Abstract
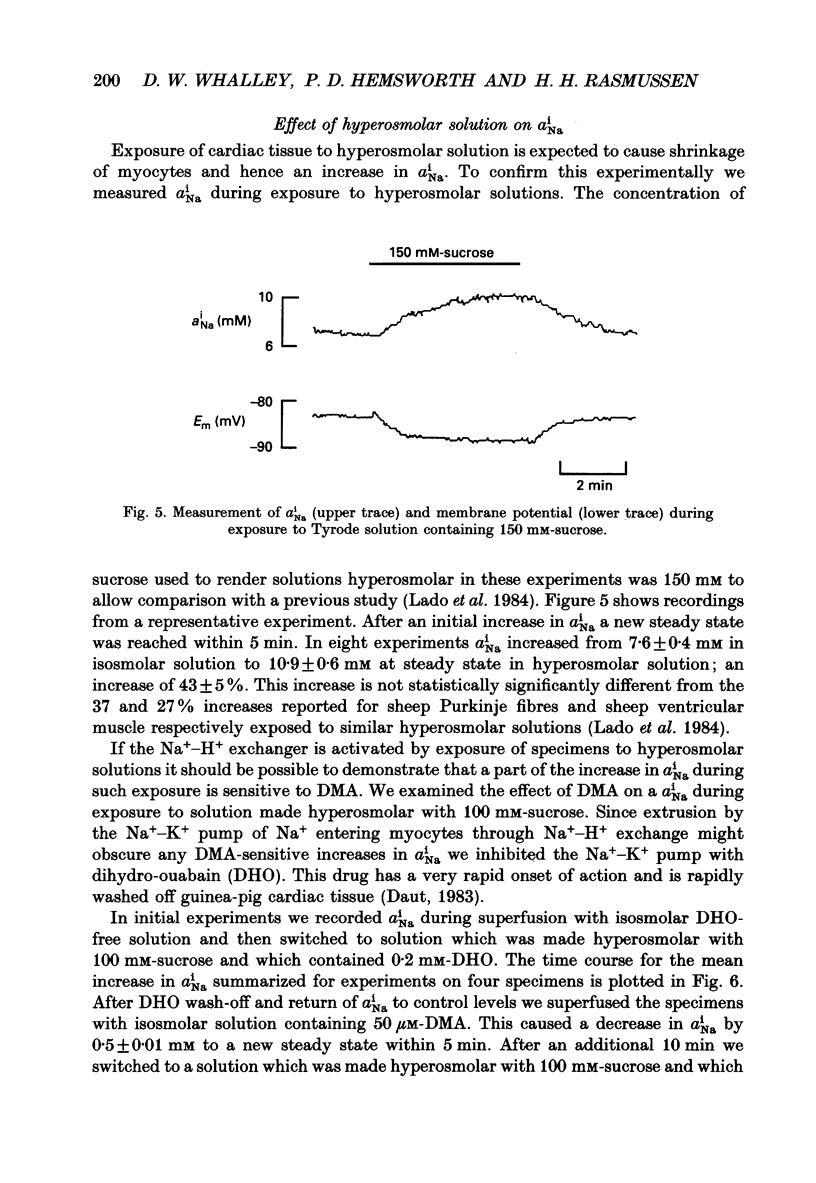
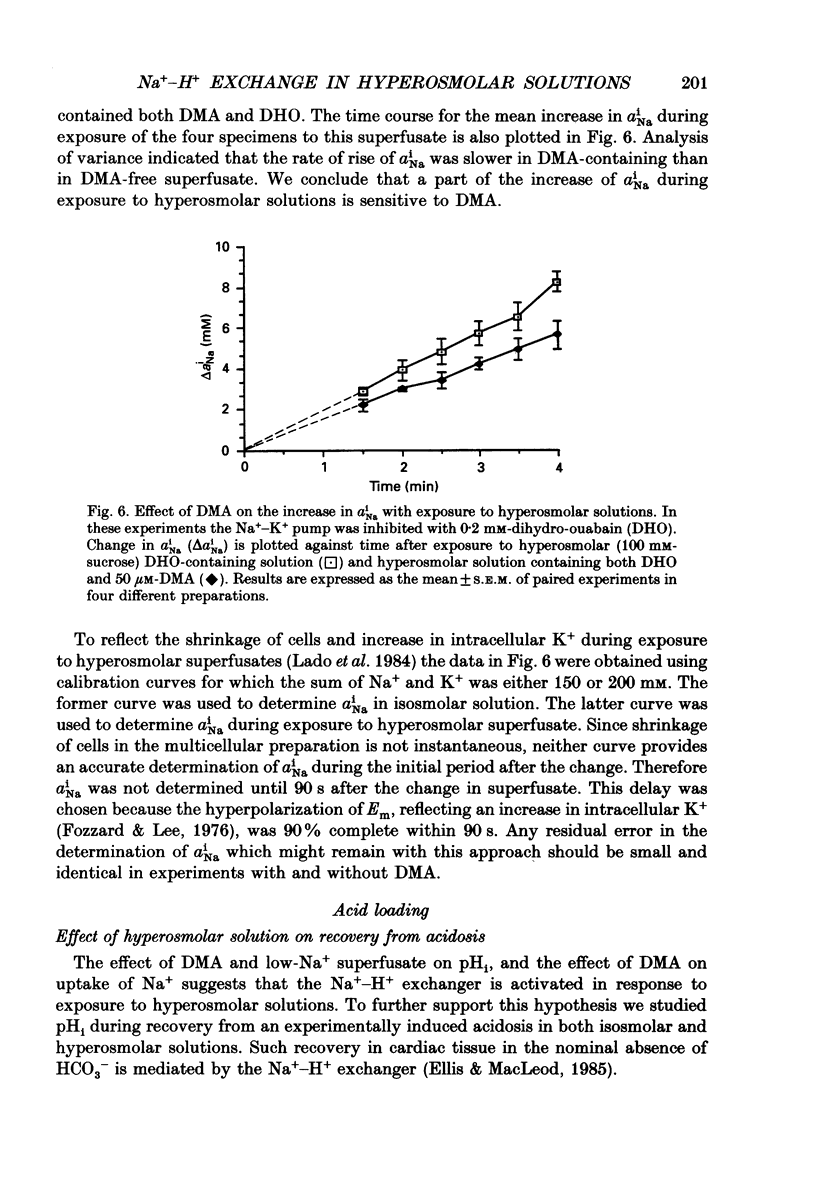
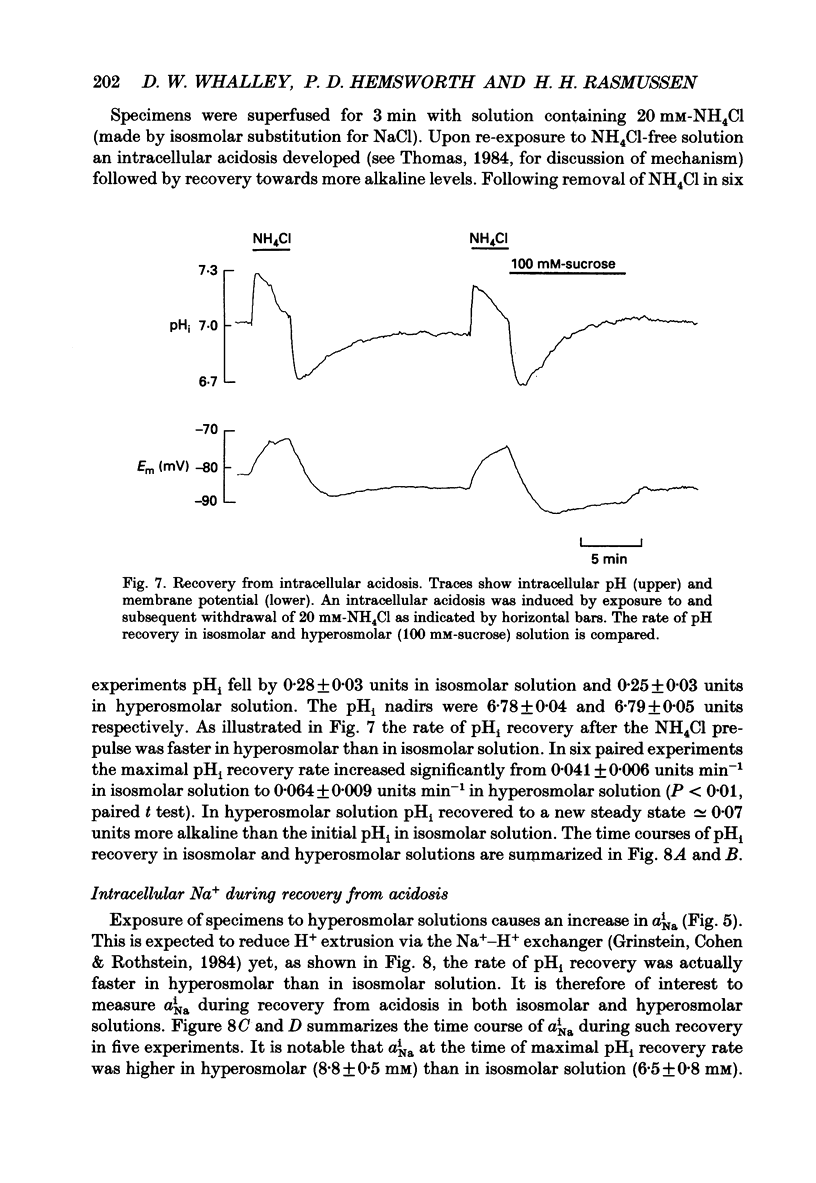
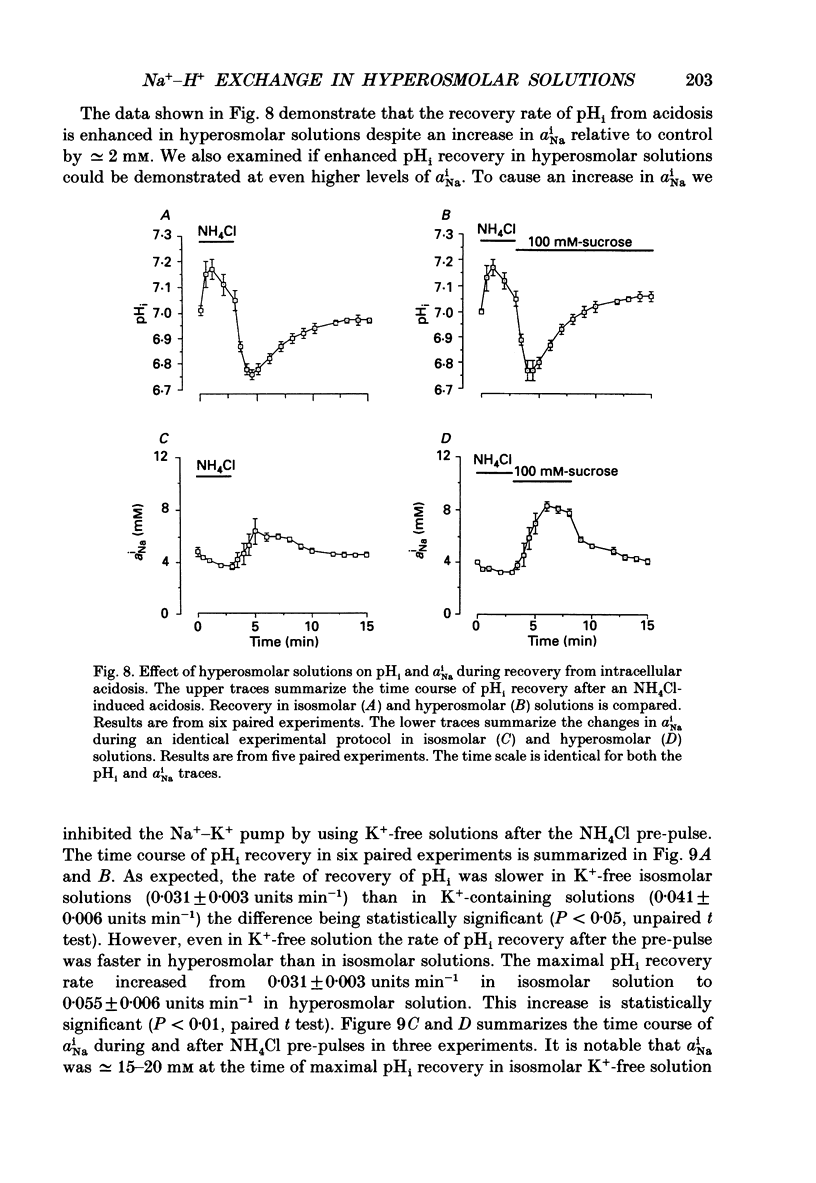
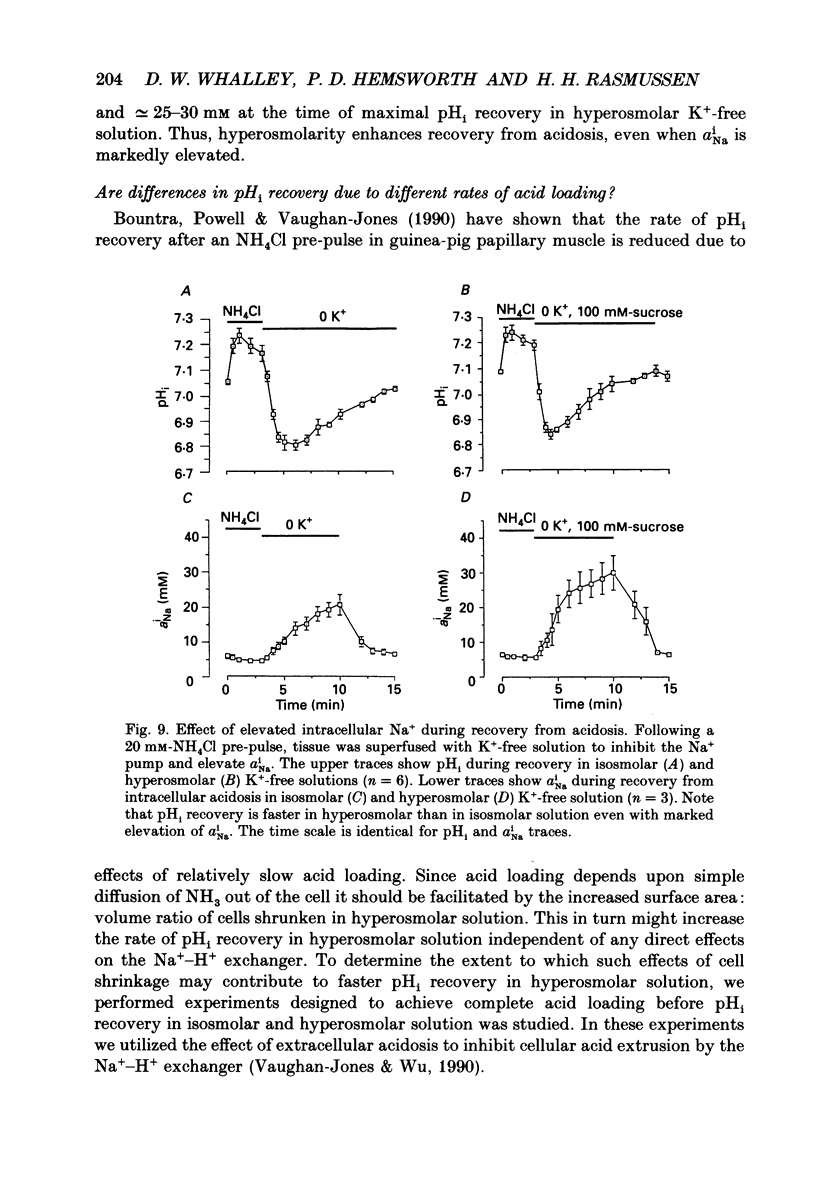
1. The effect on intracellular pH (pHi) and intracellular Na+ activity (aNai) of exposure to hyperosmolar solutions was investigated in guinea-pig ventricular muscle using ion-sensitive microelectrodes. 2. Exposure of tissue to solution made hyperosmolar by the addition of 100 mM-sucrose produced an intracellular alkalinization of 0.10 pH units and hyperpolarization of the membrane potential. 3. When extracellular Na+ was reduced to 15 mM by substitution of NaCl with choline chloride, exposure to hyperosmolar solutions caused a decrease in pHi. Identical experiments using LiCl as the sodium substitute resulted in an increase in pHi of a magnitude similar to that seen at physiological Na+ levels. 4. In the presence of 50 microM-5-(N,N-dimethyl)amiloride (DMA), an inhibitor of Na(+)-H+ exchange, pHi decreased upon exposure to hyperosmolar solution. 5. The recovery of pHi from an intracellular acidosis (induced by brief exposure to NH4Cl) was enhanced in hyperosmolar solution when compared to recovery in isosmolar solution. This enhancement was observed even when aNai was markedly elevated (greater than 25 mM) by inhibition of the Na(+)-K+ pump. 6. There was an increase in aNai during exposure to hyperosmolar solutions. When the Na(+)-K+ pump was inhibited with dihydro-ouabain a component of this increase in aNai was sensitive to DMA. 7. We conclude that exposure of cardiac tissue to hyperosmolar solutions results in an intracellular alkalosis due to activation of the sarcolemmal Na(+)-H+ exchanger. Such changes should be considered when exposure to hyperosmolar solutions is used in the study of excitation-contraction coupling and cardiac muscle mechanics.
