Abstract
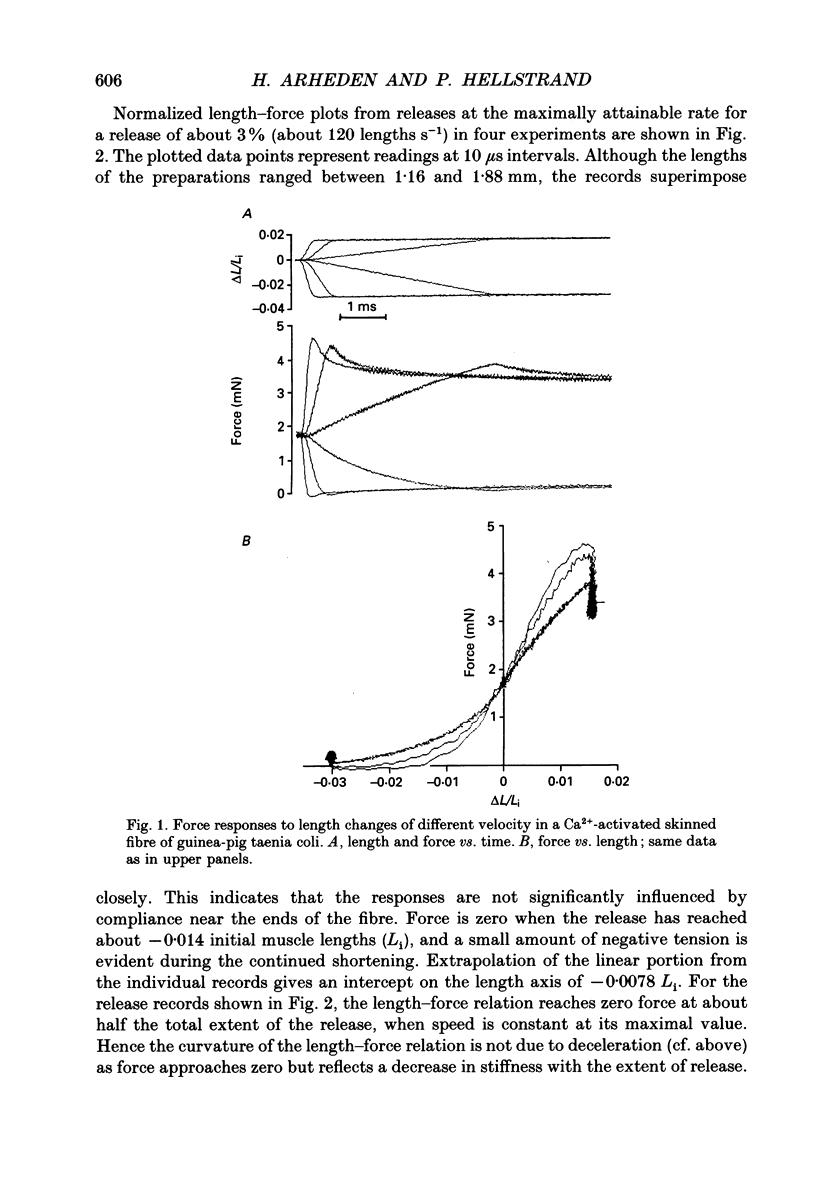
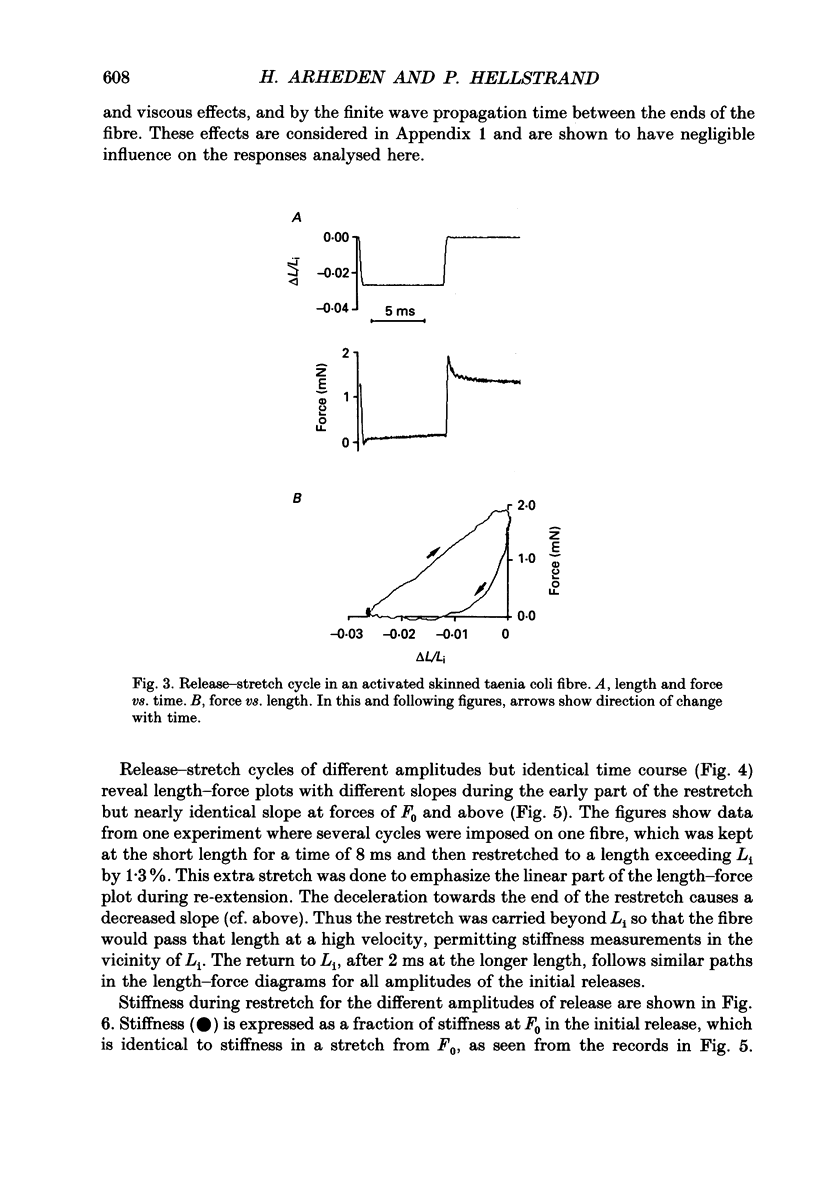
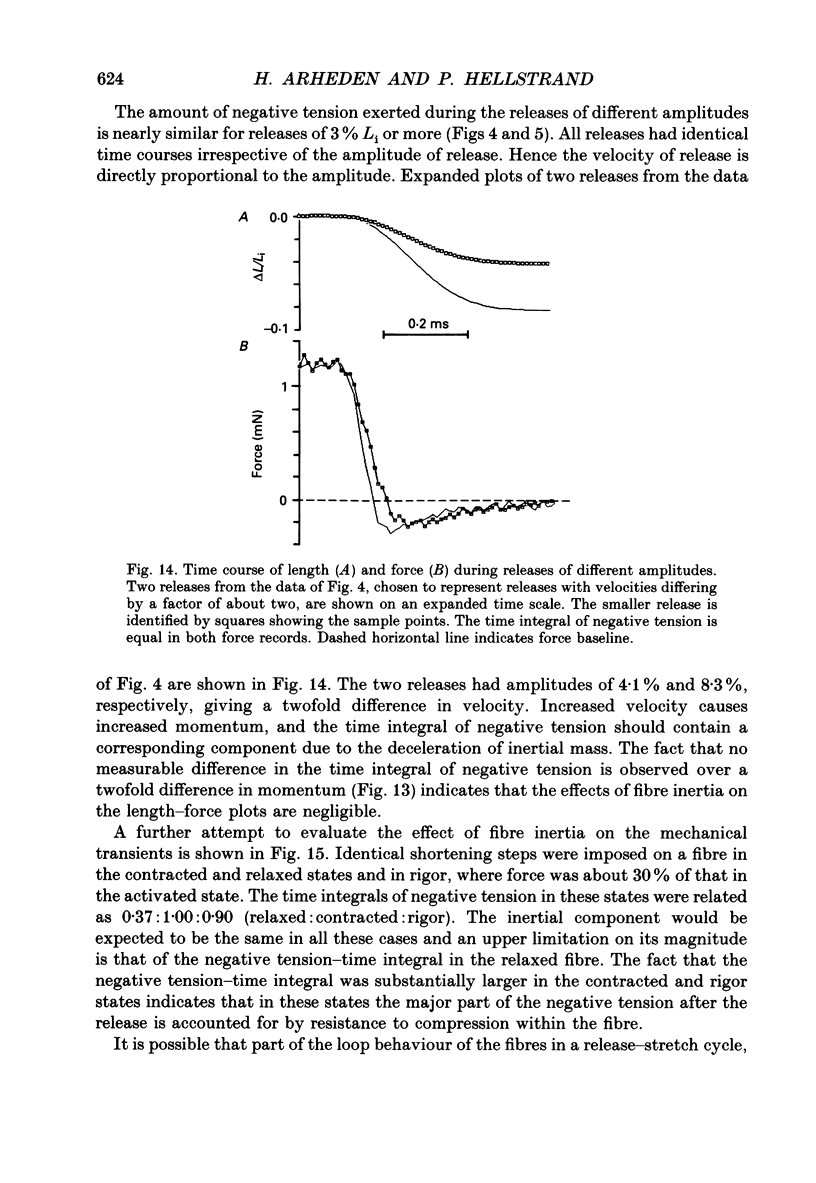
1. Mechanical transients in fibre bundles of skinned smooth muscle of guinea-pig taenia coli at 21-22 degrees C were investigated by recording tension responses to length changes of up to 9%, complete within 0.3 ms. 2. The length-force relationship, recorded continuously during rapid stretch of a Ca(2+)-activated contracted muscle, was linear up to at least 2.5 times the isometric force, corresponding to a stretch of about 1%. The slope of the relationship (stiffness) increased with the velocity of stretch. 3. During rapid release (about 120 muscle lengths s-1) the length-force relationship was linear down to about 50% of the initial isometric force, reached at about 80 microseconds after the beginning of the release. At lower force the length-force relationship was concave upwards. The linear portion extrapolated to zero force at about -0.008 muscle lengths. In large releases the length-force plot approached the force baseline under an acute angle, and negative force was transiently exerted. 4. When the muscle was stretched back to the initial length after a shortening step, force transiently rose above the isometric force, but decayed back within a few milliseconds. Stiffness at the time of restretch was compared with that in the initial shortening step by plotting force vs. length, and was found to be decreased to 63% within 0.3 ms of a step to zero force. Stiffness decreased further with time at zero force, and after 256 ms was about 29% of the isometric value. 5. In rigor, caused by the introduction of ATP-free solution during the plateau of isometric contraction, fibre tension decreased to about 30% of the active tension, whereas stiffness relative to force increased; 82% of the initial stiffness in rigor was detected in a restretch immediately after a shortening step, decreasing to 59% at 256 ms. When the fibre was activated at suboptimal [Ca2+] to cause the same force as in rigor, stiffness was lower than in rigor and decreased more after a release. 