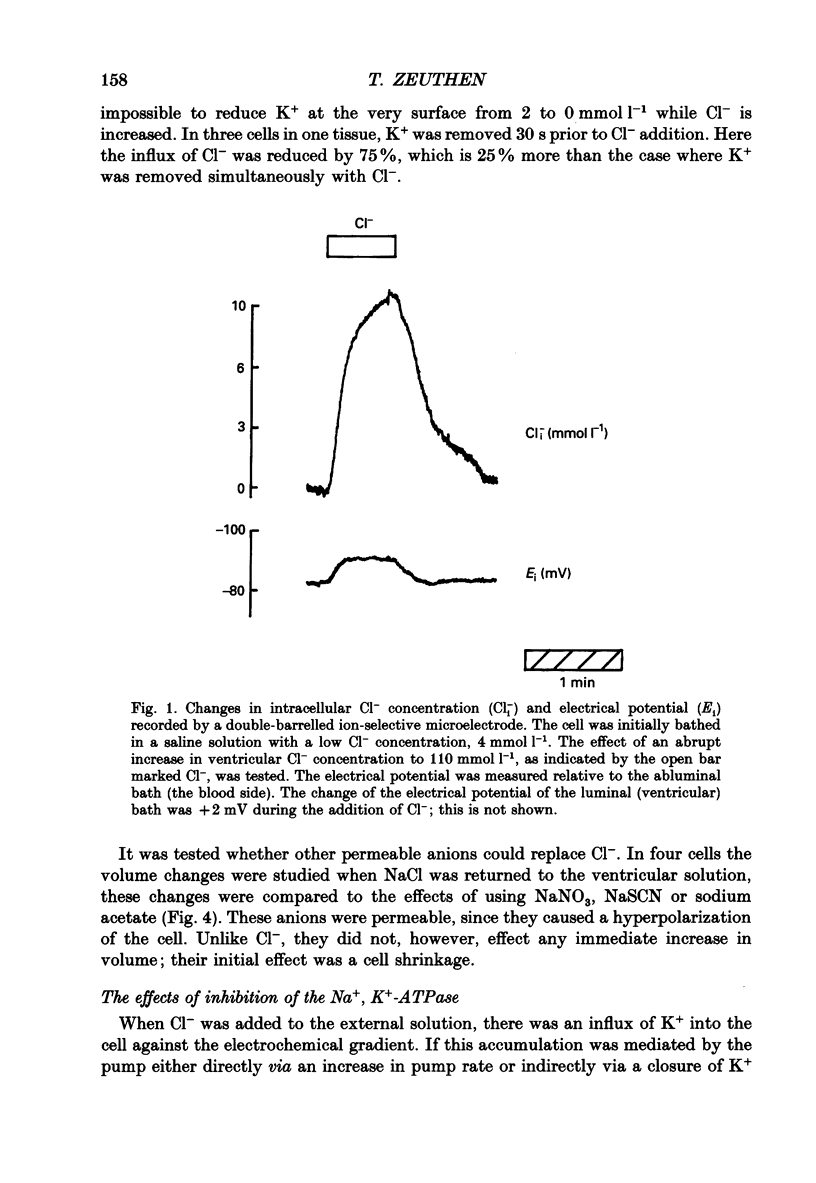
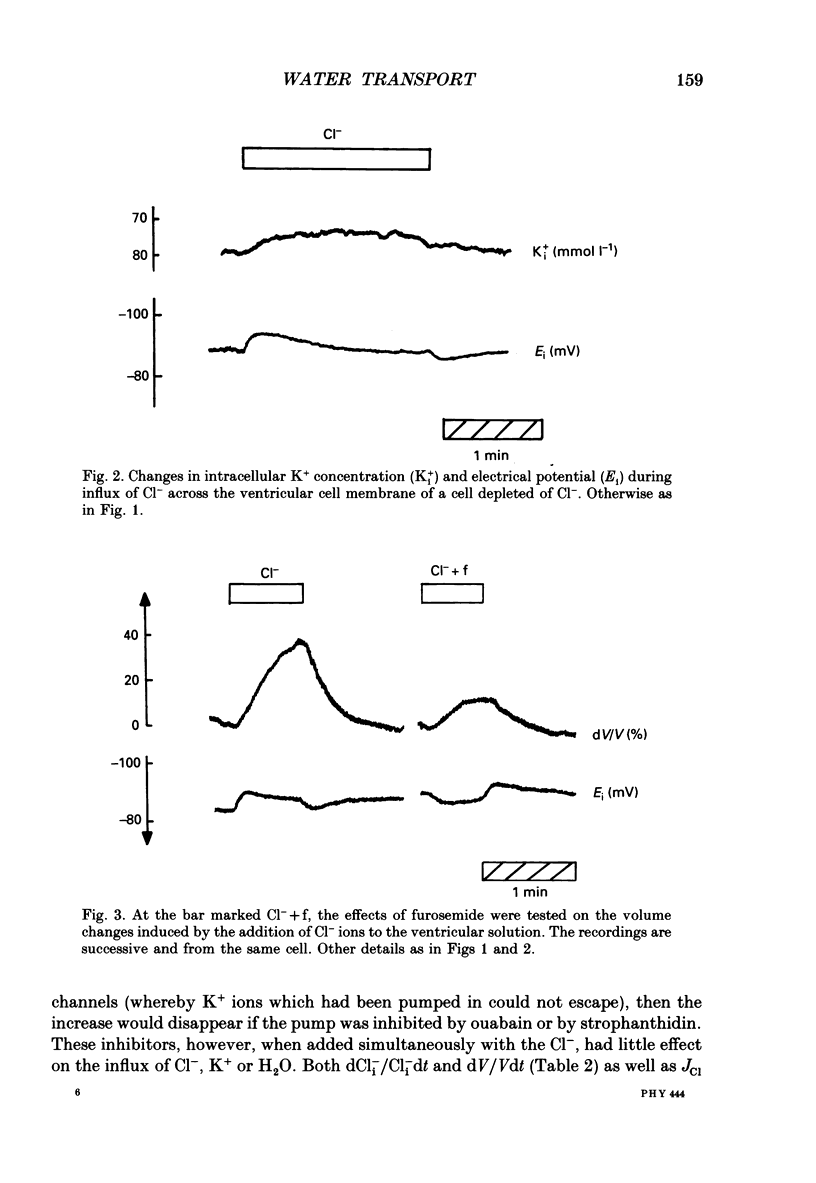
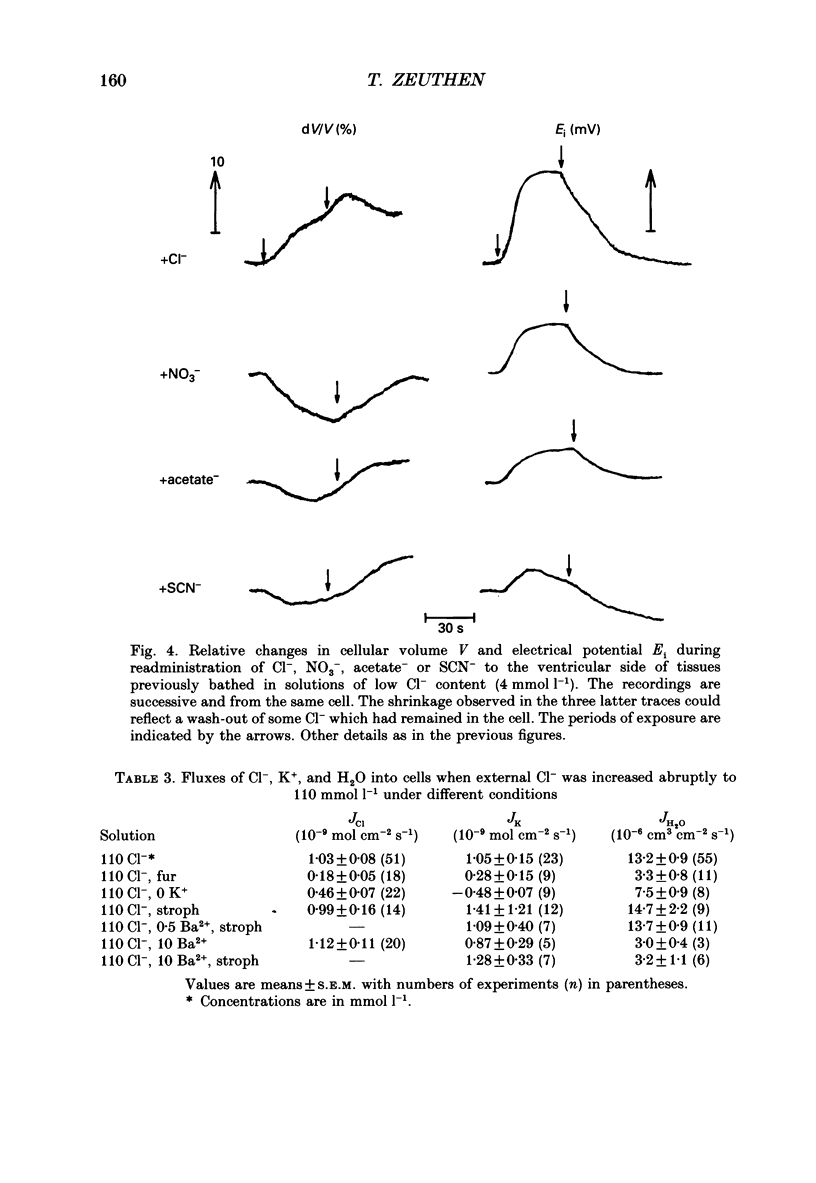
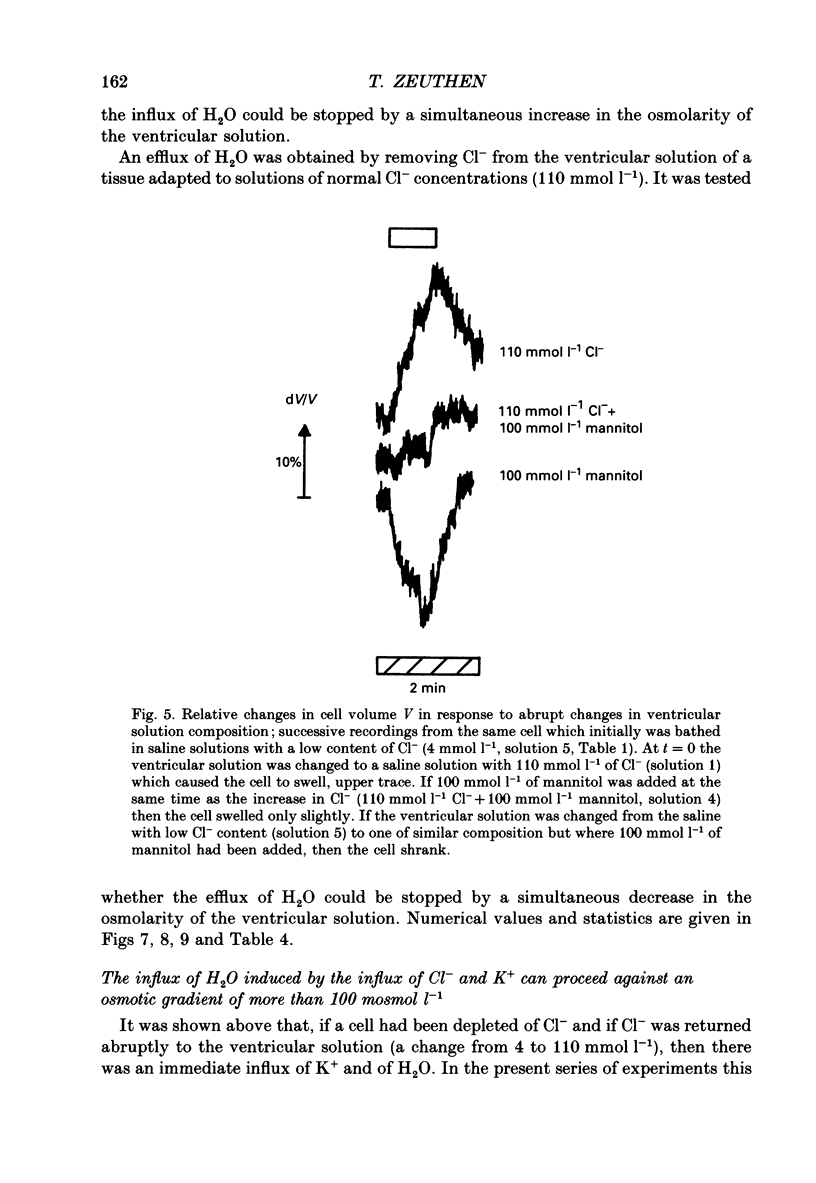
Abstract
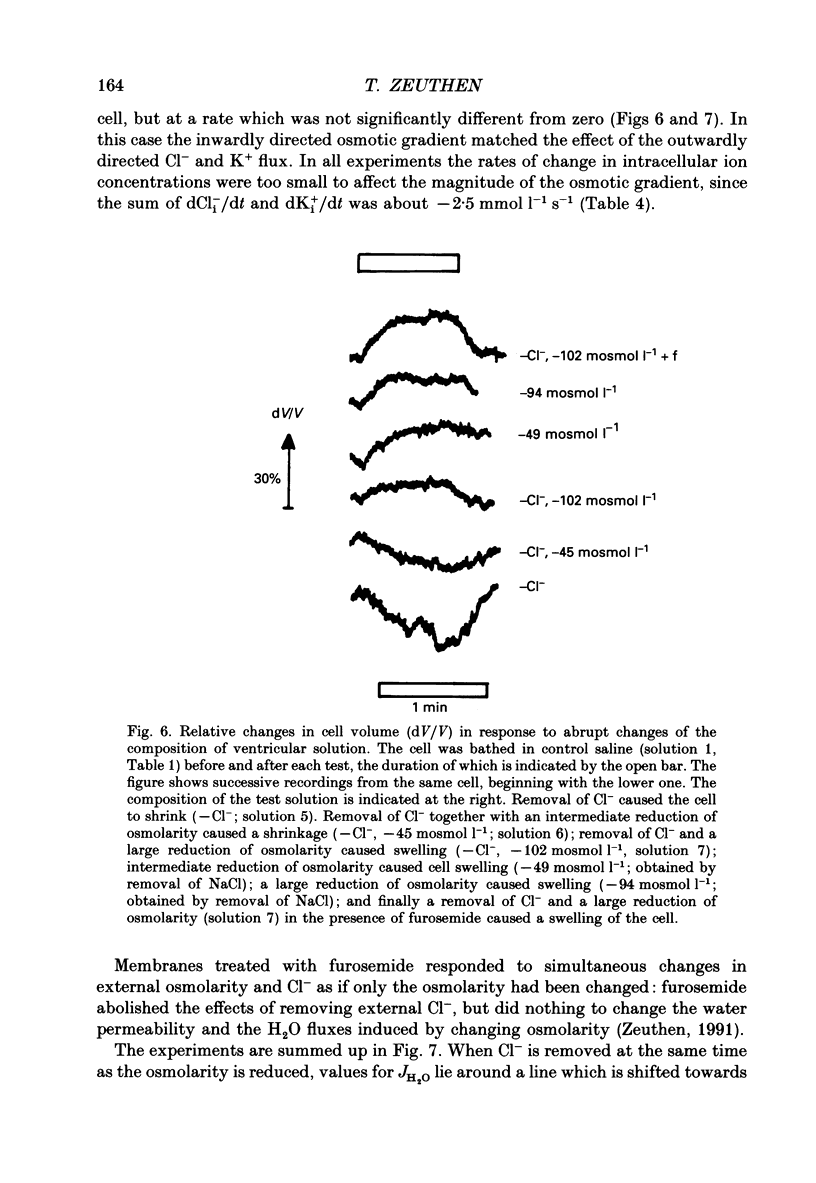
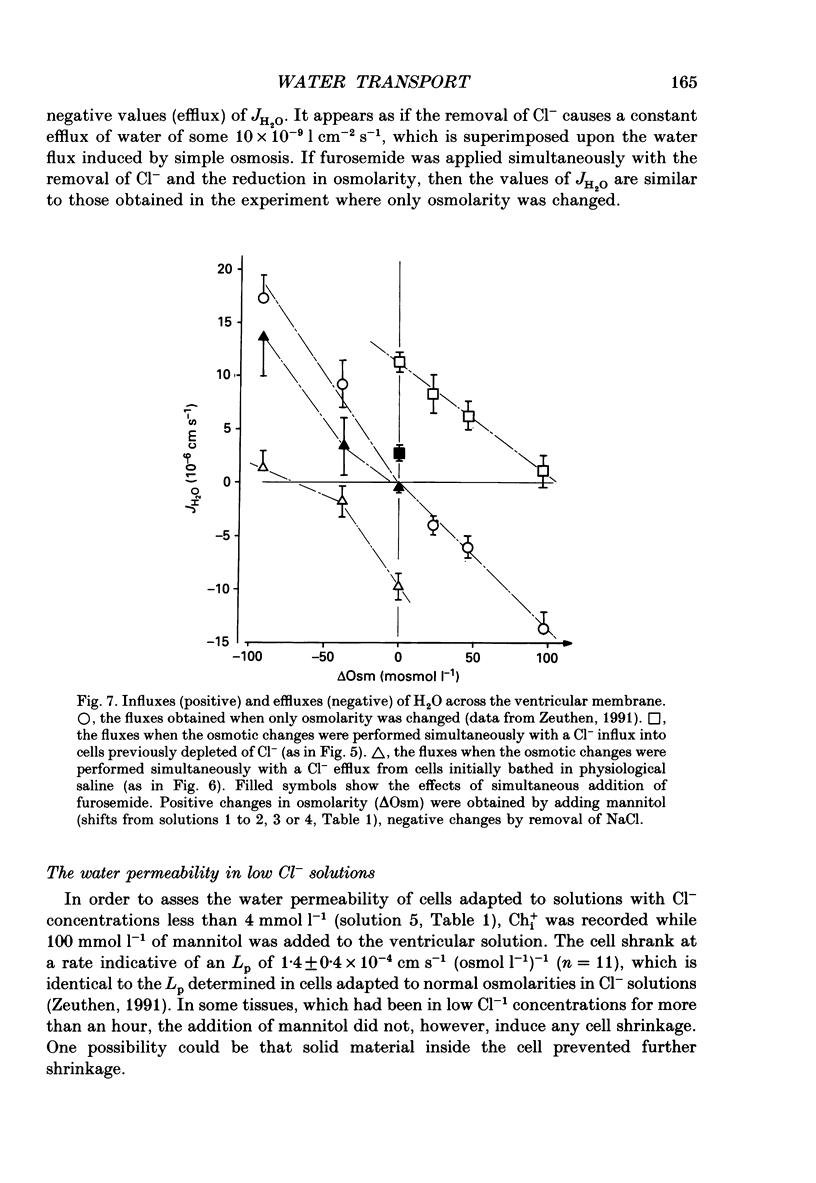
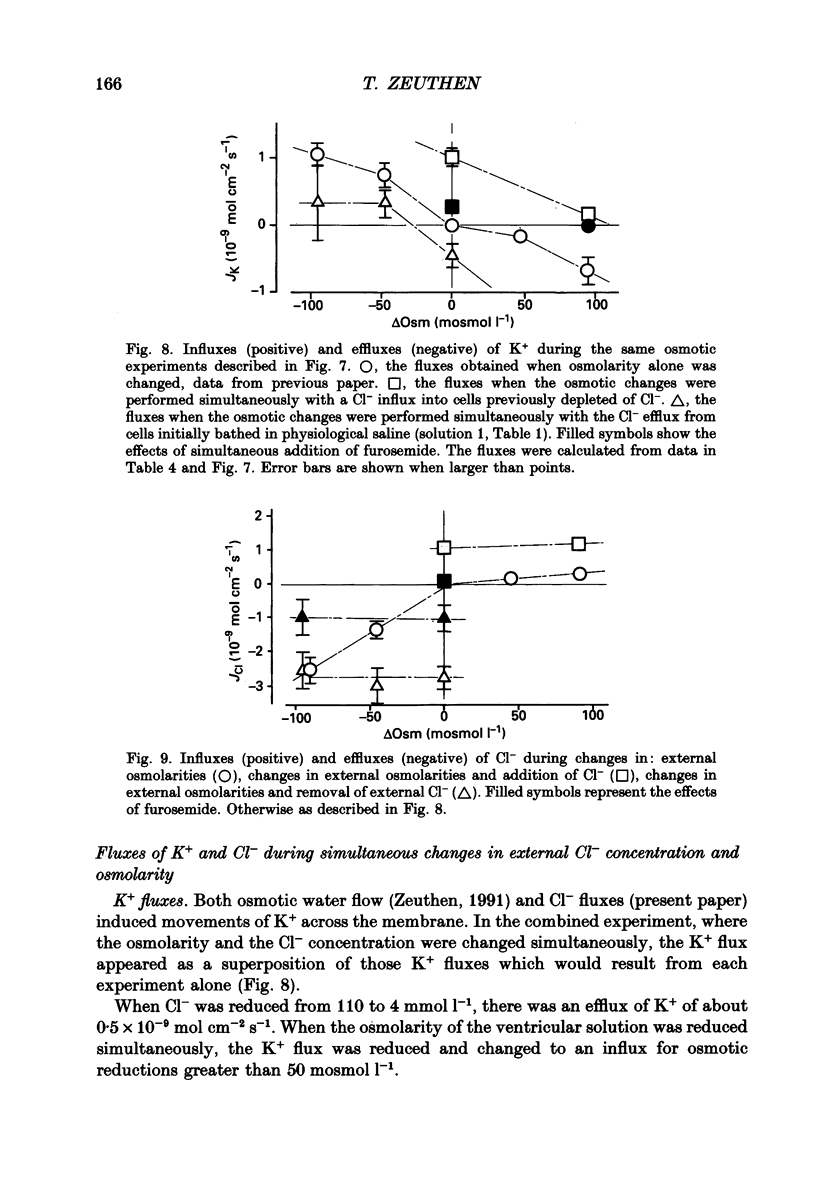
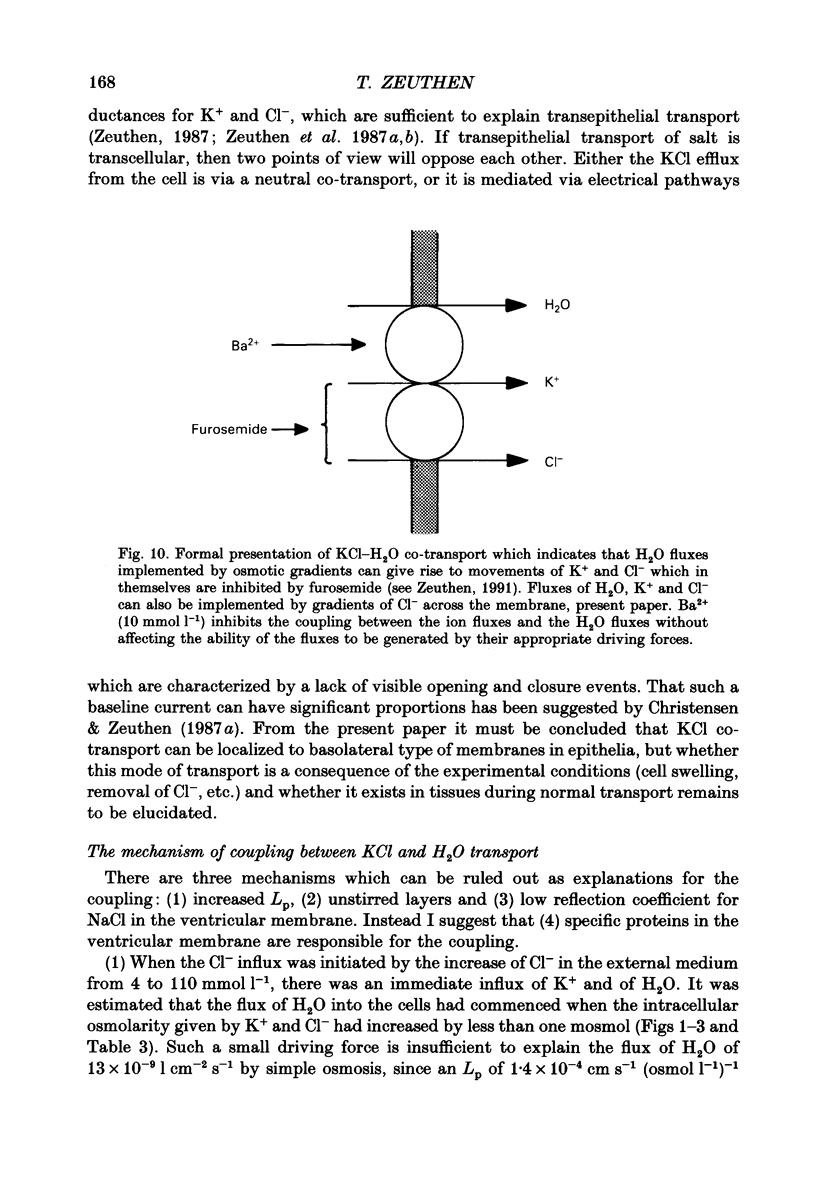
1. The interaction between Cl-, K+ and H2O fluxes were studied in the ventricular membrane of the choroid plexus epithelium from Necturus maculosus by means of ion-selective microelectrodes. The flux of H2O was measured by means of K+ electrodes as the dilution or concentration of intracellular choline ions, Ch+i. 2. In one series of experiments Cl- was readministered to the ventricular solution of tissues incubated in media with low Cl- concentrations. The resulting influx of Cl- was associated with an instantaneous influx of K+ and H2O. 3. Both the Cl- and the K+ influxes were reduced by the diuretic furosemide but were unaffected by inhibitors of Na+, K(+)-ATPase or changes in membrane potentials induced by Ba2+. Since the influx of K+ proceeds against its electrochemical gradient and is unaffected by changes in membrane potentials, the membrane exhibits secondary active, electroneutral transport of K+. 