Full text
PDF


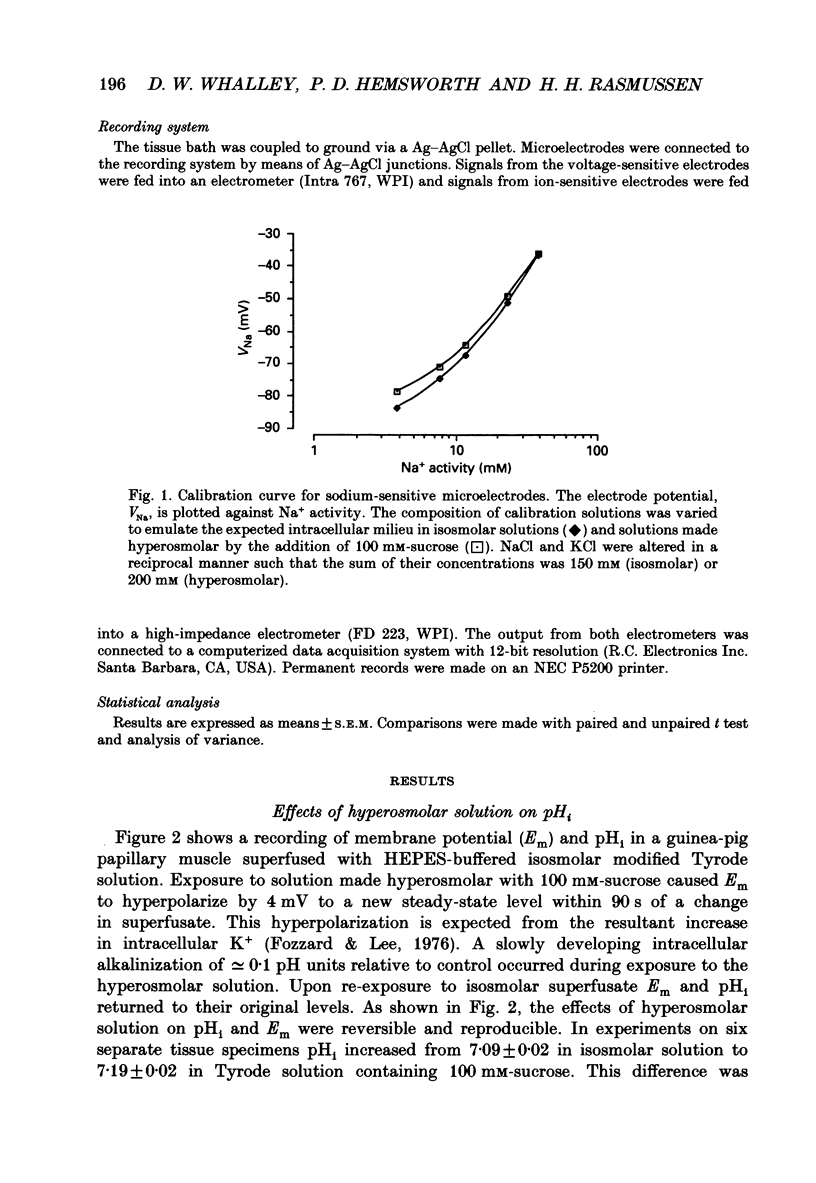
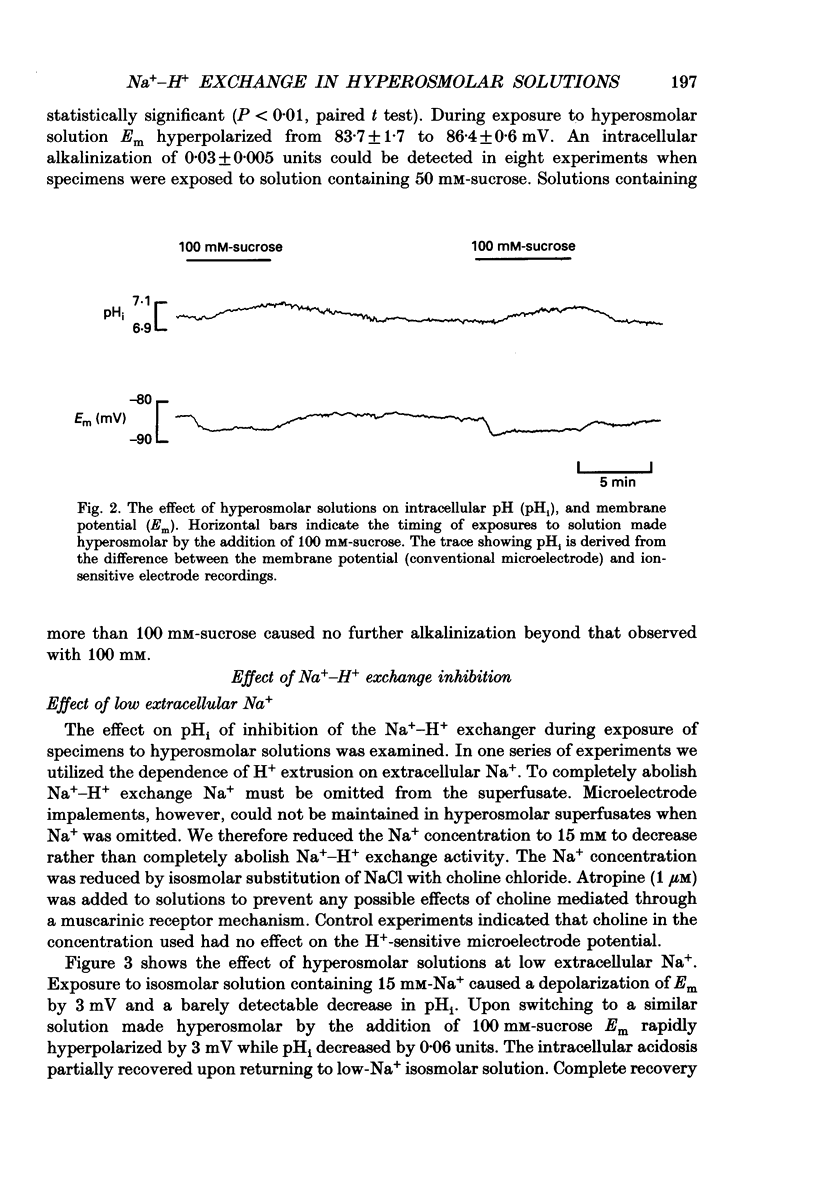
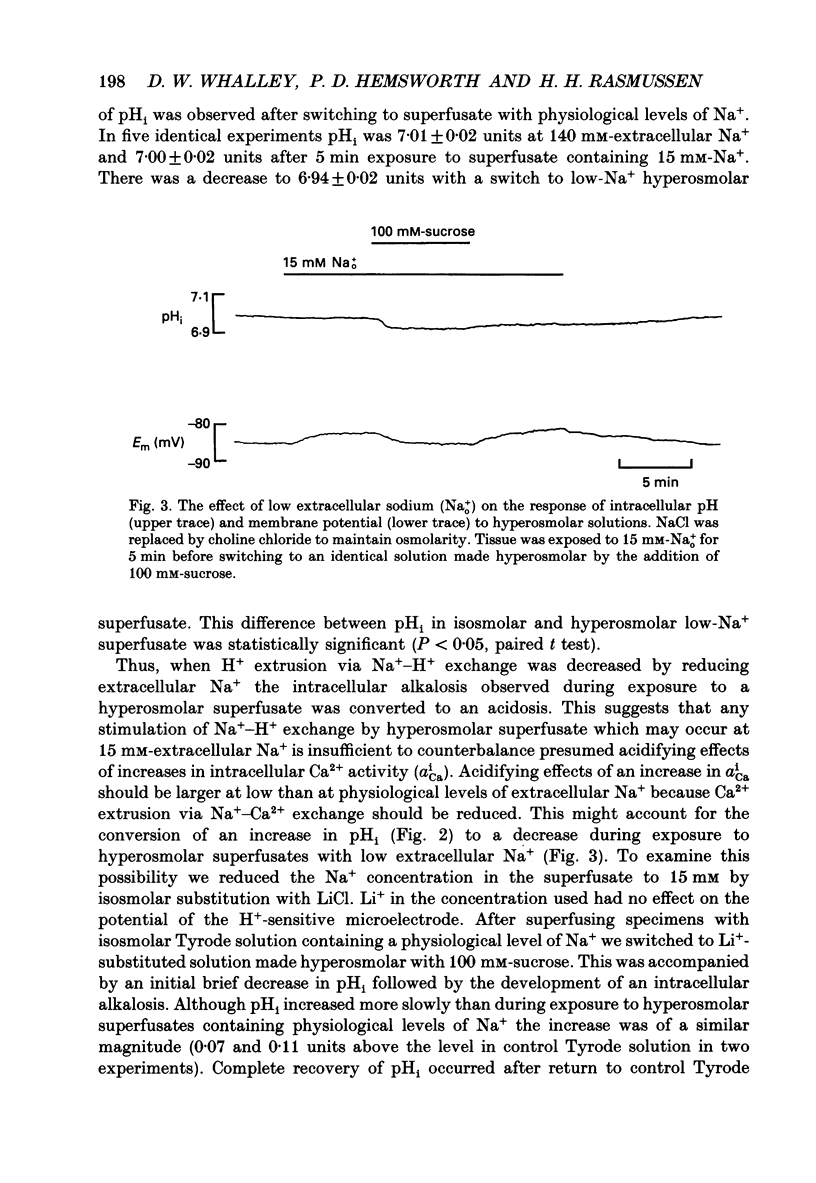
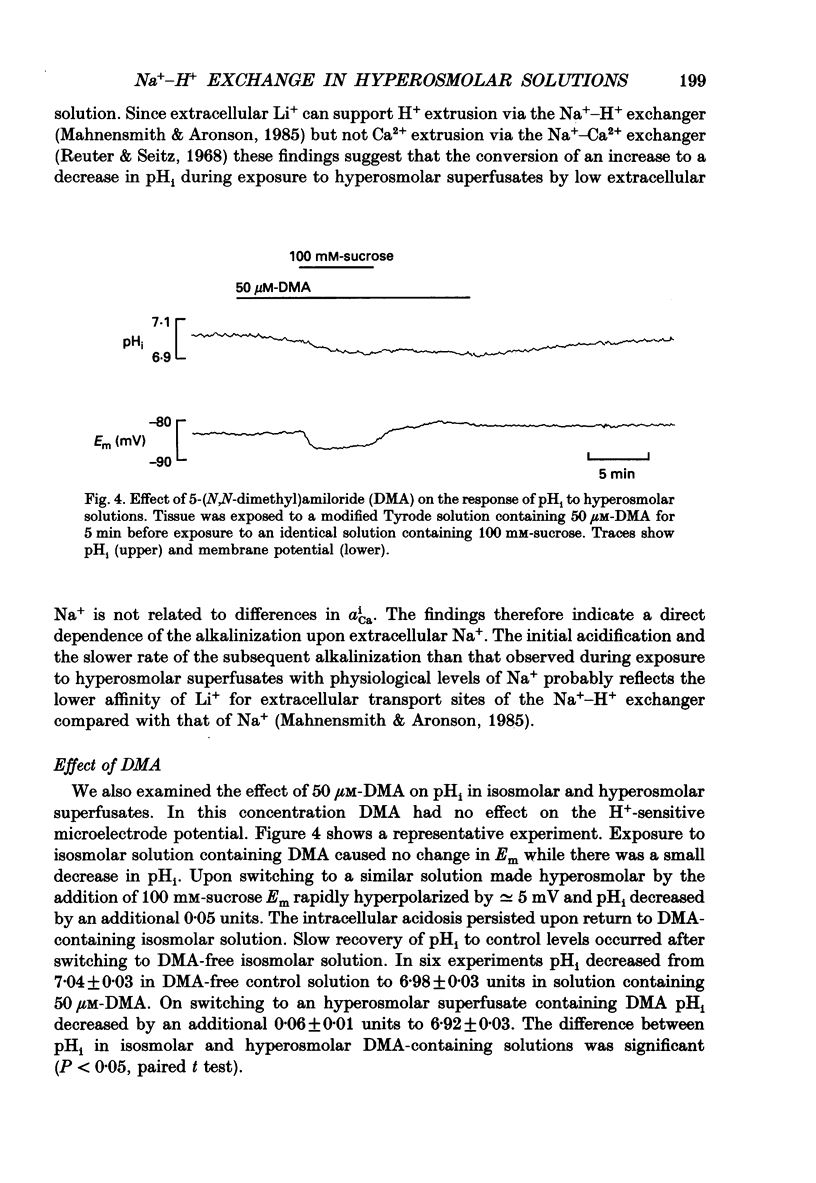






Selected References
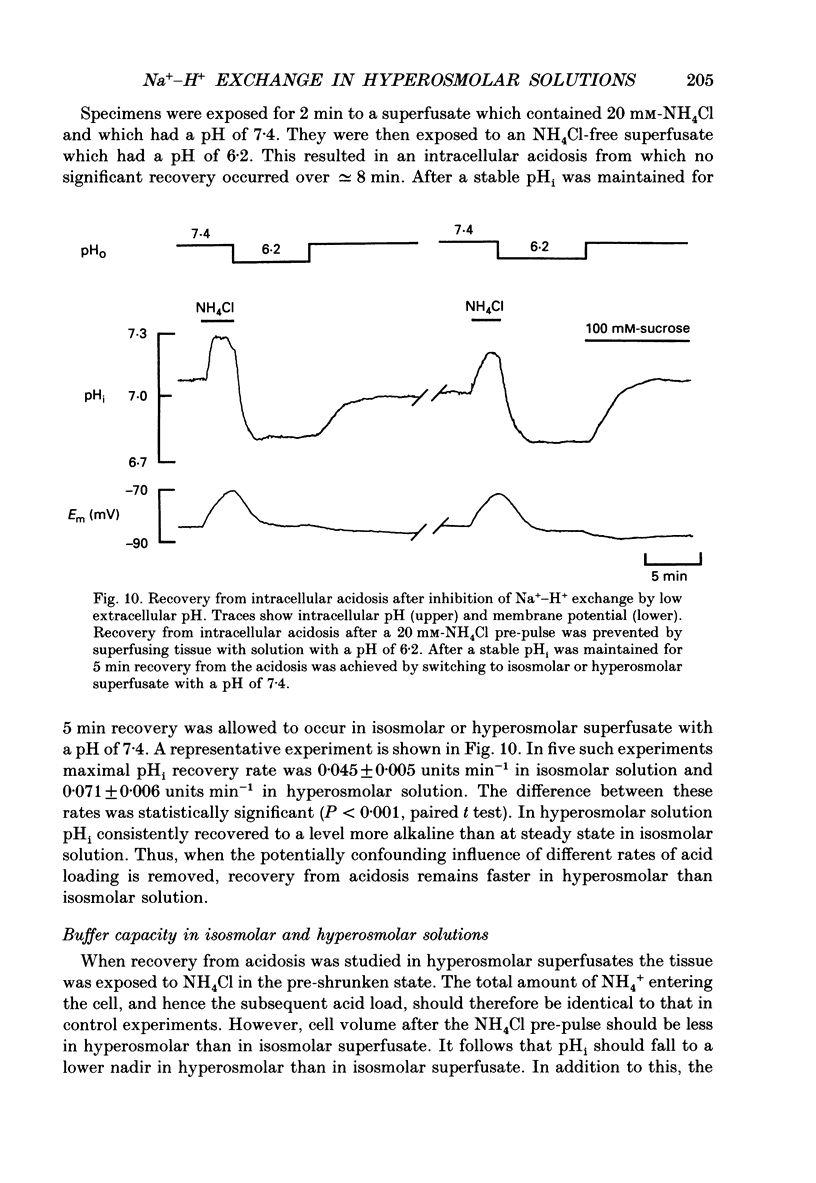
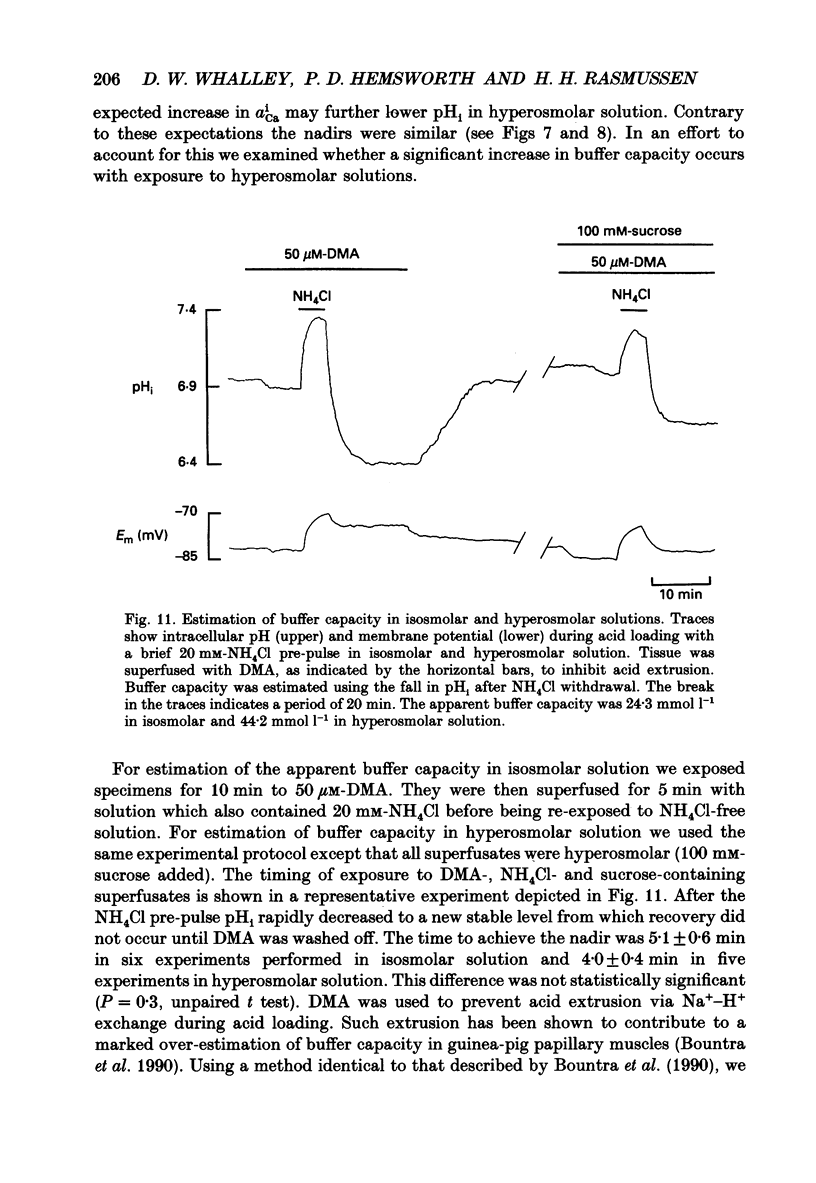
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Morris P. G., Pirolo J. S., Smith G. L. Metabolic consequences of increasing intracellular calcium and force production in perfused ferret hearts. J Physiol. 1986 Jul;376:121–141. doi: 10.1113/jphysiol.1986.sp016145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Smith G. L. The effects of hypertonicity on tension and intracellular calcium concentration in ferret ventricular muscle. J Physiol. 1987 Feb;383:425–439. doi: 10.1113/jphysiol.1987.sp016418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann D., Lanter F., Steiner R. A., Schulthess P., Shijo Y., Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem. 1981 Dec;53(14):2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
- Atkins J. M., Wildenthal K., Horwitz L. D. Cardiovascular responses to hyperosmotic mannitol in anesthetized and conscious dogs. Am J Physiol. 1973 Jul;225(1):132–137. doi: 10.1152/ajplegacy.1973.225.1.132. [DOI] [PubMed] [Google Scholar]
- Aviram A., Pfau A., Czaczkes J. W., Ullmann T. D. Hyperosmolality with hyponatremia, caused by inappropriate administration of mannitol. Am J Med. 1967 Apr;42(4):648–650. doi: 10.1016/0002-9343(67)90066-6. [DOI] [PubMed] [Google Scholar]
- Beyer T., Jepsen L. S., Lüllmann H., Ravens U. Responses to hypertonic solutions in guinea-pig atria: changes in action potentials, force of contraction and calcium content. J Mol Cell Cardiol. 1986 Jan;18(1):81–89. doi: 10.1016/s0022-2828(86)80985-3. [DOI] [PubMed] [Google Scholar]
