Abstract
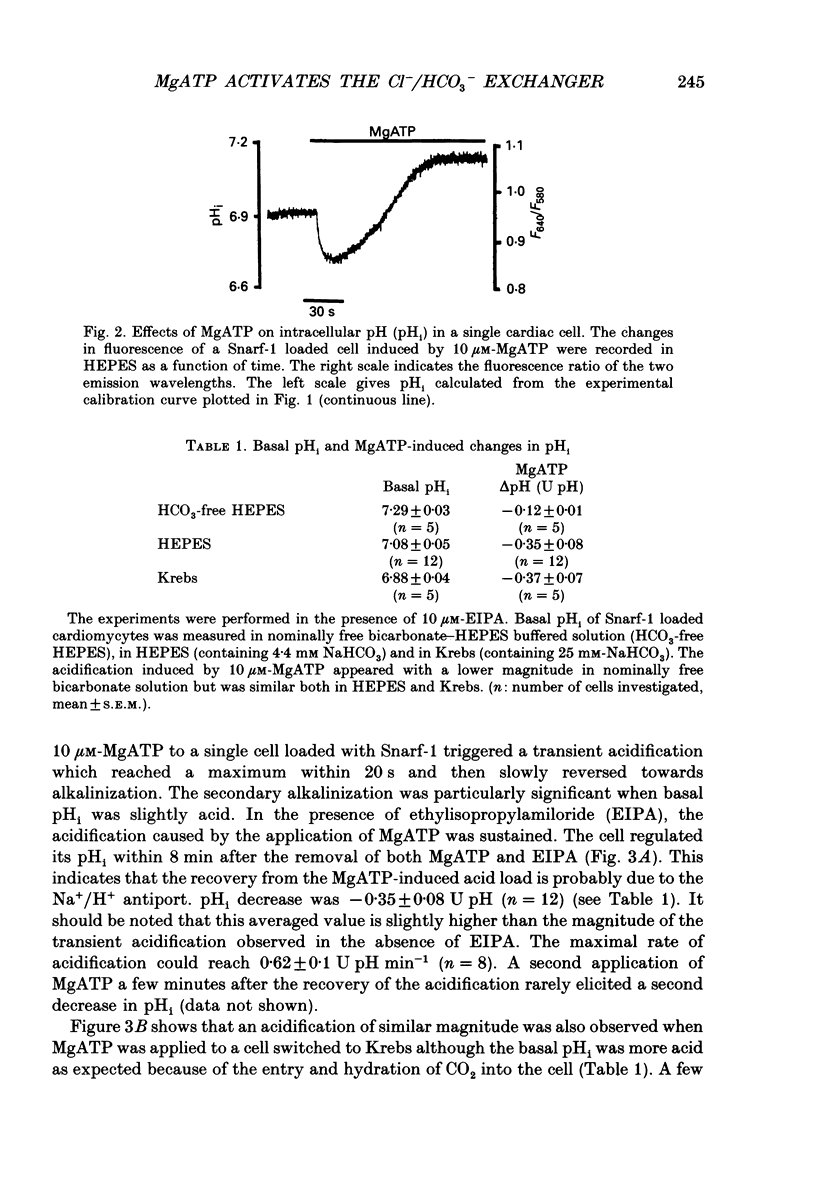
1. The effect of extracellular MgATP on cytosolic pH (pHi) was investigated in single rat cardiac cells loaded with the pH-sensitive probe Snarf-1. 2. Basal pHi in HEPES-buffered solution (containing 4.4 mM-NaHCO3) was 7.08. MgATP induced a transient acidification followed by an alkalinization. The latter is prevented by ethylisopropylamiloride (EIPA) and has been attributed to the activation of the Na+/H+ antiport. The MgATP-induced acidification reached a maximal value of 0.42 +/- 0.03 pH units (U pH). It was concentration dependent with a K0.5 of 2.6 microM-MgATP. This acidification was also observed with the same magnitude in the presence of the more physiological Krebs-bicarbonate buffer but was greatly reduced in nominally HCO3-free HEPES. 3. The MgATP-induced acidification was prevented by 4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid (DIDS), probenecid and ethacrynic acid but not by bumetanide. It was dependent upon the external chloride concentration. The K0.5[Cl-] was 9 mM and the maximal acidification required 60 mM-Cl-. 4. MgATP accelerated the recovery from an alkalinization triggered by a pulse of NH4Cl. The nucleotide also facilitated the efflux of HCO3- when the cell was switched from a Krebs-bicarbonate buffer gassed with 5% CO2 to an HEPES buffer. 5. The acidification was only evoked by MgATP and its poorly hydrolysable analogues but not by the other nucleotides (ADP, GTP (guanosine triphosphate), CTP (cytidine triphosphate) UTP (urodine triphosphate), ITP (inositol triphosphate) nor by adenosine. It required the presence of Mg2+ ions. 6. These results provide evidence that MgATP activates the Cl-/HCO3- exchanger and that this activation accounts for the acidification. Such an activation could not be related to the P1- or the P2-purinergic receptors since it requires triphosphate adenylic compounds and Mg2+ ions. This leads us to suggest the existence of a putative P3-type of purinergic receptor.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addanki A., Cahill F. D., Sotos J. F. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem. 1968 May 10;243(9):2337–2348. [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. The effect of loop diuretics on Cl- transport in smooth muscle of the guinea-pig vas deferens and taenia from the caecum. J Physiol. 1990 Feb;421:33–53. doi: 10.1113/jphysiol.1990.sp017932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S., Reinisch L., Beebe D. C. Intracellular pH measurement using single excitation-dual emission fluorescence ratios. Am J Physiol. 1990 Jan;258(1 Pt 1):C171–C178. doi: 10.1152/ajpcell.1990.258.1.C171. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Meghji P. The effect of adenyl compounds on the rat heart. Br J Pharmacol. 1983 May;79(1):211–218. doi: 10.1111/j.1476-5381.1983.tb10514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T., Williams C. A. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J Physiol. 1977 Jun;268(2):371–390. doi: 10.1113/jphysiol.1977.sp011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin C., Vigne P., Ladoux A., Lazdunski M. The regulation of the intracellular pH in cells from vertebrates. Eur J Biochem. 1988 May 16;174(1):3–14. doi: 10.1111/j.1432-1033.1988.tb14055.x. [DOI] [PubMed] [Google Scholar]
- Ganz M. B., Boyarsky G., Sterzel R. B., Boron W. F. Arginine vasopressin enhances pHi regulation in the presence of HCO3- by stimulating three acid-base transport systems. Nature. 1989 Feb 16;337(6208):648–651. doi: 10.1038/337648a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Yamaguchi D. T., Kleeman C. R., Muallem S. Cytosolic pH regulation in osteoblasts. Regulation of anion exchange by intracellular pH and Ca2+ ions. J Gen Physiol. 1990 Jan;95(1):121–145. doi: 10.1085/jgp.95.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazono T., Takeshige K., Cragoe E. J., Jr, Minakami S. Involvement of calcium and protein kinase C in the activation of the Na+/H+ exchanger in cultured bovine aortic endothelial cells stimulated by extracellular ATP. Biochim Biophys Acta. 1989 Sep 19;1013(2):152–158. doi: 10.1016/0167-4889(89)90043-8. [DOI] [PubMed] [Google Scholar]
- Legssyer A., Poggioli J., Renard D., Vassort G. ATP and other adenine compounds increase mechanical activity and inositol trisphosphate production in rat heart. J Physiol. 1988 Jul;401:185–199. doi: 10.1113/jphysiol.1988.sp017157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Jacob R., Piwnica-Worms D., Lieberman M. (Na + K + 2Cl) cotransport in cultured embryonic chick heart cells. Am J Physiol. 1987 Nov;253(5 Pt 1):C721–C730. doi: 10.1152/ajpcell.1987.253.5.C721. [DOI] [PubMed] [Google Scholar]
- Liu S., Piwnica-Worms D., Lieberman M. Intracellular pH regulation in cultured embryonic chick heart cells. Na(+)-dependent Cl-/HCO3- exchange. J Gen Physiol. 1990 Dec;96(6):1247–1269. doi: 10.1085/jgp.96.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus I. H., Olsnes S. Selective inhibition of sodium-linked and sodium-independent bicarbonate/chloride antiport in Vero cells. J Biol Chem. 1987 Jun 5;262(16):7486–7491. [PubMed] [Google Scholar]
- Muallem S., Loessberg P. A. Intracellular pH-regulatory mechanisms in pancreatic acinar cells. II. Regulation of H+ and HCO3- transporters by Ca2(+)-mobilizing agonists. J Biol Chem. 1990 Aug 5;265(22):12813–12819. [PubMed] [Google Scholar]