4. The influx of water, initiated simultaneously with the influx of K+ and Cl-, commenced before these ions had changed the osmolarity of the intracellular solution significantly. The influx of H2O could proceed against an osmotic gradient. The influx stopped when 100 mmol l-1 of mannitol was added to the ventricular solution at the same time as the Cl- ions. The influx of H2O was inhibited by K+ removal, furosemide or high external Ba2+ (10 mmol l-1), but not by strophanthidin, ouabain or low concentrations of Ba2+ (0.5 mmol l-1). The influx could not continue with other permeable anions, NO3-, acetate- or SCN-, replacing Cl-. 5. In another series of experiments Cl- was removed from the ventricular solution of tissues bathed in saline solutions with normal concentrations of Cl-. The resulting efflux of Cl- was associated with an instantaneous efflux of K+ and H2O. This efflux of H2O could proceed against an osmotic gradient of up to 70 mosmol l-1. This effect was inhibited by furosemide, in which case the water fluxes were entirely dependent on the osmotic gradients and the osmotic water permeability Lp of the ventricular membrane. 6. The data suggest that there is a coupling between the flux of KCl and of water in the ventricular membrane, which implies that the reflection coefficient sigma for KCl under the given circumstances is less than one. I suggest that the ability of leaky epithelia to transport against osmotic gradients depends on such a coupling, which derives from the properties of the proteins through which K+, Cl- and H2O leave the cell.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomsztyk K., Wright F. S. Dependence of ion fluxes on fluid transport by rat proximal tubule. Am J Physiol. 1986 Apr;250(4 Pt 2):F680–F689. doi: 10.1152/ajprenal.1986.250.4.F680. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Loo D. D., Wright E. M. Ca2+-activated K+ channels in the apical membrane of Necturus choroid plexus. J Membr Biol. 1988 Nov;105(3):207–219. doi: 10.1007/BF01870998. [DOI] [PubMed] [Google Scholar]
- Cherksey B. D., Zeuthen T. A membrane protein with a K+ and a Cl- channel. Acta Physiol Scand. 1987 Jan;129(1):137–138. doi: 10.1111/j.1748-1716.1987.tb08048.x. [DOI] [PubMed] [Google Scholar]
- Christensen O., Simon M., Randlev T. Anion channels in a leaky epithelium. A patch-clamp study of choroid plexus. Pflugers Arch. 1989 Oct;415(1):37–46. doi: 10.1007/BF00373139. [DOI] [PubMed] [Google Scholar]