6. After completion of a release-stretch cycle, stiffness was rapidly restored to the same value as in isometric contraction. Test stretches at different points in time after completion of the cycle revealed that most of the stiffness had been restored within 1 ms of the restretch, occurring concomitantly with a decay in force.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arheden H., Arner A., Hellstrand P. Cross-bridge behaviour in skinned smooth muscle of the guinea-pig taenia coli at altered ionic strength. J Physiol. 1988 Sep;403:539–558. doi: 10.1113/jphysiol.1988.sp017263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner A., Goody R. S., Rapp G., Rüegg J. C. Relaxation of chemically skinned guinea pig taenia coli smooth muscle from rigor by photolytic release of adenosine-5'-triphosphate. J Muscle Res Cell Motil. 1987 Oct;8(5):377–385. doi: 10.1007/BF01578427. [DOI] [PubMed] [Google Scholar]
- Arner A., Hellstrand P. Effects of calcium and substrate on force-velocity relation and energy turnover in skinned smooth muscle of the guinea-pig. J Physiol. 1985 Mar;360:347–365. doi: 10.1113/jphysiol.1985.sp015621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner A. Mechanical characteristics of chemically skinned guinea-pig taenia coli. Pflugers Arch. 1982 Dec;395(4):277–284. doi: 10.1007/BF00580790. [DOI] [PubMed] [Google Scholar]
- Ashton F. T., Somlyo A. V., Somlyo A. P. The contractile apparatus of vascular smooth muscle: intermediate high voltage stereo electron microscopy. J Mol Biol. 1975 Oct 15;98(1):17–29. doi: 10.1016/s0022-2836(75)80098-2. [DOI] [PubMed] [Google Scholar]
- Brenner B., Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci U S A. 1986 May;83(10):3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler B. H., Dusik L. A., Menard M. R. Tension responses of frog skeletal muscle fibres to rapid shortening and lengthening steps. J Physiol. 1988 Mar;397:631–641. doi: 10.1113/jphysiol.1988.sp017022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomo F., Lombardi V., Piazzesi G. The recovery of tension in transients during steady lengthening of frog muscle fibres. Pflugers Arch. 1989 Jun;414(2):245–247. doi: 10.1007/BF00580970. [DOI] [PubMed] [Google Scholar]
