Abstract
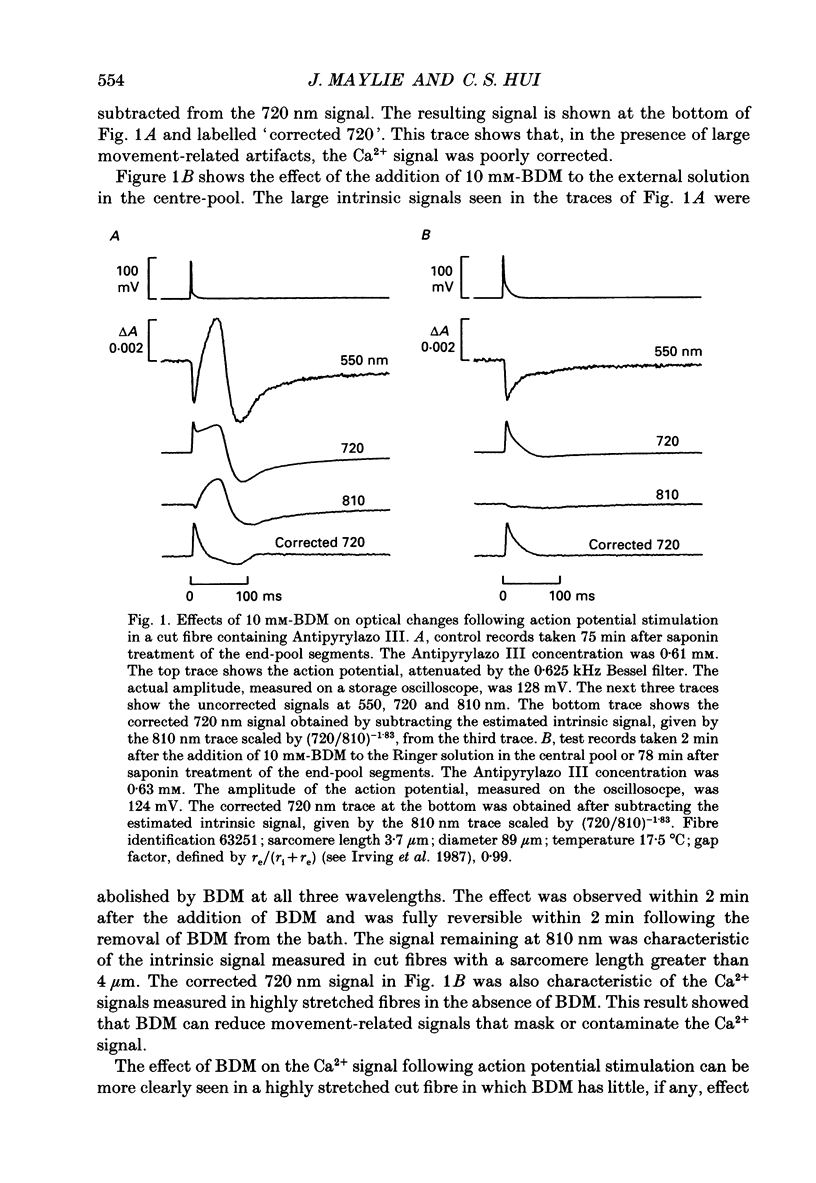
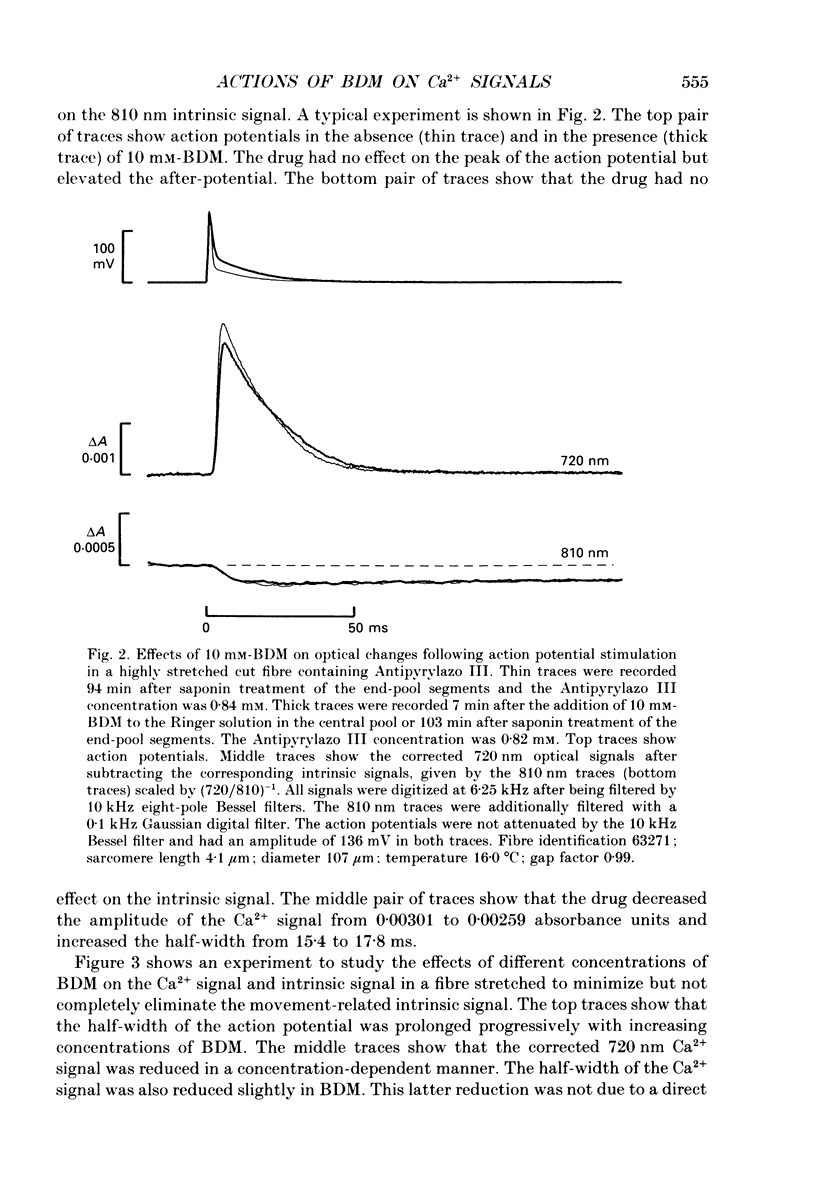
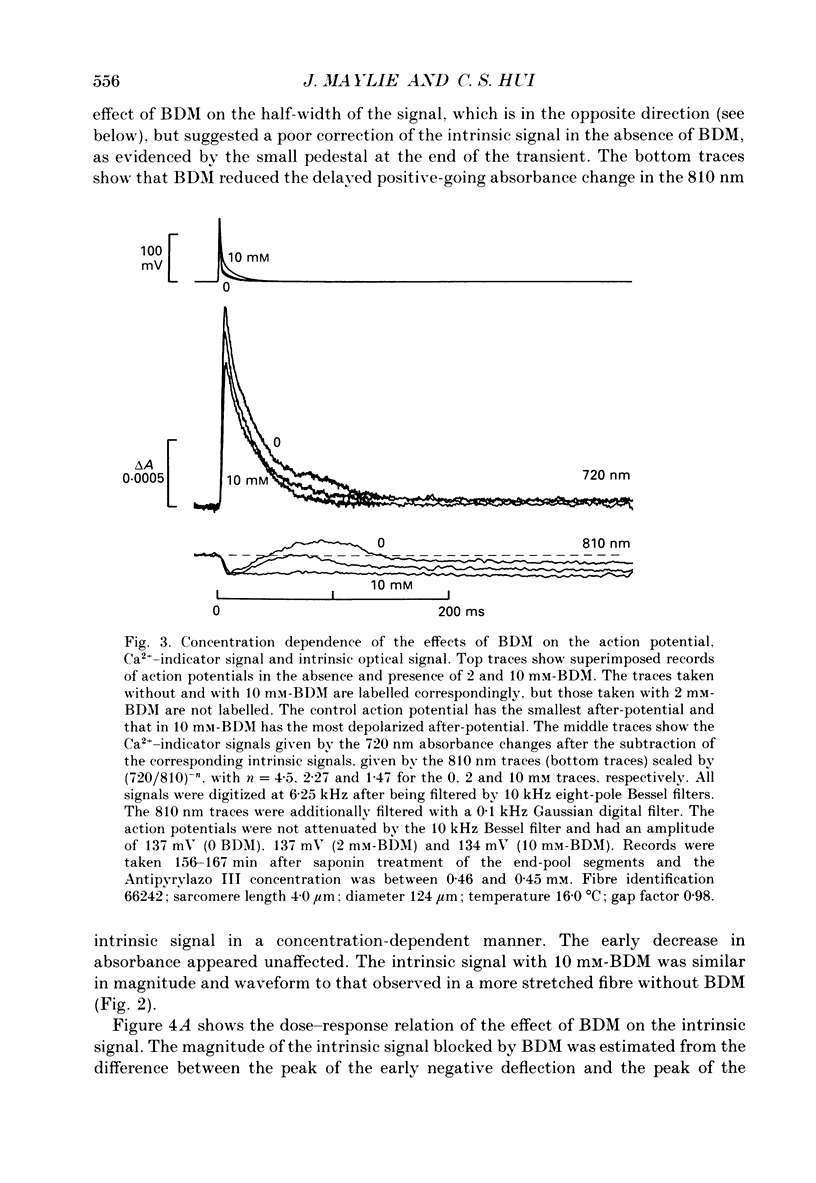
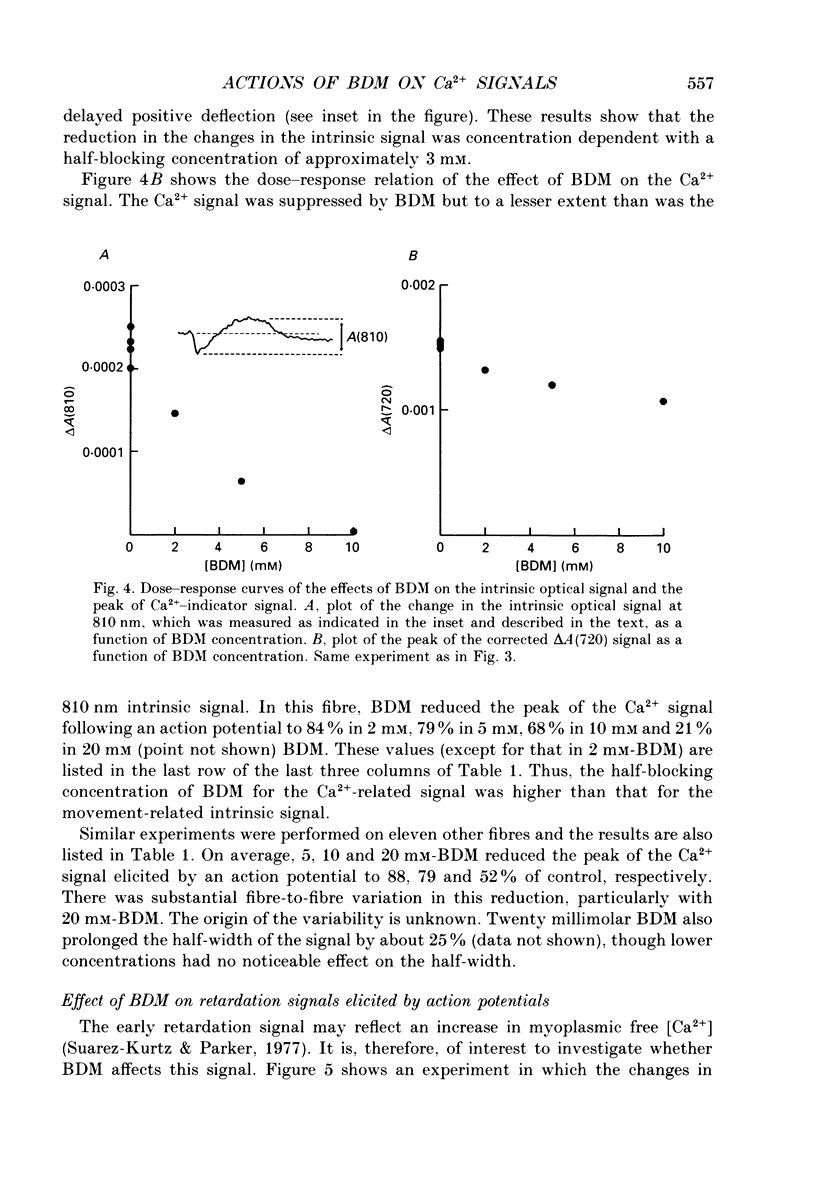
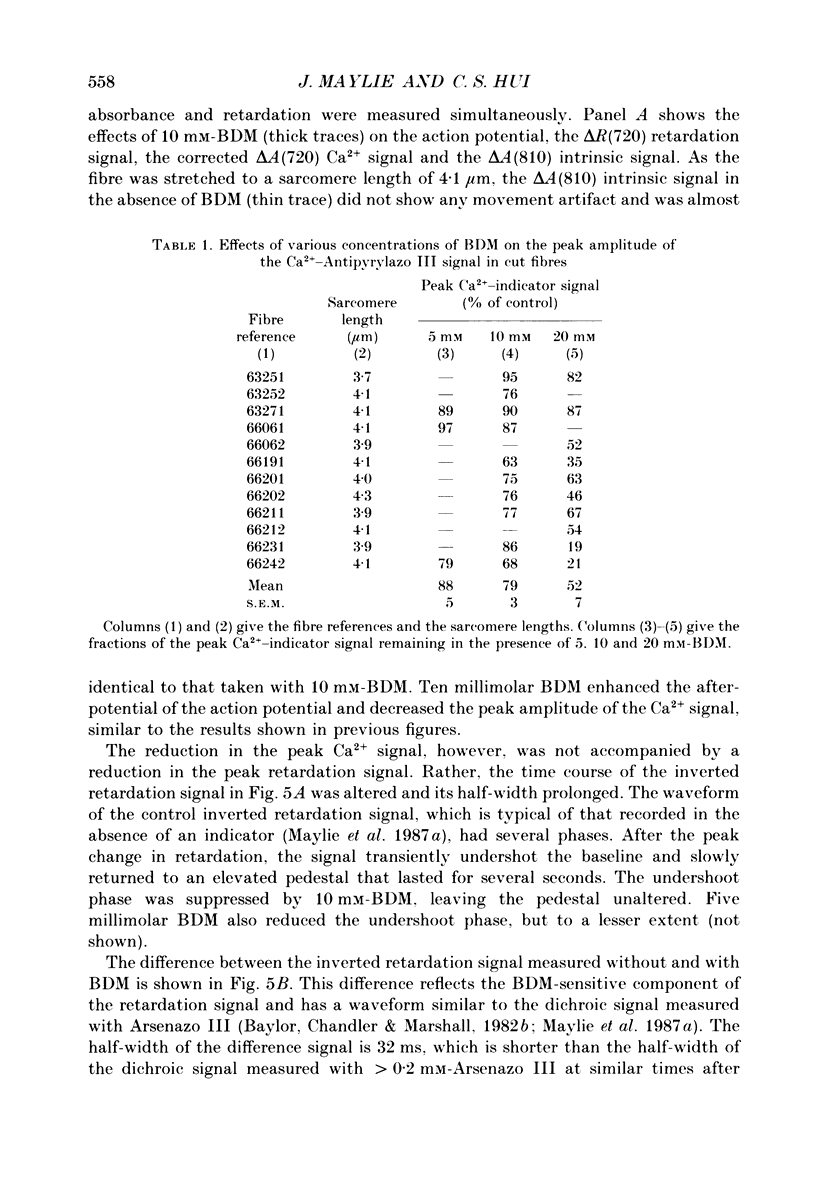
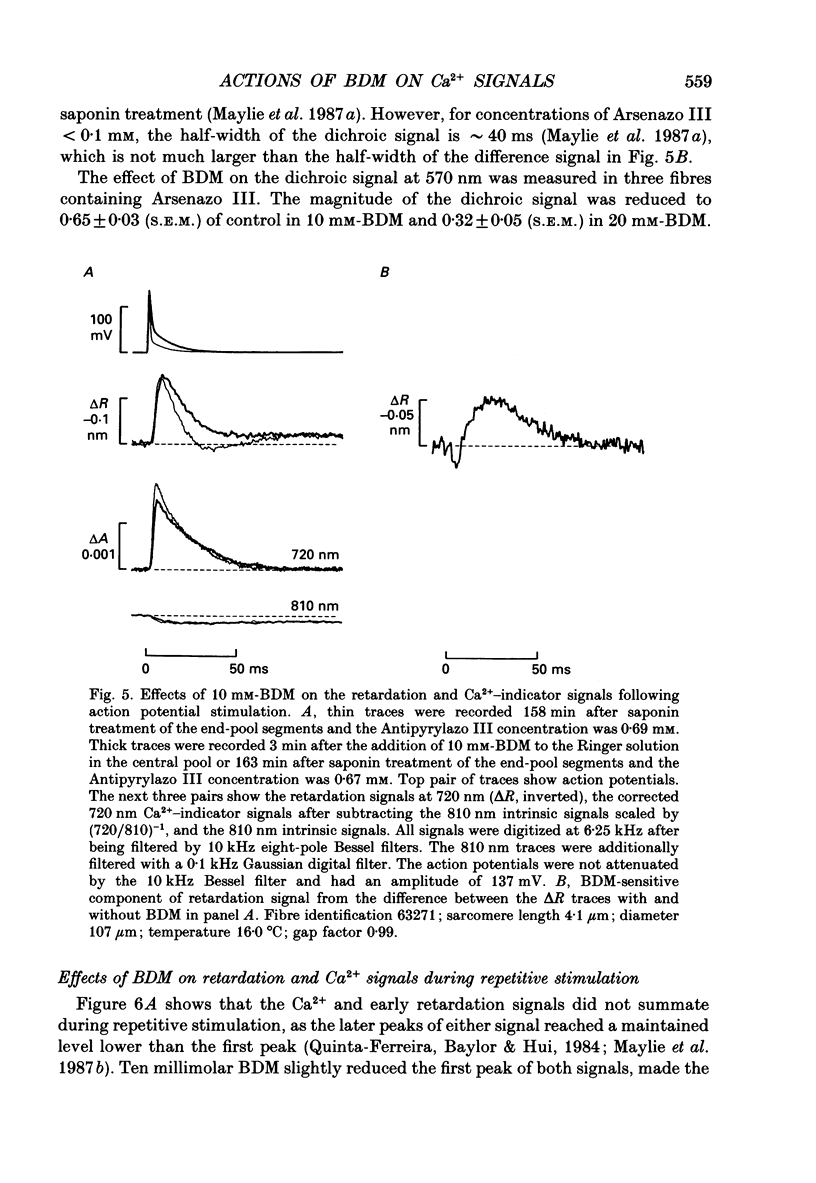
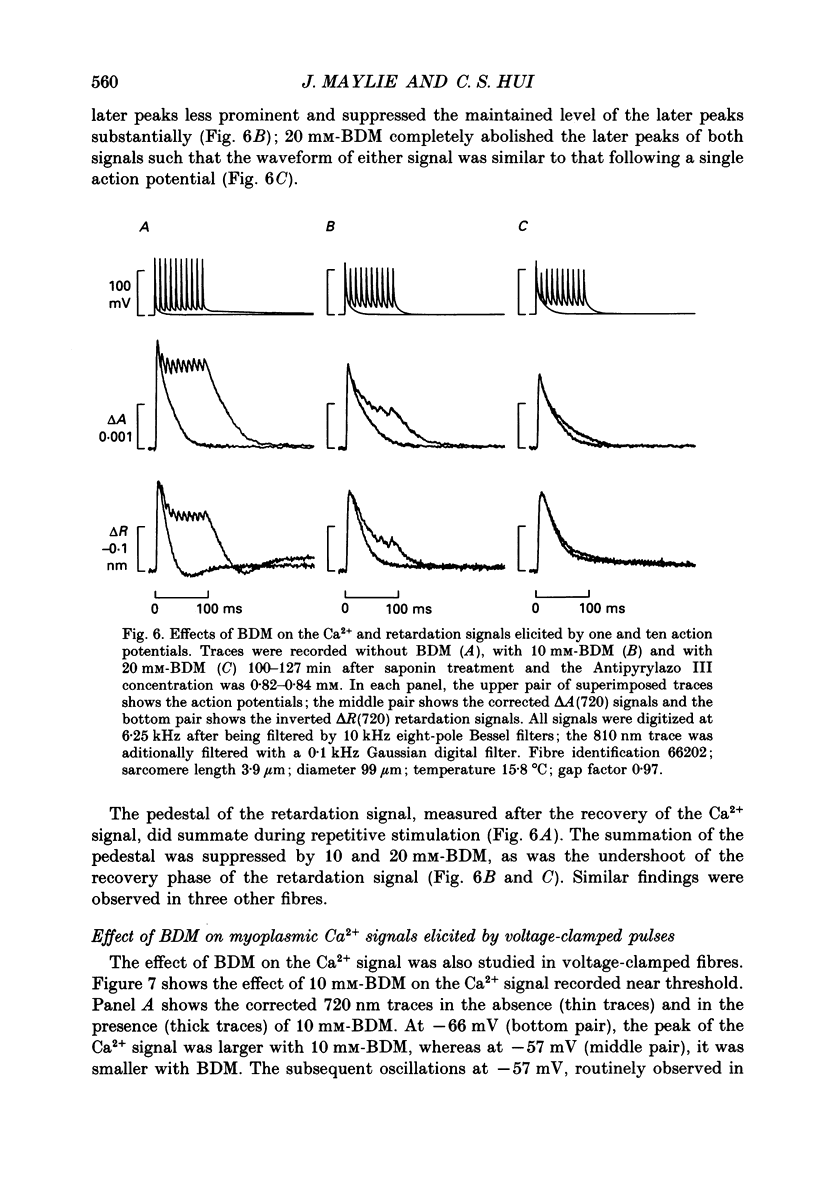
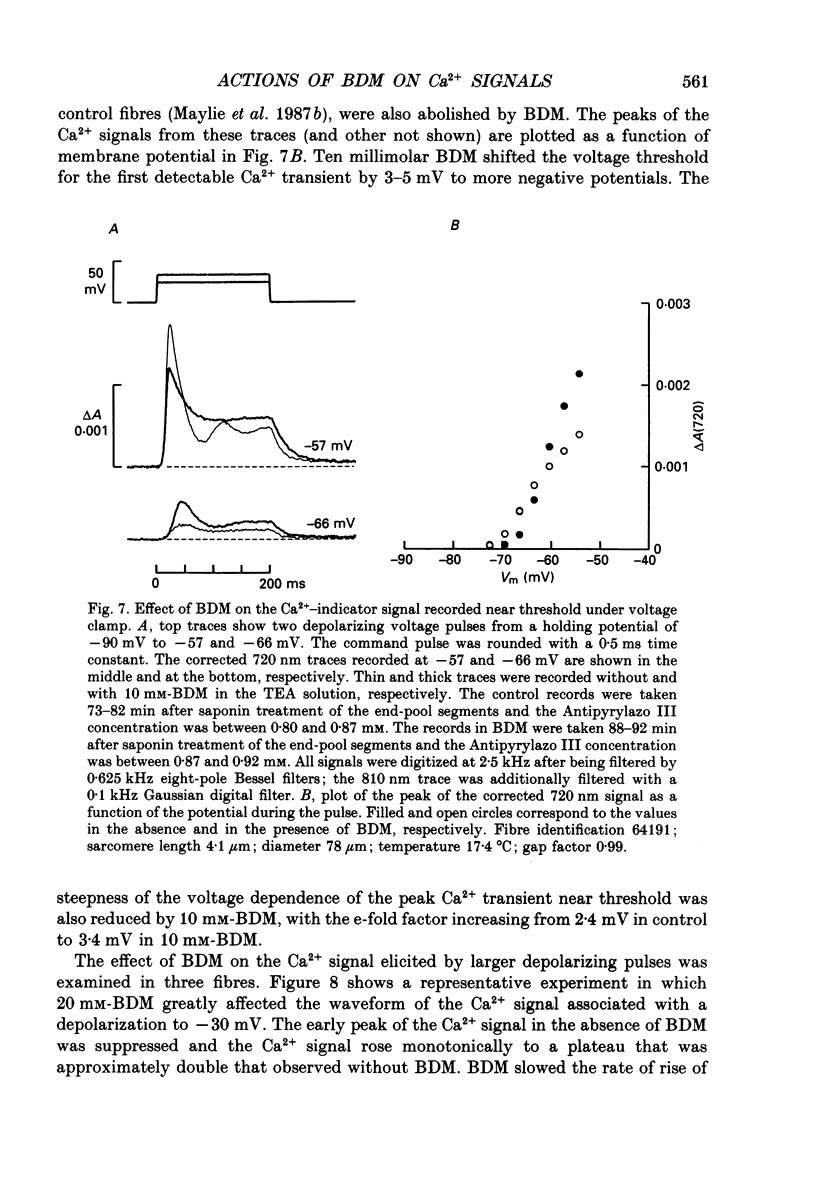
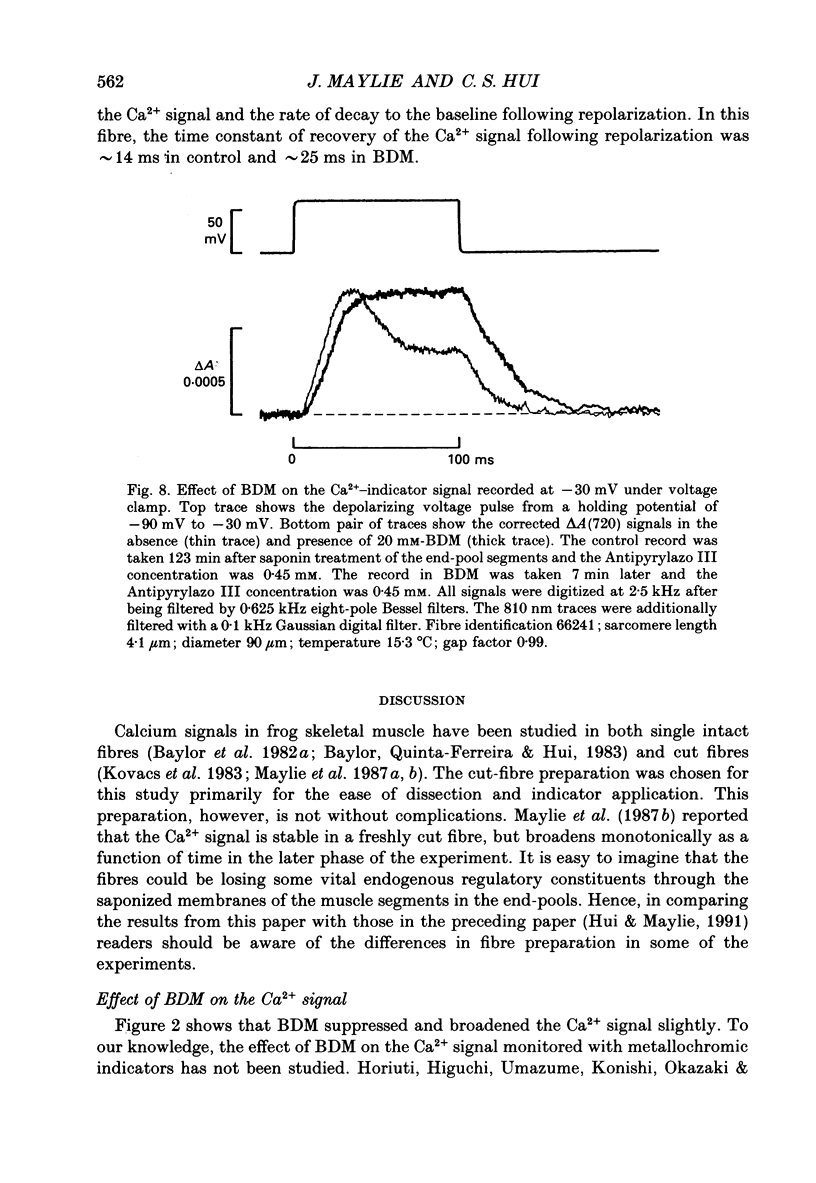
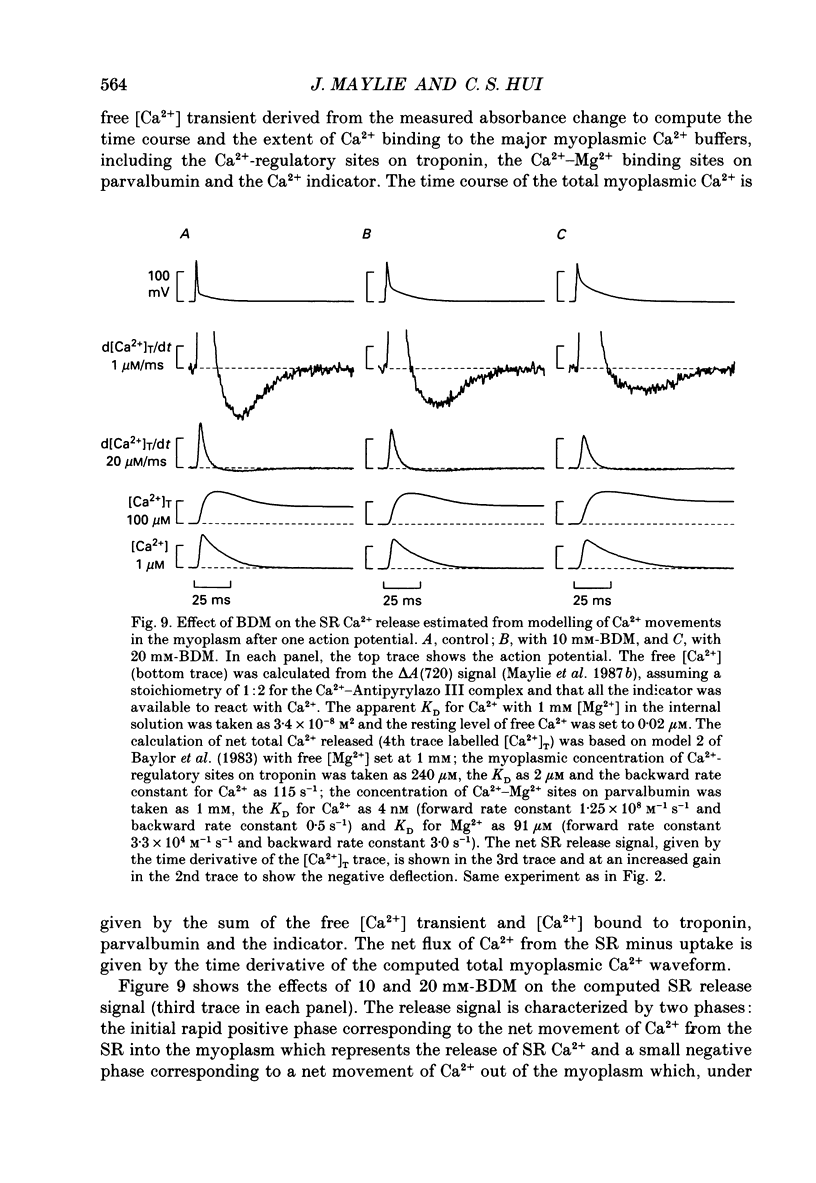
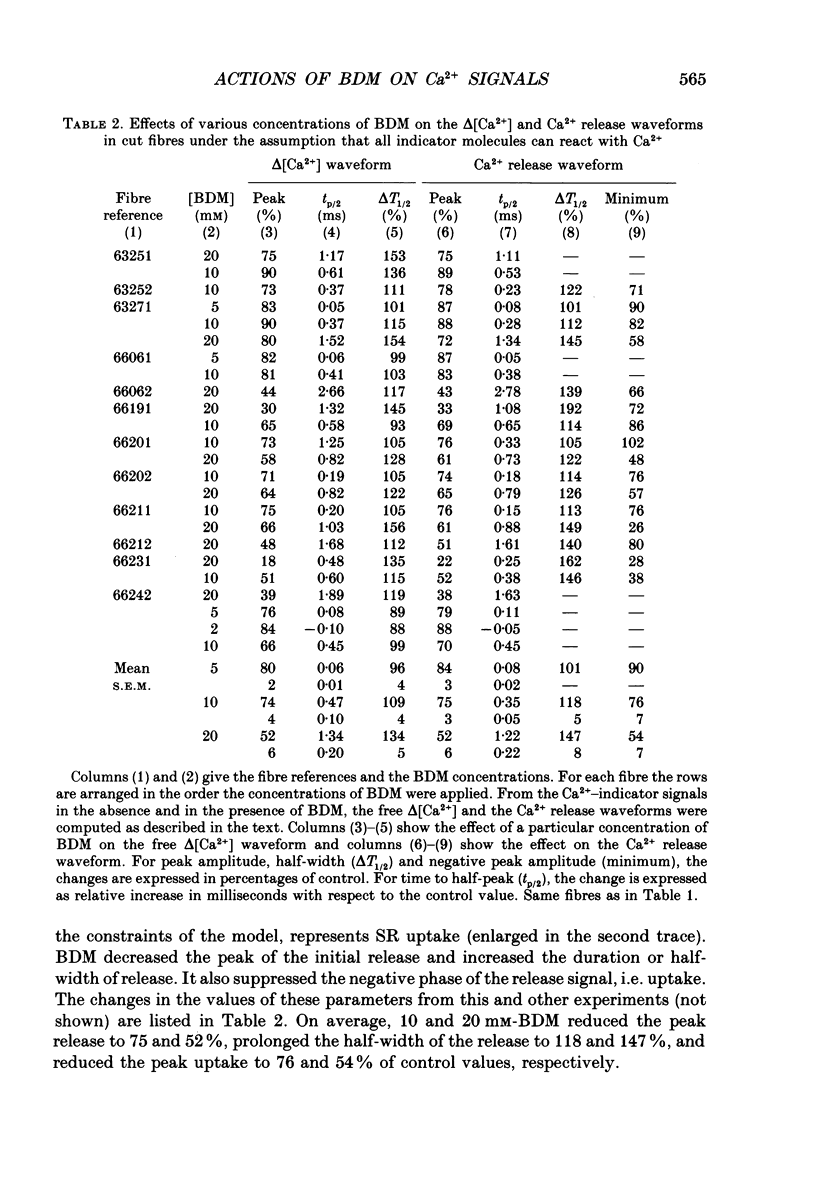
1. The effects of 2,3-butanedione monoxime (BDM) on the optical retardation and myoplasmic Ca2+ signal were studied in twitch fibres of Rana temporaria. The myoplasmic Ca2+ transient in response to action potential stimulation was monitored in cut fibres containing Antipyrylazo III under current clamp in a double Vaseline-gap chamber. 