- Bountra C., Powell T., Vaughan-Jones R. D. Comparison of intracellular pH transients in single ventricular myocytes and isolated ventricular muscle of guinea-pig. J Physiol. 1990 May;424:343–365. doi: 10.1113/jphysiol.1990.sp018071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C., Vaughan-Jones R. D. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989 Nov;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cala P. M. Volume regulation by Amphiuma red blood cells. The membrane potential and its implications regarding the nature of the ion-flux pathways. J Gen Physiol. 1980 Dec;76(6):683–708. doi: 10.1085/jgp.76.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. B., Borg T. K. The collagen network of the heart. Lab Invest. 1979 Mar;40(3):364–372. [PubMed] [Google Scholar]
- Chapman R. A. The effects of changes of the tonicity of the bathing fluid upon the tension generated by atrial trabeculae isolated from the heart of the frog, Rana pipiens. Q J Exp Physiol Cogn Med Sci. 1978 Oct;63(4):301–314. doi: 10.1113/expphysiol.1978.sp002444. [DOI] [PubMed] [Google Scholar]
- Daut J. Inhibition of the sodium pump in guinea-pig ventricular muscle by dihydro-ouabain: effects of external potassium and sodium. J Physiol. 1983 Jun;339:643–662. doi: 10.1113/jphysiol.1983.sp014740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowska K., Baumgarten C. M. Regulation of cellular volume in rabbit ventricular myocytes: bumetanide, chlorothiazide, and ouabain. Am J Physiol. 1991 Jan;260(1 Pt 1):C122–C131. doi: 10.1152/ajpcell.1991.260.1.C122. [DOI] [PubMed] [Google Scholar]