- Cooke P. H., Fay F. S., Craig R. Myosin filaments isolated from skinned amphibian smooth muscle cells are side-polar. J Muscle Res Cell Motil. 1989 Jun;10(3):206–220. doi: 10.1007/BF01739811. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Greene L. E. The relation of muscle biochemistry to muscle physiology. Annu Rev Physiol. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney F. W., Hirst D. G. Cross-bridge detachment and sarcomere 'give' during stretch of active frog's muscle. J Physiol. 1978 Mar;276:449–465. doi: 10.1113/jphysiol.1978.sp012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension transients during steady shortening of frog muscle fibres. J Physiol. 1985 Apr;361:131–150. doi: 10.1113/jphysiol.1985.sp015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunst S. J. Contractile force of canine airway smooth muscle during cyclical length changes. J Appl Physiol Respir Environ Exerc Physiol. 1983 Sep;55(3):759–769. doi: 10.1152/jappl.1983.55.3.759. [DOI] [PubMed] [Google Scholar]
- Güth K., Kuhn H. J., Herzig J. W., Rüegg J. C. Evidence for cross bridge slippage in a stretched muscle fibre. Experientia. 1978 Sep 15;34(9):1183–1184. doi: 10.1007/BF01922946. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hellstrand P., Arheden H., Sjölin L., Arner A. Stiffness and the energetics of active shortening in chemically skinned smooth muscle. Prog Clin Biol Res. 1987;245:333–345. [PubMed] [Google Scholar]
- Hellstrand P., Arner A. Myosin light chain phosphorylation and the cross-bridge cycle at low substrate concentration in chemically skinned guinea pig Taenia coli. Pflugers Arch. 1985 Dec;405(4):323–328. doi: 10.1007/BF00595684. [DOI] [PubMed] [Google Scholar]
- Hellstrand P., Johansson B. Analysis of the length response to a force step in smooth muscle from rabbit urinary bladder. Acta Physiol Scand. 1979 Jun;106(2):221–238. doi: 10.1111/j.1748-1716.1979.tb06392.x. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemt P., Peiper U., Speden R. N., Zilker F. The kinetics of post-vibration tension recovery of the isolated rat portal vein. J Physiol. 