Abstract
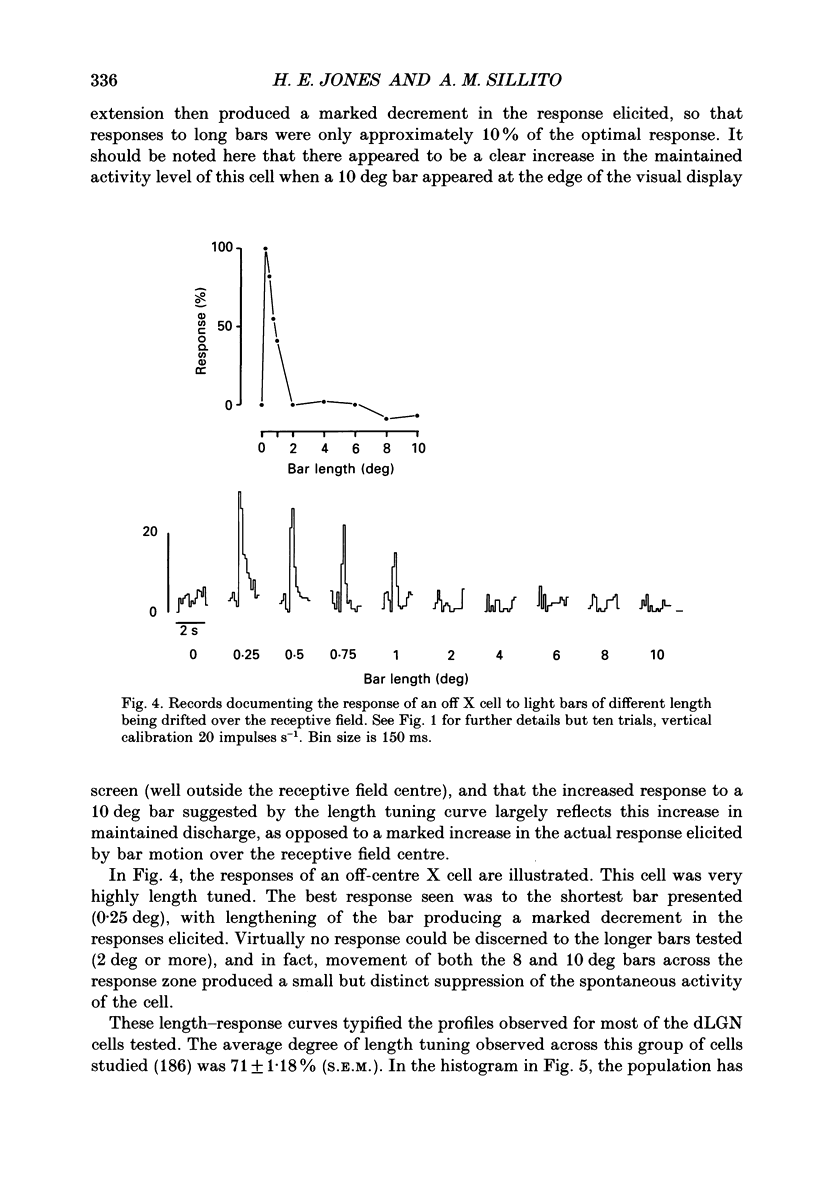
1. In this report we have systematically examined the length-response properties of a large population of cells recorded in the cat dorsal lateral geniculate nucleus (dLGN). The responses of A laminae dLGN cells were assessed by the use of conventional single-unit extracellular recording techniques. The length preference of these cells was examined by plotting multihistogram length tuning curves to moving bars of light. Bar length was randomized in an interleaved fashion under computer control. The other stimulus parameters were standardized within the limits of those routinely used to assess the length preference of cortical cells. 2. The majority of cells (186/198), whose length-response properties are considered in detail in this report, exhibited strong centre-surround antagonism and a mean degree of length tuning equivalent to, or exceeding, that seen in most cortical hypercomplex cells (71 +/- 1.18%, S.E.M., n = 186). 3. The values for X cells (74 +/- 1.41%, S.E.M., n = 100) and Y cells (67 +/- 2.13%, S.E.M., n = 74) were very similar, as were those of the on-centre (71 +/- 1.51%, S.E.M., n = 123) and off-centre (71 +/- 1.85%, S.E.M., n = 63) subgroups. 4. A distinct subgroup of the Y cell population was identified. These comprised the remaining twelve out of the 198 cells examined and their response properties were sufficiently distinct to merit classification as a discrete subpopulation of cells which we have termed nlY cells. They were characterized by very poor levels of both centre-surround antagonism and length tuning, and were most frequently encountered close to laminar borders. Their response properties have been described in detail elsewhere. 5. We quantitatively compared the degree of length tuning seen with moving bars to the strength of centre-surround antagonism assessed with flashing spots. The degree of length tuning did not necessarily follow the level of centre-surround antagonism. 6. Examination of the effects of unilaterally extending bar length to one or other side of the receptive field did not reveal the type of asymmetry frequently seen in cortical hypercomplex cells. 7. The high degree of length tuning seen in this study underlines the potential importance of geniculate response properties to the generation of the length-response properties of cortical hypercomplex cells. The findings are discussed in relation to the synaptic mechanisms contributing to the generation of length tuning at subcortical and cortical levels.