2. In fibres not stretched adequately to suppress all the contraction, BDM blocked the movement-related intrinsic optical signal at 810 nm very effectively. 3. In fibres stretched to sarcomere lengths greater than or equal to 4 microns to reduce the contraction to below detectable levels, the effect of BDM on the Ca(2+)-Antipyrylazo III signal was studied after correcting for the instrinsic signal unrelated to movement. With increasing concentrations of BDM, the peak of the Ca(2+)-Antipyrylazo III signal was suppressed progressively. Concomitantly, the half-width was prolonged somewhat. On average, 5, 10 and 20 mM-BDM reduced the peak amplitude to 88, 78 and 54% of control, respectively. 4. BDM had no effect on the rising phase or the peak amplitude of the retardation signal measured at 720 nm, but suppressed the undershoot in the decay phase of the signal in a dose-dependent manner. BDM also had no effect on the late pedestal level of the signal. 5. During repetitive stimulation by a train of ten action potentials, 10 mM-BDM suppressed the second to the tenth peaks of the Ca2+ signal and of the retardation signal more effectively than the first peak. Twenty millimolar BDM almost completely suppressed the later peaks of both signals such that the signals decayed with a time course similar to that elicited by a single action potential. 6. The effect of BDM on the Ca(2+)-Antipyrylazo III signal was also studied in fibres under voltage clamp; 10 mM-BDM lowered the threshold for the Ca(2+)-Antipyrylazo III transient by a few millivolts and reduced the steepness of the peak amplitude versus voltage plot near threshold. 7. Based on a model used by Baylor, Chandler & Marshall (1983) to estimate the net Ca2+ release from the sarcoplasmic reticulum, 10 and 20 mM-BDM were found to reduce the peak release to 75 and 52%, to prolong the half-width of the release waveform to 118 and 147%, and to reduce the peak uptake to 76 and 54% of control values, respectively. 8. It is concluded that BDM affects the optical retardation and myoplasmic Ca2+ signal monitored with Antipyrylazo III in a dose-dependent manner.