Abstract
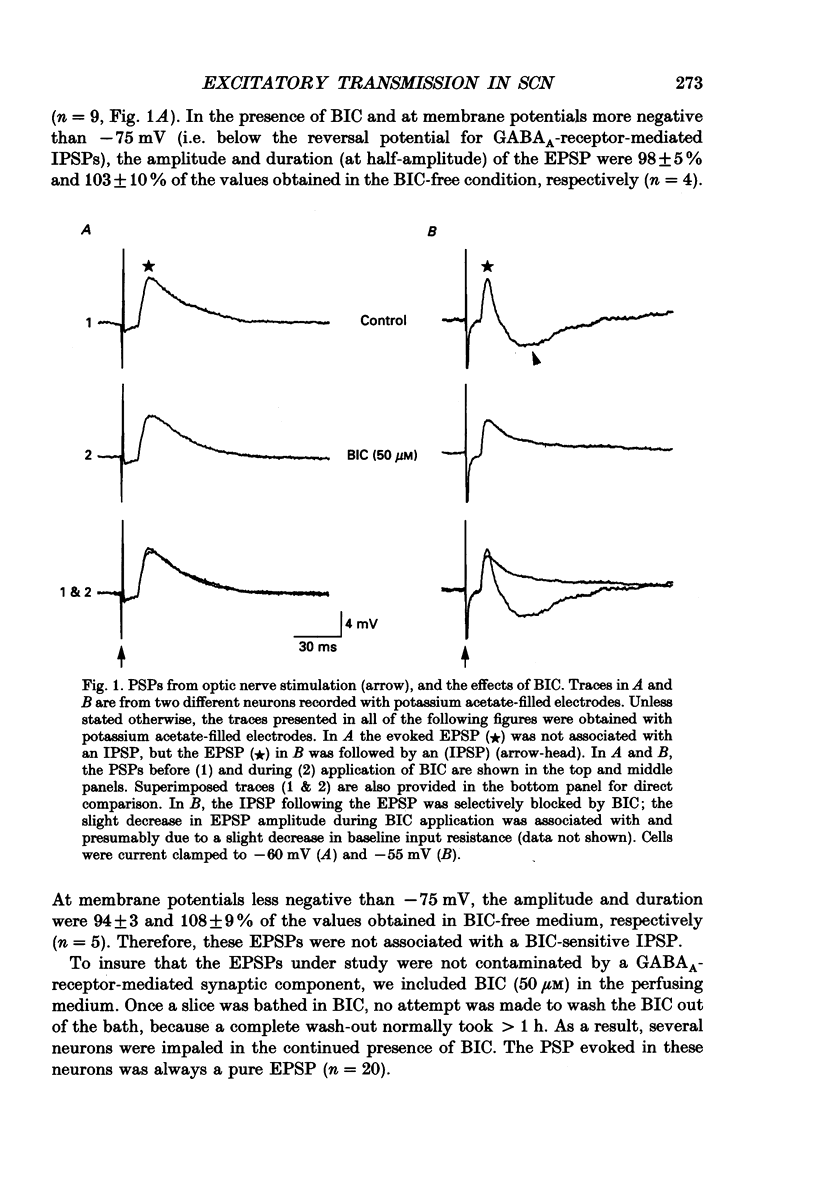
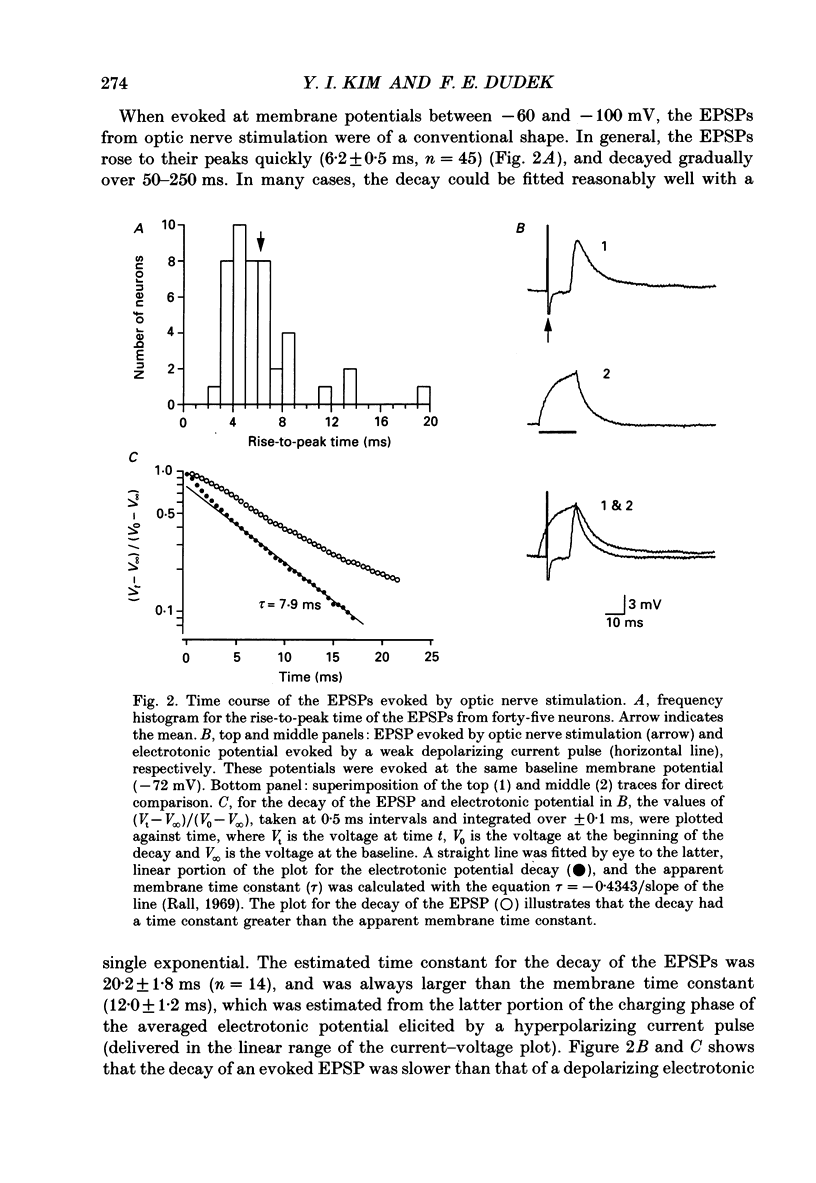
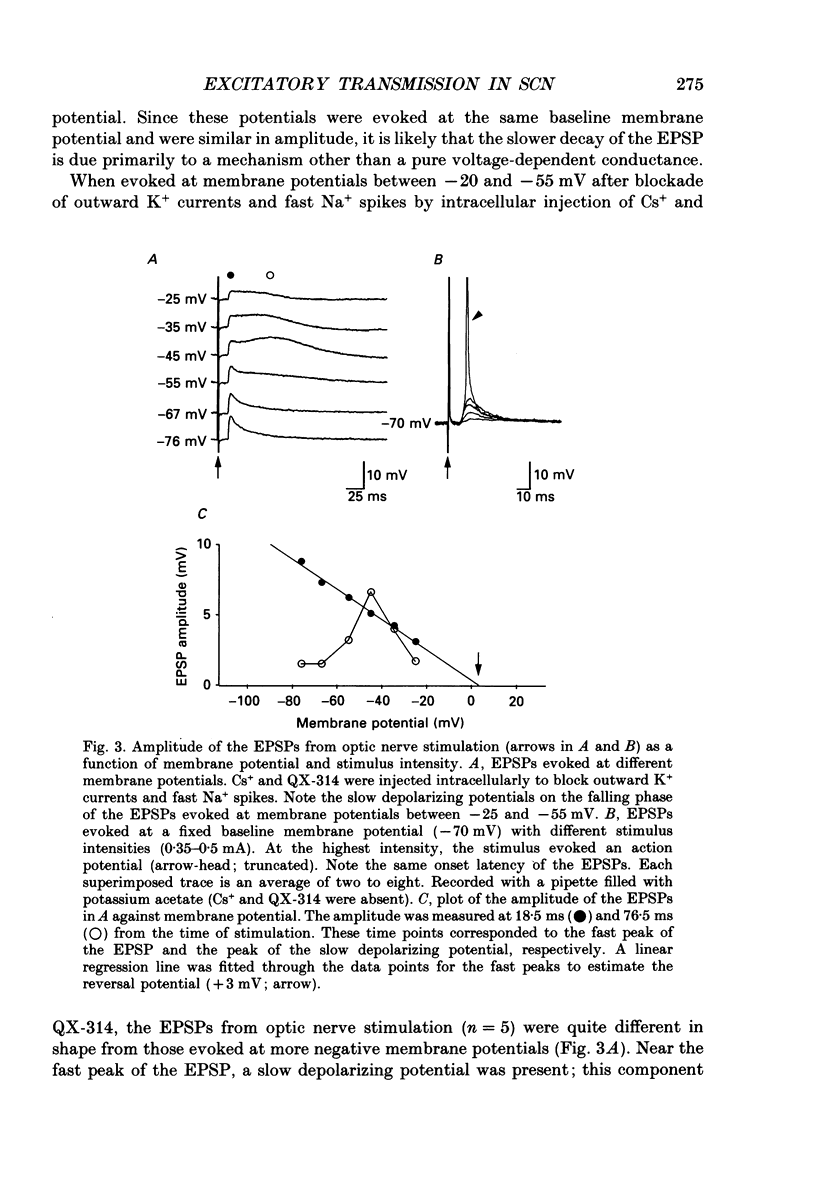
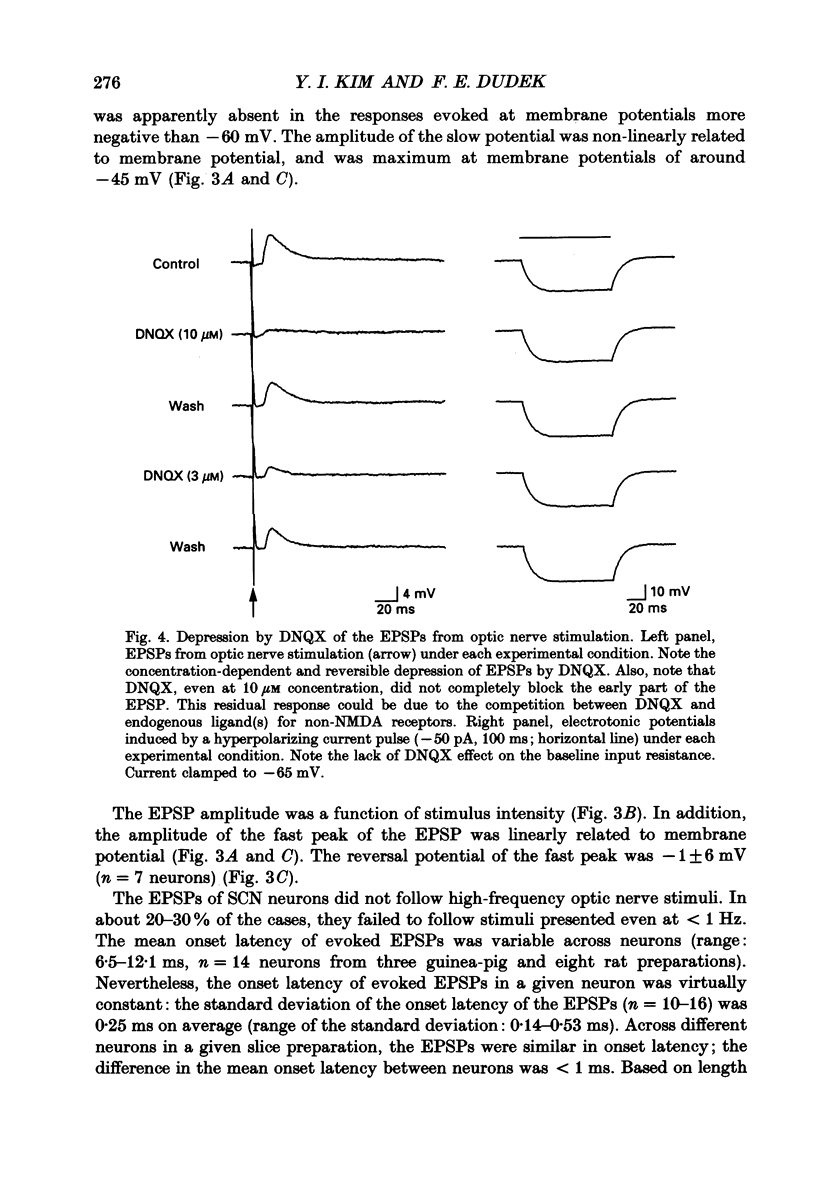
1. To study the synaptic mechanisms of excitatory transmission in the suprachiasmatic nucleus (SCN), we assessed the effects of excitatory amino acid receptor antagonists on excitatory postsynaptic potentials (EPSPs) recorded from SCN neurons in horizontal and parasagittal hypothalamic slice preparations from rats and guinea-pigs. The EPSPs were evoked by electrical stimulation of either optic nerve or a site near the SCN. 2. When evoked at membrane potentials between -60 and -100 mV, the EPSPs from optic nerve stimulation were conventional in shape; they rose to the peak quickly (6.2 +/- 0.5 ms, mean +/- S.E.M.; n = 45) and decayed gradually over 50-250 ms. When evoked at membrane potentials between -20 and -55 mV after blockade of outward K+ currents and fast Na+ spikes by intracellular injection of Cs+ and QX-314 (n = 5 neurons), a slow depolarizing potential emerged near the fast peak of the EPSP. This slow potential, unlike the fast peak, was not linearly related to membrane potential. 