- Ellis D., MacLeod K. T. Sodium-dependent control of intracellular pH in Purkinje fibres of sheep heart. J Physiol. 1985 Feb;359:81–105. doi: 10.1113/jphysiol.1985.sp015576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., Thomas R. C. Direct measurement of the intracellular pH of mammalian cardiac muscle. J Physiol. 1976 Nov;262(3):755–771. doi: 10.1113/jphysiol.1976.sp011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A., Lee C. O. Influence of changes in external potassium and chloride ions on membrane potential and intracellular potassium ion activity in rabbit ventricular muscle. J Physiol. 1976 Apr;256(3):663–689. doi: 10.1113/jphysiol.1976.sp011345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Martin M. M., Recant L. Clinical and metabolic characteristics of hyperosmolar nonketotic coma. Diabetes. 1971 Apr;20(4):228–238. doi: 10.2337/diab.20.4.228. [DOI] [PubMed] [Google Scholar]
- Goethals M. A., Adèle S. M., Brutsaert D. L. Contractility in mammalian heart muscle; calcium and osmolality. Circ Res. 1975 Jan;36(1):27–33. doi: 10.1161/01.res.36.1.27. [DOI] [PubMed] [Google Scholar]
- Green J., Yamaguchi D. T., Kleeman C. R., Muallem S. Cytosolic pH regulation in osteoblasts. Interaction of Na+ and H+ with the extracellular and intracellular faces of the Na+/H+ exchanger. J Gen Physiol. 1988 Aug;92(2):239–261. doi: 10.1085/jgp.92.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Yamaguchi D. T., Kleeman C. R., Muallem S. Selective modification of the kinetic properties of Na+/H+ exchanger by cell shrinkage and swelling. J Biol Chem. 1988 Apr 15;263(11):5012–5015. [PubMed] [Google Scholar]
