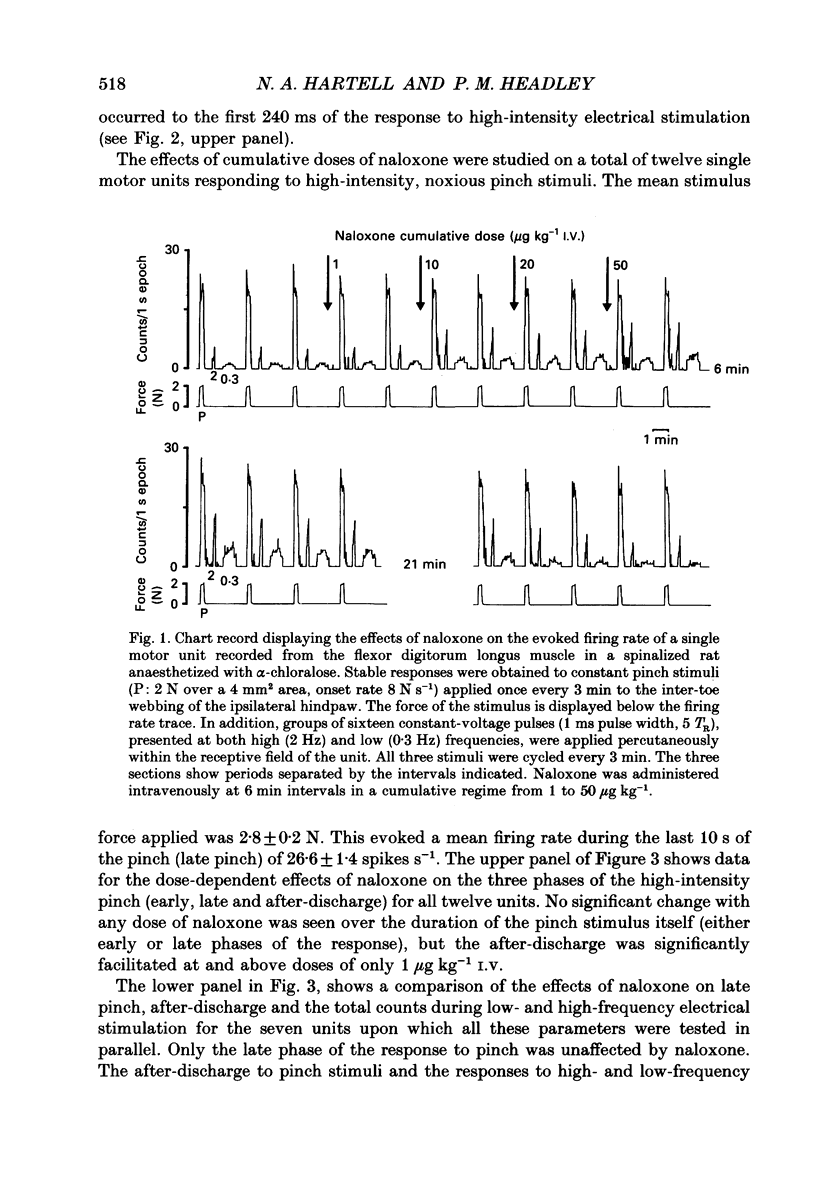





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basbaum A. I., Fields H. L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bell J. A., Martin W. R. The effect of the narcotic antagonists naloxone, naltrexone and nalorphine on spinal cord C-fiber reflexes evoked by electrical stimulation or radiant heat. Eur J Pharmacol. 1977 Mar 21;42(2):147–154. doi: 10.1016/0014-2999(77)90354-5. [DOI] [PubMed] [Google Scholar]
- Bell J. A., Sharpe L. G., Pickworth W. B. Electrophysiologically recorded C-fiber reflexes in intact and acute decerebrate-spinal cats: absence of naloxone facilitation in intact cats. Neuropharmacology. 1985 Jun;24(6):555–559. doi: 10.1016/0028-3908(85)90063-2. [DOI] [PubMed] [Google Scholar]
- Catley D. M., Clarke R. W., Pascoe J. E. Naloxone enhancement of spinal reflexes in the rabbit. J Physiol. 1983 Jun;339:61–73. doi: 10.1113/jphysiol.1983.sp014702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain S., Lewis D. M. Contractile characteristics and innervation ratio of rat soleus motor units. J Physiol. 1989 May;412:1–21. doi: 10.1113/jphysiol.1989.sp017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. W., Ford T. W. The contributions of mu-, delta- and kappa-opioid receptors to the actions of endogenous opioids on spinal reflexes in the rabbit. Br J Pharmacol. 1987 Jul;91(3):579–589. doi: 10.1111/j.1476-5381.1987.tb11251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A. W., Hall J. G., Headley P. M., Griersmith B. T. The effect of naloxone on the excitation of dorsal horn neurones of the cat by noxious and non-noxious cutaneous stimuli. Brain Res. 1977 Dec 9;138(1):185–189. doi: 10.1016/0006-8993(77)90796-x. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Morton C. R., Johnson S. M., Zhao Z. Q. Opioid antagonists and spinal reflexes in the anaesthetized cat. Brain Res. 1984 Apr 9;297(1):33–40. doi: 10.1016/0006-8993(84)90540-7. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., North R. A. Electrophysiology of opioids. Pharmacol Rev. 1983 Dec;35(4):219–281. [PubMed] [Google Scholar]
- Fitzgerald M., Woolf C. J. The stereospecific effect of naloxone on rat dorsal horn neurones; inhibition in superficial laminae and excitation in deeper laminae. Pain. 1980 Dec;9(3):293–306. doi: 10.1016/0304-3959(80)90044-5. [DOI] [PubMed] [Google Scholar]
- Goldstein A., Pryor G. T., Otis L. S., Larsen F. On the role of endogenous opioid peptides: failure of naloxone to influence shock escape threshold in the rat. Life Sci. 1976 Mar 15;18(6):599–604. doi: 10.1016/0024-3205(76)90339-8. [DOI] [PubMed] [Google Scholar]
- Hartell N. A., Headley P. M. Spinal effects of four injectable anaesthetics on nociceptive reflexes in rats: a comparison of electrophysiological and behavioural measurements. Br J Pharmacol. 1990 Nov;101(3):563–568. doi: 10.1111/j.1476-5381.1990.tb14121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. L. Naloxone excites nociceptive units in the lumbar dorsal horn of the spinal cat. Neuroscience. 1979;4(10):1485–1491. doi: 10.1016/0306-4522(79)90053-8. [DOI] [PubMed] [Google Scholar]
- Jacob J. J., Tremblay E. C., Colombel M. C. Facilitation de réactions nociceptives par la naloxone chez la souris et chez le rat. Psychopharmacologia. 1974 Jul 11;37(3):217–223. doi: 10.1007/BF00421535. [DOI] [PubMed] [Google Scholar]