3. An antagonist for kainate- and quisqualate-type excitatory amino acid receptors, 6,7-dinitroquinoxaline-2,3-dione (DNQX 1-10 microM), depressed in a concentration-dependent and reversible manner the EPSPs evoked by optic nerve stimulation at membrane potentials between -60 and -100 mV (n = 9). The effects of DNQX were not associated with any significant changes in the baseline input resistance or membrane potential of the postsynaptic neurons. The selective N-methyl-D-aspartate (NMDA) receptor antagonist, DL-2-amino-5-phosphonopentanoic acid (AP5, 50-100 microM), did not affect significantly and consistently the EPSPs evoked at these membrane potentials (n = 7). On the other hand, AP5 (50 microM) blocked or depressed the slow depolarizing component of the EPSPs evoked at membrane potentials between -20 and -55 mV (n = 4). No significant changes in baseline input resistance or membrane potential accompanied the effects of AP5. 4. Stimulation of a site lateral or dorsocaudal to the SCN evoked EPSPs distinct from those evoked by optic nerve stimulation. Again, DNQX (0.3-10 microM) depressed the EPSPs evoked at membrane potentials between -60 and -100 mV (n = 4) whereas AP5 (50 microM) had no effect (n = 5). When evoked at less negative membrane potentials (i.e. -20 to -55 mV) after intracellular injection of Cs+ and QX-314, the EPSPs had a slow depolarizing potential, similar to the EPSPs from optic nerve stimulation.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