- Christensen O., Zeuthen T. Maxi K+ channels in leaky epithelia are regulated by intracellular Ca2+, pH and membrane potential. Pflugers Arch. 1987 Mar;408(3):249–259. doi: 10.1007/BF02181467. [DOI] [PubMed] [Google Scholar]
- Corcia A., Armstrong W. M. KCl cotransport: a mechanism for basolateral chloride exit in Necturus gallbladder. J Membr Biol. 1983;76(2):173–182. doi: 10.1007/BF02000617. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. M. TRANSPORT OF SALT AND WATER IN RABBIT AND GUINEA PIG GALL BLADDER. J Gen Physiol. 1964 Sep;48:1–14. doi: 10.1085/jgp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. The mechanism of water transport by the gall-bladder. J Physiol. 1962 May;161:503–527. doi: 10.1113/jphysiol.1962.sp006900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. S., Spring K. R. Intracellular activities during volume regulation by Necturus gallbladder. J Membr Biol. 1984;78(3):187–199. doi: 10.1007/BF01925967. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Properties of the basolateral membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney. A model for secondary active chloride transport. Pflugers Arch. 1983 Mar;396(4):325–334. doi: 10.1007/BF01063938. [DOI] [PubMed] [Google Scholar]
- HEISEY S. R., HELD D., PAPPENHEIMER J. R. Bulk flow and diffusion in the cerebrospinal fluid system of the goat. Am J Physiol. 1962 Nov;203:775–781. doi: 10.1152/ajplegacy.1962.203.5.775. [DOI] [PubMed] [Google Scholar]
- Ikonomov O., Simon M., Frömter E. Electrophysiological studies on lateral intercellular spaces of Necturus gallbladder epithelium. Pflugers Arch. 1985 Mar;403(3):301–307. doi: 10.1007/BF00583604. [DOI] [PubMed] [Google Scholar]
- Jessen F., Cherksey B. D., Zeuthen T., Hoffmann E. K. Isolation and reconstitution of furosemide-binding proteins from Ehrlich ascites tumor cells. J Membr Biol. 1989 May;108(2):139–151. doi: 10.1007/BF01871025. [DOI] [PubMed] [Google Scholar]
- Larson M., Spring K. R. Volume regulation by Necturus gallbladder: basolateral KCl exit. J Membr Biol. 1984;81(3):219–232. doi: 10.1007/BF01868715. [DOI] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Loo D. D., Brown P. D., Wright E. M. Ca2+-activated K+ currents in Necturus choroid plexus. J Membr Biol. 1988 Nov;105(3):221–231. doi: 10.1007/BF01870999. [DOI] [PubMed] [Google Scholar]