- Orchard C. H., Kentish J. C. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990 Jun;258(6 Pt 1):C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms D., Lieberman M. Microfluorometric monitoring of pHi in cultured heart cells: Na+-H+ exchange. Am J Physiol. 1983 May;244(5):C422–C428. doi: 10.1152/ajpcell.1983.244.5.C422. [DOI] [PubMed] [Google Scholar]
- Puceat M., Clement O., Lechene P., Pelosin J. M., Ventura-Clapier R., Vassort G. Neurohormonal control of calcium sensitivity of myofilaments in rat single heart cells. Circ Res. 1990 Aug;67(2):517–524. doi: 10.1161/01.res.67.2.517. [DOI] [PubMed] [Google Scholar]
- Pucéat M., Clément O., Scamps F., Vassort G. Extracellular ATP-induced acidification leads to cytosolic calcium transient rise in single rat cardiac myocytes. Biochem J. 1991 Feb 15;274(Pt 1):55–62. doi: 10.1042/bj2740055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Jobson T. M., Rink T. J. Agonist-evoked changes in cytosolic pH and calcium concentration in human platelets: studies in physiological bicarbonate. J Physiol. 1990 Jan;420:31–45. doi: 10.1113/jphysiol.1990.sp017900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scamps F., Vassort G. Mechanism of extracellular ATP-induced depolarization in rat isolated ventricular cardiomyocytes. Pflugers Arch. 1990 Nov;417(3):309–316. doi: 10.1007/BF00370997. [DOI] [PubMed] [Google Scholar]
- Szatkowski M. S. The effect of extracellular weak acids and bases on the intracellular buffering power of snail neurones. J Physiol. 1989 Feb;409:103–120. doi: 10.1113/jphysiol.1989.sp017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A., Leusen I. Analysis of Cl- -HCO3(-) exchange during recovery from intracellular acidosis in cardiac Purkinje strands. Am J Physiol. 1984 May;246(5 Pt 1):C391–C400. doi: 10.1152/ajpcell.1984.246.5.C391. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. An investigation of chloride-bicarbonate exchange in the sheep cardiac Purkinje fibre. J Physiol. 1986 Oct;379:377–406. doi: 10.1113/jphysiol.1986.sp016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of intracellular pH in cardiac muscle. Ciba Found Symp. 1988;139:23–46. doi: 10.1002/9780470513699.ch3. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. pH dependence of intrinsic H+ buffering power in the sheep cardiac Purkinje fibre. J Physiol. 1990 Jun;425:429–448. doi: 10.1113/jphysiol.1990.sp018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallert M. A., Fröhlich O. Na+-H+ exchange in isolated myocytes from adult rat heart. Am J Physiol. 1989 Aug;257(2 Pt 1):C207–C213. doi: 10.1152/ajpcell.1989.257.2.C207. [DOI] [PubMed] [Google Scholar]