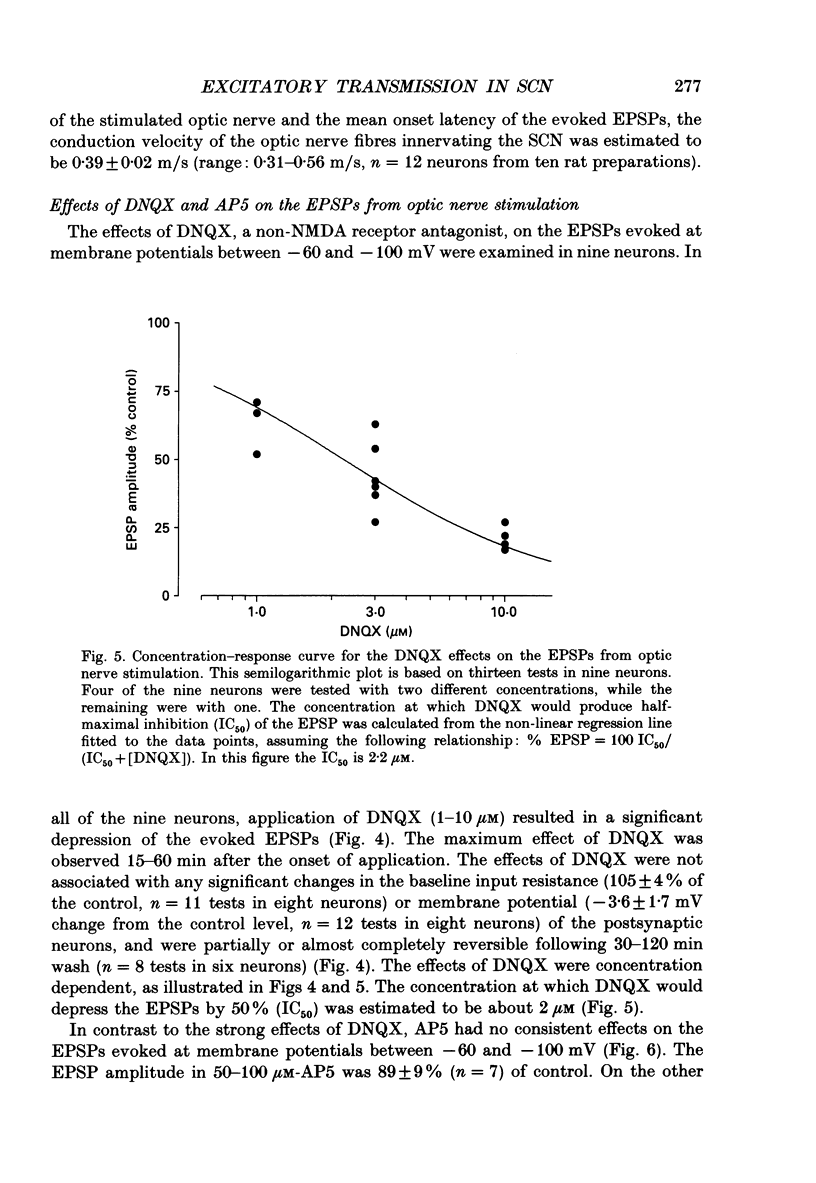
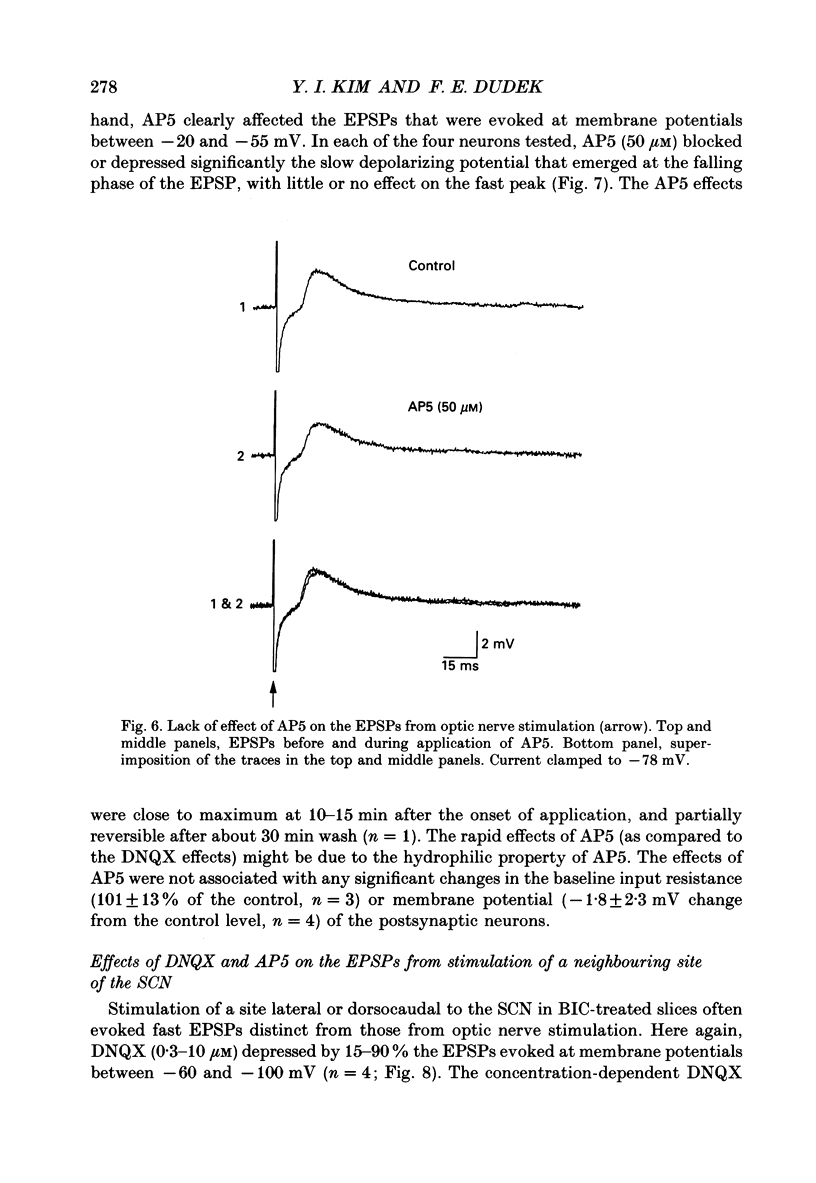
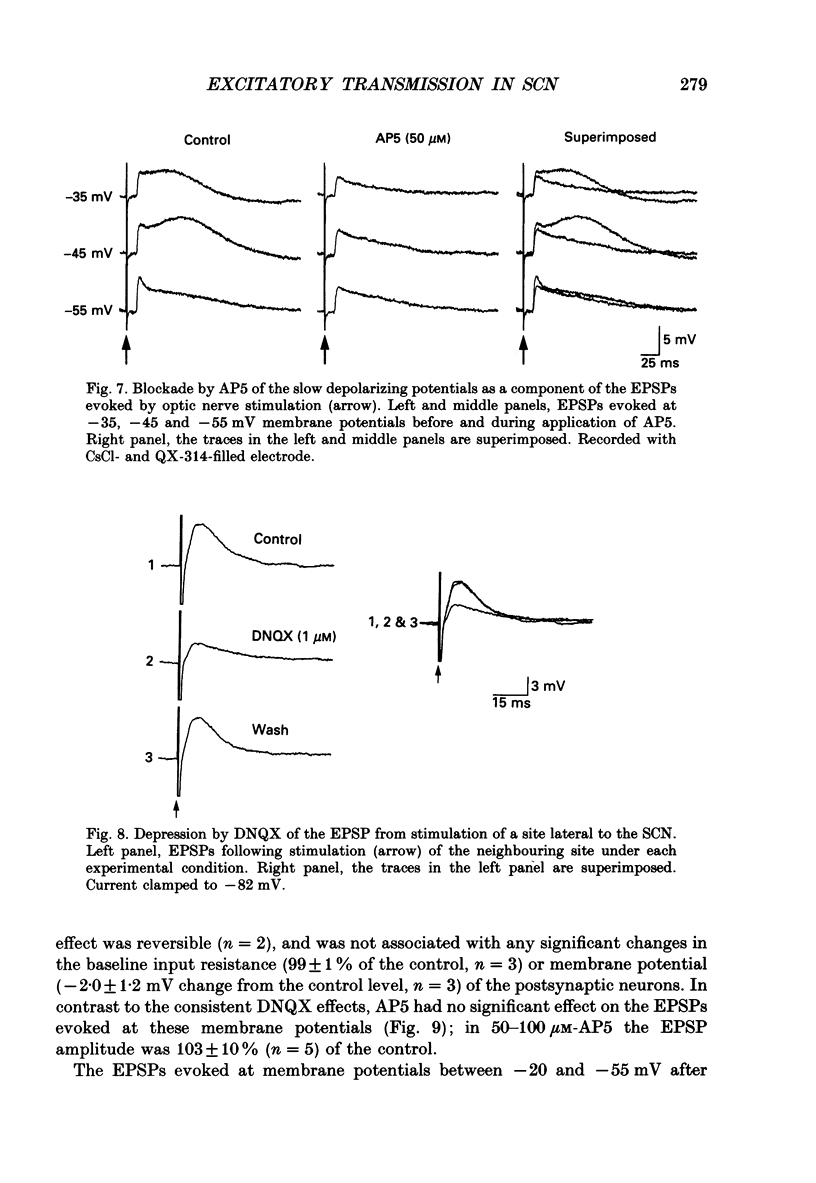
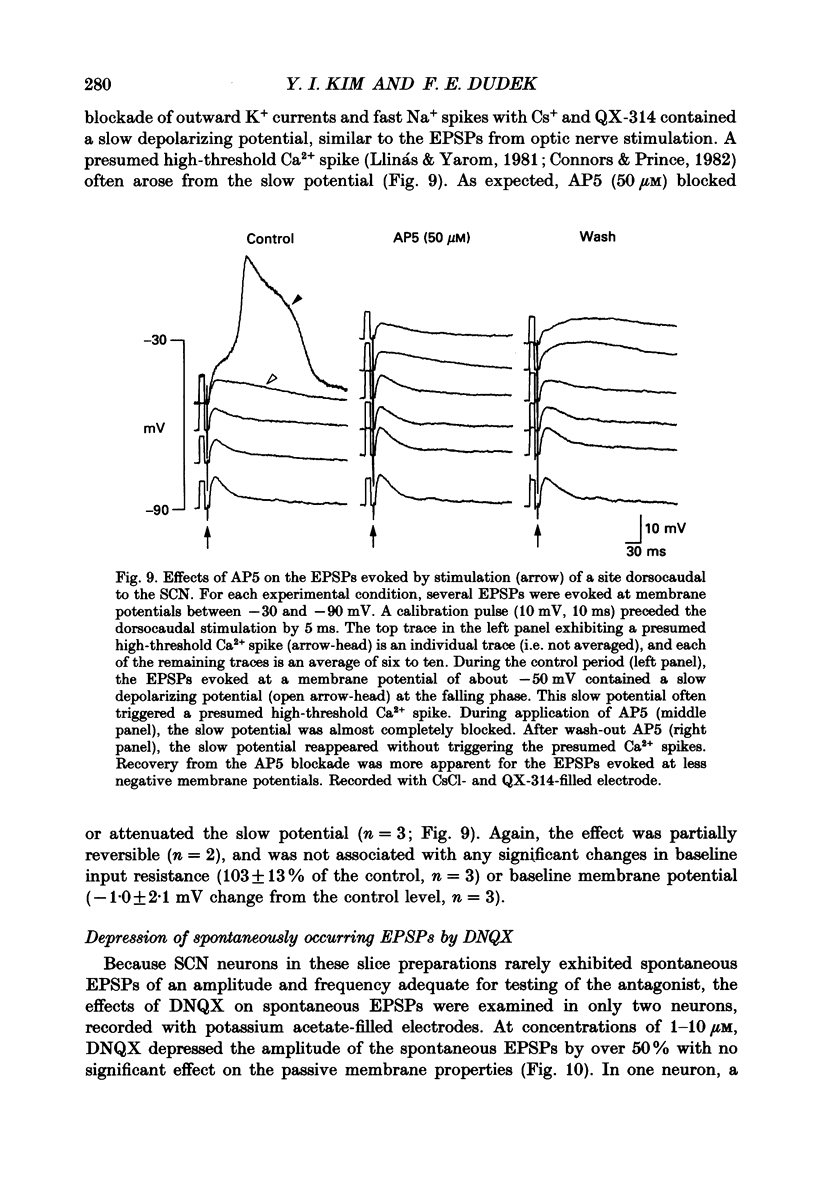
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birch P. J., Grossman C. J., Hayes A. G. 6,7-Dinitro-quinoxaline-2,3-dion and 6-nitro,7-cyano-quinoxaline-2,3-dion antagonise responses to NMDA in the rat spinal cord via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988 Oct 26;156(1):177–180. doi: 10.1016/0014-2999(88)90163-x. [DOI] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Johnston D. Voltage-clamp analysis of mossy fiber synaptic input to hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):487–507. doi: 10.1152/jn.1983.50.2.487. [DOI] [PubMed] [Google Scholar]
- Cahill G. M., Menaker M. Effects of excitatory amino acid receptor antagonists and agonists on suprachiasmatic nucleus responses to retinohypothalamic tract volleys. Brain Res. 1989 Feb 6;479(1):76–82. doi: 10.1016/0006-8993(89)91337-1. [DOI] [PubMed] [Google Scholar]
- Cahill G. M., Menaker M. Kynurenic acid blocks suprachiasmatic nucleus responses to optic nerve stimulation. Brain Res. 1987 Apr 28;410(1):125–129. doi: 10.1016/s0006-8993(87)80032-x. [DOI] [PubMed] [Google Scholar]