1981 Mar;312:281–296. doi: 10.1113/jphysiol.1981.sp013629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Tewes A., Gagelmann M., Güth K., Arner A., Rüegg J. C. Temporal relationship between force, ATPase activity, and myosin phosphorylation during a contraction/relaxation cycle in a skinned smooth muscle. Pflugers Arch. 1990 Jul;416(5):512–518. doi: 10.1007/BF00382683. [DOI] [PubMed] [Google Scholar]
- Meiss R. A. Transient responses and continuous behavior of active smooth muscle during controlled stretches. Am J Physiol. 1982 Mar;242(3):C146–C158. doi: 10.1152/ajpcell.1982.242.3.C146. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Warshaw D. M. The anatomical location of the series elastic component in rat vascular smooth muscle. J Physiol. 1981 May;314:321–330. doi: 10.1113/jphysiol.1981.sp013710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E., Cooke R. A model of crossbridge action: the effects of ATP, ADP and Pi. J Muscle Res Cell Motil. 1989 Jun;10(3):181–196. doi: 10.1007/BF01739809. [DOI] [PubMed] [Google Scholar]
- Pfitzer G., Peterson J. W., Rüegg J. C. Length dependence of calcium activated isometric force and immediate stiffness in living and glycerol extracted vascular smooth muscle. Pflugers Arch. 1982 Aug;394(2):174–181. doi: 10.1007/BF00582921. [DOI] [PubMed] [Google Scholar]
- Stienen G. J., Blangé T. Tension responses to rapid length changes in skinned muscle fibres of the frog. Pflugers Arch. 1985 Sep;405(1):5–11. doi: 10.1007/BF00591090. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Güth K., Kuhn H. J., Rüegg J. C. Decrease in stiffness during shortening in calcium activated skinned muscle fibers. Pflugers Arch. 1982 Feb;392(4):322–326. doi: 10.1007/BF00581626. [DOI] [PubMed] [Google Scholar]
- Warshaw D. M., Fay F. S. Cross-bridge elasticity in single smooth muscle cells. J Gen Physiol. 1983 Aug;82(2):157–199. doi: 10.1085/jgp.82.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw D. M., Rees D. D., Fay F. S. Characterization of cross-bridge elasticity and kinetics of cross-bridge cycling during force development in single smooth muscle cells. J Gen Physiol. 1988 Jun;91(6):761–779. doi: 10.1085/jgp.91.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]