- PARSONS D. S., WINGATE D. L. The effect of osmotic gradients on fluid transfer across rat intestine in vitro. Biochim Biophys Acta. 1961 Jan 1;46:170–183. doi: 10.1016/0006-3002(61)90660-6. [DOI] [PubMed] [Google Scholar]
- Pearce D., Verkman A. S. NaCl reflection coefficients in proximal tubule apical and basolateral membrane vesicles. Measurement by induced osmosis and solvent drag. Biophys J. 1989 Jun;55(6):1251–1259. doi: 10.1016/S0006-3495(89)82920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B. E., Spring K. R. Gallbladder epithelial cell hydraulic water permeability and volume regulation. J Gen Physiol. 1982 Mar;79(3):481–505. doi: 10.1085/jgp.79.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratz J., Ripoche P., Corman B. Evidence for proteic water pathways in the luminal membrane of kidney proximal tubule. Biochim Biophys Acta. 1986 Apr 14;856(2):259–266. doi: 10.1016/0005-2736(86)90035-0. [DOI] [PubMed] [Google Scholar]
- Reuss L. Basolateral KCl co-transport in a NaCl-absorbing epithelium. Nature. 1983 Oct 20;305(5936):723–726. doi: 10.1038/305723a0. [DOI] [PubMed] [Google Scholar]
- Tripathi S., Boulpaep E. L. Mechanisms of water transport by epithelial cells. Q J Exp Physiol. 1989 Jul;74(4):385–417. doi: 10.1113/expphysiol.1989.sp003288. [DOI] [PubMed] [Google Scholar]