- Cahill G. M., Menaker M. Responses of the suprachiasmatic nucleus to retinohypothalamic tract volleys in a slice preparation of the mouse hypothalamus. Brain Res. 1989 Feb 6;479(1):65–75. doi: 10.1016/0006-8993(89)91336-x. [DOI] [PubMed] [Google Scholar]
- Colwell C. S., Ralph M. R., Menaker M. Do NMDA receptors mediate the effects of light on circadian behavior? Brain Res. 1990 Jul 16;523(1):117–120. doi: 10.1016/0006-8993(90)91643-u. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Prince D. A. Effects of local anesthetic QX-314 on the membrane properties of hippocampal pyramidal neurons. J Pharmacol Exp Ther. 1982 Mar;220(3):476–481. [PubMed] [Google Scholar]
- Crunelli V., Kelly J. S., Leresche N., Pirchio M. On the excitatory post-synaptic potential evoked by stimulation of the optic tract in the rat lateral geniculate nucleus. J Physiol. 1987 Mar;384:603–618. doi: 10.1113/jphysiol.1987.sp016472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. P., Hasuo H. Excitatory amino acid-receptor-mediated EPSPs in rat dorsolateral septal nucleus neurones in vitro. J Physiol. 1989 Nov;418:353–365. doi: 10.1113/jphysiol.1989.sp017845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groos G. A., Meijer J. H. Effects of illumination on suprachiasmatic nucleus electrical discharge. Ann N Y Acad Sci. 1985;453:134–146. doi: 10.1111/j.1749-6632.1985.tb11806.x. [DOI] [PubMed] [Google Scholar]