- Grinstein S., Cohen S. Cytoplasmic [Ca2+] and intracellular pH in lymphocytes. Role of membrane potential and volume-activated Na+/H+ exchange. J Gen Physiol. 1987 Feb;89(2):185–213. doi: 10.1085/jgp.89.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Goetz J. D., Rothstein A. Na+/H+ exchange in volume regulation and cytoplasmic pH homeostasis in lymphocytes. Fed Proc. 1985 Jun;44(9):2508–2512. [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Rothstein A. Cytoplasmic pH regulation in thymic lymphocytes by an amiloride-sensitive Na+/H+ antiport. J Gen Physiol. 1984 Mar;83(3):341–369. doi: 10.1085/jgp.83.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean T., Frelin C., Vigne P., Lazdunski M. The Na+/H+ exchange system in glial cell lines. Properties and activation by an hyperosmotic shock. Eur J Biochem. 1986 Oct 15;160(2):211–219. doi: 10.1111/j.1432-1033.1986.tb09959.x. [DOI] [PubMed] [Google Scholar]
- KOCH-WESER J. Influence of osmolarity of perfusate on contractility of mammalian myocardium. Am J Physiol. 1963 Jun;204:957–962. doi: 10.1152/ajplegacy.1963.204.6.957. [DOI] [PubMed] [Google Scholar]
- Kaila K., Vaughan-Jones R. D. Influence of sodium-hydrogen exchange on intracellular pH, sodium and tension in sheep cardiac Purkinje fibres. J Physiol. 1987 Sep;390:93–118. doi: 10.1113/jphysiol.1987.sp016688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish J. C. The inhibitory effects of monovalent ions on force development in detergent-skinned ventricular muscle from guinea-pig. J Physiol. 1984 Jul;352:353–374. doi: 10.1113/jphysiol.1984.sp015296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Lado M. G., Sheu S. S., Fozzard H. A. Effects of tonicity on tension and intracellular sodium and calcium activities in sheep heart. Circ Res. 1984 May;54(5):576–585. doi: 10.1161/01.res.54.5.576. [DOI] [PubMed] [Google Scholar]