- Van der Goot F. G., Podevin R. A., Corman B. J. Water permeabilities and salt reflection coefficients of luminal, basolateral and intracellular membrane vesicles isolated from rabbit kidney proximal tubule. Biochim Biophys Acta. 1989 Nov 27;986(2):332–340. doi: 10.1016/0005-2736(89)90485-9. [DOI] [PubMed] [Google Scholar]
- Welling L. W., Welling D. J., Ochs T. J. Video measurement of basolateral NaCl reflection coefficient in proximal tubule. Am J Physiol. 1987 Aug;253(2 Pt 2):F290–F298. doi: 10.1152/ajprenal.1987.253.2.F290. [DOI] [PubMed] [Google Scholar]
- Zeuthen T., Christensen O., Baerentsen J. H., la Cour M. The mechanism of electrodiffusive K+ transport in leaky epithelia and some of its consequences for anion transport. Pflugers Arch. 1987 Mar;408(3):260–266. doi: 10.1007/BF02181468. [DOI] [PubMed] [Google Scholar]
- Zeuthen T., Christensen O., Cherksey B. Electrodiffusion of Cl- and K+ in epithelial membranes reconstituted into planar lipid bilayers. Pflugers Arch. 1987 Mar;408(3):275–281. doi: 10.1007/BF02181470. [DOI] [PubMed] [Google Scholar]
- Zeuthen T. Ion activities in the lateral intercellular spaces of gallbladder epithelium transporting at low external osmolarities. J Membr Biol. 1983;76(2):113–122. doi: 10.1007/BF02000611. [DOI] [PubMed] [Google Scholar]