- Groos G., Mason R., Meijer J. Electrical and pharmacological properties of the suprachiasmatic nuclei. Fed Proc. 1983 Aug;42(11):2790–2795. [PubMed] [Google Scholar]
- Güldner F. H. Synapses of optic nerve afferents in the rat suprachiasmatic nucleus. I. Identification, qualitative description, development and distribution. Cell Tissue Res. 1978 Nov 9;194(1):17–35. doi: 10.1007/BF00209231. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Wagoner N., Cowan W. M. An autoradiographic and electron microscopic study of retino-hypothalamic connections. Z Zellforsch Mikrosk Anat. 1972;135(1):1–26. doi: 10.1007/BF00307084. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Lester R. A., Quarum M. L., Parker J. D., Weber E., Jahr C. E. Interaction of 6-cyano-7-nitroquinoxaline-2,3-dione with the N-methyl-D-aspartate receptor-associated glycine binding site. Mol Pharmacol. 1989 May;35(5):565–570. [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981 Jun;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Meijer J. H., Groos G. A., Rusak B. Luminance coding in a circadian pacemaker: the suprachiasmatic nucleus of the rat and the hamster. Brain Res. 1986 Sep 10;382(1):109–118. doi: 10.1016/0006-8993(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Meijer J. H., Rietveld W. J. Neurophysiology of the suprachiasmatic circadian pacemaker in rodents. Physiol Rev. 1989 Jul;69(3):671–707. doi: 10.1152/physrev.1989.69.3.671. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Eichler V. B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972 Jul 13;42(1):201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Lenn N. J. A retinohypothalamic projection in the rat. J Comp Neurol. 1972 Sep;146(1):1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- Moore R. Y. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc. 1983 Aug;42(11):2783–2789. [PubMed] [Google Scholar]
- Nishino H., Kiyomi K., Brooks C. M. The role of suprachiasmatic nuclei of the hypothalamus in the production of circadian rhythm. Brain Res. 1976 Aug 6;112(1):45–59. doi: 10.1016/0006-8993(76)90333-4. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J. 1969 Dec;9(12):1483–1508. doi: 10.1016/S0006-3495(69)86467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph M. R., Foster R. G., Davis F. C., Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990 Feb 23;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Perlow M. J., Ungerleider L. G., Mishkin M., Tamarkin L., Orloff D. G., Hoffman H. J., Klein D. C. Effects of damage to the suprachiasmatic area of the anterior hypothalamus on the daily melatonin and cortisol rhythms in the rhesus monkey. J Neurosci. 