- Kayser V., Benoist J. M., Neil A., Gautron M., Guilbaud G. Behavioural and electrophysiological studies on the paradoxical antinociceptive effects of an extremely low dose of naloxone in an animal model of acute and localized inflammation. Exp Brain Res. 1988;73(2):402–410. doi: 10.1007/BF00248233. [DOI] [PubMed] [Google Scholar]
- Kayser V., Besson J. M., Guilbaud G. Paradoxical effects of low doses of naloxone in experimental models of inflammatory pain. Prog Brain Res. 1988;77:301–312. doi: 10.1016/s0079-6123(08)62796-x. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Chitour D., Kraus E., Dickenson A. H., Besson J. M. Effect of naloxone upon diffuse noxious inhibitory controls (DNIC) in the rat. Brain Res. 1981 Jan 12;204(2):387–402. doi: 10.1016/0006-8993(81)90597-7. [DOI] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Millan M. J., Millan M. H., Członkowski A., Höllt V., Pilcher C. W., Herz A., Colpaert F. C. A model of chronic pain in the rat: response of multiple opioid systems to adjuvant-induced arthritis. J Neurosci. 1986 Apr;6(4):899–906. doi: 10.1523/JNEUROSCI.06-04-00899.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C. G., Headley P. M. Spinal antinociceptive actions of mu- and kappa-opioids: the importance of stimulus intensity in determining 'selectivity' between reflexes to different modalities of noxious stimulus. Br J Pharmacol. 1989 Oct;98(2):523–532. doi: 10.1111/j.1476-5381.1989.tb12626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C. G., West D. C., Headley P. M. Spinal antinociceptive actions and naloxone reversibility of intravenous mu- and kappa-opioids in spinalized rats: potency mismatch with values reported for spinal administration. Br J Pharmacol. 1989 Oct;98(2):533–543. doi: 10.1111/j.1476-5381.1989.tb12627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivot J. P., Chaouch A., Besson J. M. The influence of naloxone on the C fiber response of dorsal horn neurons and their inhibitory control by raphe magnus stimulation. Brain Res. 1979 Nov 2;176(2):355–364. doi: 10.1016/0006-8993(79)90989-2. [DOI] [PubMed] [Google Scholar]
- Standaert D. G., Watson S. J., Houghten R. A., Saper C. B. Opioid peptide immunoreactivity in spinal and trigeminal dorsal horn neurons projecting to the parabrachial nucleus in the rat. J Neurosci. 1986 May;6(5):1220–1226. doi: 10.1523/JNEUROSCI.06-05-01220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman F. S., Hirst M., Smith P. Brain and serum levels of naloxone following peripheral administration. Life Sci. 1983 Sep 12;33(11):1091–1096. doi: 10.1016/0024-3205(83)90665-3. [DOI] [PubMed] [Google Scholar]
- Willer J. C., Bussel B. Evidence for a direct spinal mechanism in morphine-induced inhibition of nociceptive reflexes in humans. Brain Res. 1980 Apr 7;187(1):212–215. doi: 10.1016/0006-8993(80)90507-7. [DOI] [PubMed] [Google Scholar]
- Woolf C. J. Analgesia and hyperalgesia produced in the rat by intrathecal naloxone. Brain Res. 1980 May 12;189(2):593–597. doi: 10.1016/0006-8993(80)90375-3. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Mitchell D., Barrett G. D. Antinociceptive effect of peripheral segmental electrical stimulation in the rat. Pain. 1980 Apr;8(2):237–252. doi: 10.1016/0304-3959(88)90011-5. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Wall P. D. Morphine-sensitive and morphine-insensitive actions of C-fibre input on the rat spinal cord. Neurosci Lett. 1986 Feb 28;64(2):221–225. doi: 10.1016/0304-3940(86)90104-7. [DOI] [PubMed] [Google Scholar]