- Zeuthen T. Relations between intracellular ion activities and extracellular osmolarity in Necturus gallbladder epithelium. J Membr Biol. 1982;66(2):109–121. doi: 10.1007/BF01868487. [DOI] [PubMed] [Google Scholar]
- Zeuthen T. The effects of chloride ions on electrodiffusion in the membrane of a leaky epithelium. Studies of intact tissue by microelectrodes. Pflugers Arch. 1987 Mar;408(3):267–274. doi: 10.1007/BF02181469. [DOI] [PubMed] [Google Scholar]
- Zeuthen T. Water permeability of ventricular cell membrane in choroid plexus epithelium from Necturus maculosus. J Physiol. 1991 Dec;444:133–151. doi: 10.1113/jphysiol.1991.sp018870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T., Wright E. M. Epithelial potassium transport: tracer and electrophysiological studies in choroid plexus. J Membr Biol. 1981;60(2):105–128. doi: 10.1007/BF01870414. [DOI] [PubMed] [Google Scholar]
- van Heeswijk M. P., van Os C. H. Osmotic water permeabilities of brush border and basolateral membrane vesicles from rat renal cortex and small intestine. J Membr Biol. 1986;92(2):183–193. doi: 10.1007/BF01870707. [DOI] [PubMed] [Google Scholar]