- Little G. R., Sleator W. W. Calcium exchange and contraction strength of guinea pig atrium in normal and hypertonic media. J Gen Physiol. 1969 Oct;54(4):494–511. doi: 10.1085/jgp.54.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnensmith R. L., Aronson P. S. The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res. 1985 Jun;56(6):773–788. doi: 10.1161/01.res.56.6.773. [DOI] [PubMed] [Google Scholar]
- Orchard C. H., Kentish J. C. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990 Jun;258(6 Pt 1):C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Pine M. B., Brooks W. W., Nosta J. J., Abelmann W. H. Hydrostatic forces limit swelling of rat ventricular myocardium. Am J Physiol. 1981 Nov;241(5):H740–H747. doi: 10.1152/ajpheart.1981.241.5.H740. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol. 1984 Sep;354:3P–22P. doi: 10.1113/jphysiol.1984.sp015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Eisner D. A., Lederer W. J. Effects of changes of intracellular pH on contraction in sheep cardiac Purkinje fibers. J Gen Physiol. 1987 Jun;89(6):1015–1032. doi: 10.1085/jgp.89.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. Extracellular H+ inactivation of Na(+)-H+ exchange in the sheep cardiac Purkinje fibre. J Physiol. 1990 Sep;428:441–466. doi: 10.1113/jphysiol.1990.sp018221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenthal K., Mierzwiak D. S., Mitchell J. H. Acute effects of increased serum osmolality on left ventricular performance. Am J Physiol. 1969 Apr;216(4):898–904. doi: 10.1152/ajplegacy.1969.216.4.898. [DOI] [PubMed] [Google Scholar]