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor S. M., Chandler W. K., Marshall M. W. Dichroic components of Arsenazo III and dichlorophosphonazo III signals in skeletal muscle fibres. J Physiol. 1982 Oct;331:179–210. doi: 10.1113/jphysiol.1982.sp014369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Use of metallochromic dyes to measure changes in myoplasmic calcium during activity in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:139–177. doi: 10.1113/jphysiol.1982.sp014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Quinta-Ferreira M. E., Hui C. S. Comparison of isotropic calcium signals from intact frog muscle fibers injected with Arsenazo III or Antipyrylazo III. Biophys J. 1983 Oct;44(1):107–112. doi: 10.1016/S0006-3495(83)84282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer M. W., Gage P. W., Neering I. R., Dulhunty A. F., Lamb G. D. Paralysis of skeletal muscle by butanedione monoxime, a chemical phosphatase. Pflugers Arch. 1988 Jan;411(1):76–79. doi: 10.1007/BF00581649. [DOI] [PubMed] [Google Scholar]
- Fryer M. W., Neering I. R., Stephenson D. G. Effects of 2,3-butanedione monoxime on the contractile activation properties of fast- and slow-twitch rat muscle fibres. J Physiol. 1988 Dec;407:53–75. doi: 10.1113/jphysiol.1988.sp017403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuti K., Higuchi H., Umazume Y., Konishi M., Okazaki O., Kurihara S. Mechanism of action of 2, 3-butanedione 2-monoxime on contraction of frog skeletal muscle fibres. J Muscle Res Cell Motil. 1988 Apr;9(2):156–164. doi: 10.1007/BF01773737. [DOI] [PubMed] [Google Scholar]
- Hui C. S., Maylie J. Multiple actions of 2,3-butanedione monoxime on contractile activation in frog twitch fibres. J Physiol. 1991 Oct;442:527–549. doi: 10.1113/jphysiol.1991.sp018807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Intrinsic optical and passive electrical properties of cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):1–40. doi: 10.1085/jgp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs L., Rios E., Schneider M. F. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. J Physiol. 1983 Oct;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Calcium signals recorded from cut frog twitch fibers containing antipyrylazo III. J Gen Physiol. 1987 Jan;89(1):83–143. doi: 10.1085/jgp.89.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Comparison of arsenazo III optical signals in intact and cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):41–81. doi: 10.1085/jgp.89.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetliker H. An appraisal of the evidence for a sarcoplasmic reticulum membrane potential and its relation to calcium release in skeletal muscle. J Muscle Res Cell Motil. 1982 Sep;3(3):247–272. doi: 10.1007/BF00713037. [DOI] [PubMed] [Google Scholar]
- Suarez-Kurtz G., Parker I. Birefringence signals and calcium transients in skeletal muscle. Nature. 1977 Dec 22;270(5639):746–748. doi: 10.1038/270746a0. [DOI] [PubMed] [Google Scholar]