1981 Dec;1(12):1414–1425. doi: 10.1523/JNEUROSCI.01-12-01414.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusak B., Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979 Jul;59(3):449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Sawaki Y. Suprachiasmatic nucleus neurones: excitation and inhibition mediated by the direct retino-hypothalamic projection in female rats. Exp Brain Res. 1979 Sep;37(1):127–138. doi: 10.1007/BF01474259. [DOI] [PubMed] [Google Scholar]
- Shibata S., Liou S. Y., Ueki S. Influence of excitatory amino acid receptor antagonists and of baclofen on synaptic transmission in the optic nerve to the suprachiasmatic nucleus in slices of rat hypothalamus. Neuropharmacology. 1986 Apr;25(4):403–409. doi: 10.1016/0028-3908(86)90235-2. [DOI] [PubMed] [Google Scholar]
- Shibata S., Oomura Y., Hattori K., Kita H. Responses of suprachiasmatic nucleus neurons to optic nerve stimulation in rat hypothalamic slice preparation. Brain Res. 1984 Jun 4;302(1):83–89. doi: 10.1016/0006-8993(84)91287-3. [DOI] [PubMed] [Google Scholar]
- Stephan F. K., Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. W., Cowan W. M., Jones E. G. An autoradiographic study of the efferent connections of the ventral lateral geniculate nucleus in the albino rat and the cat. J Comp Neurol. 1974 Jul;156(2):143–163. doi: 10.1002/cne.901560203. [DOI] [PubMed] [Google Scholar]
- Takahashi J. S., Zatz M. Regulation of circadian rhythmicity. Science. 1982 Sep 17;217(4565):1104–1111. doi: 10.1126/science.6287576. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. Glycine modulation of the NMDA receptor/channel complex. Trends Neurosci. 1989 Sep;12(9):349–353. doi: 10.1016/0166-2236(89)90042-8. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C. Factors affecting slow regular firing in the suprachiasmatic nucleus in vitro. J Biol Rhythms. 1990 Spring;5(1):59–75. doi: 10.1177/074873049000500106. [DOI] [PubMed] [Google Scholar]
- Turek F. W. Circadian neural rhythms in mammals. Annu Rev Physiol. 1985;47:49–64. doi: 10.1146/annurev.ph.47.030185.000405. [DOI] [PubMed] [Google Scholar]
- Van den Pol A. N. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980 Jun 15;191(4):661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Wheal H. V., Thomson A. M. The electrical properties of neurones of the rat suprachiasmatic nucleus recorded intracellularly in vitro. Neuroscience. 1984 Sep;13(1):97–104. doi: 10.1016/0306-4522(84)90262-8. [DOI] [PubMed] [Google Scholar]