Full text
PDF

















Selected References
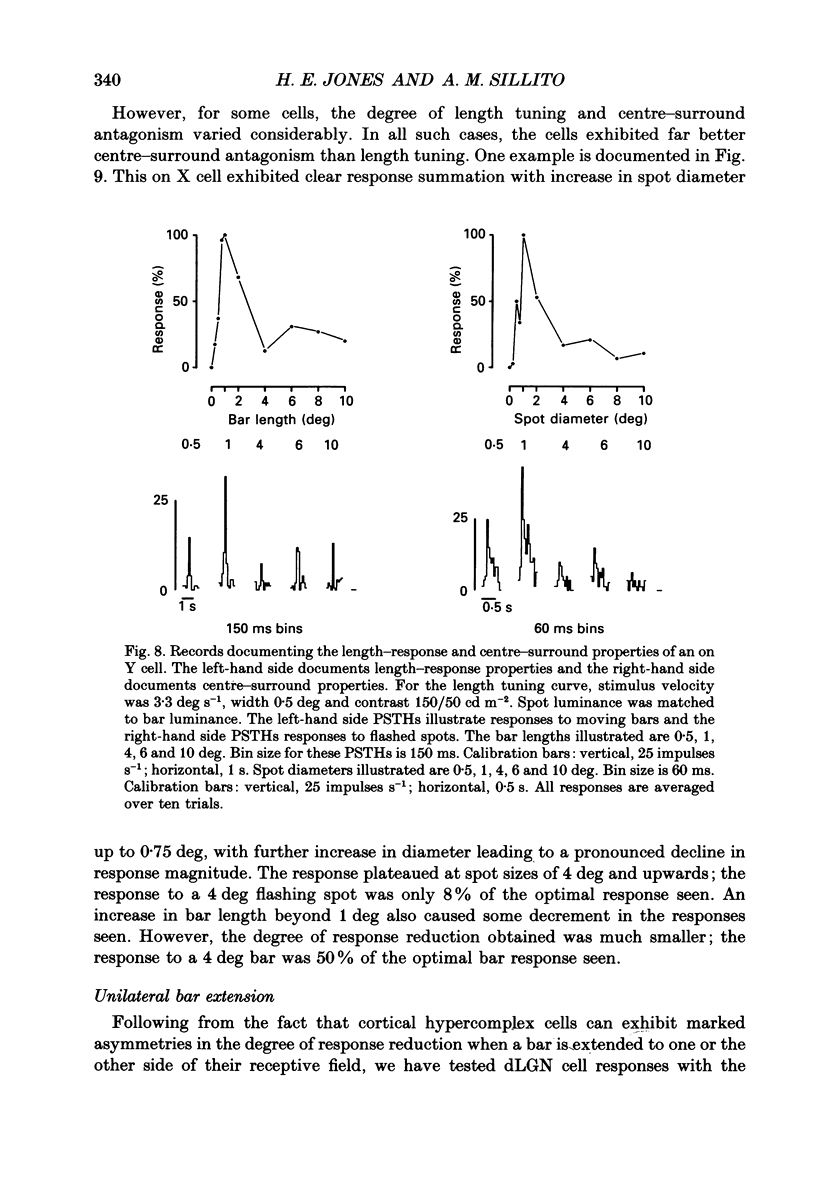
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K., Wolf W., Beckman R. Orientation bias in the response of kitten LGNd neurons to moving light bars. Brain Res. 1983 Feb;282(3):308–313. doi: 10.1016/0165-3806(83)90071-8. [DOI] [PubMed] [Google Scholar]
- Bolz J., Gilbert C. D. Generation of end-inhibition in the visual cortex via interlaminar connections. 1986 Mar 27-Apr 2Nature. 320(6060):362–365. doi: 10.1038/320362a0. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Lee B. B., Vidyasagar T. R. Response of neurons in the cat's lateral geniculate nucleus to moving bars of different length. J Neurosci. 1983 Jan;3(1):108–116. doi: 10.1523/JNEUROSCI.03-01-00108.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels J. D., Norman J. L., Pettigrew J. D. Biases for oriented moving bars in lateral geniculate nucleus neurons of normal and stripe-reared cats. Exp Brain Res. 1977 Aug 31;29(2):155–172. doi: 10.1007/BF00237039. [DOI] [PubMed] [Google Scholar]
- Dreher B. Hypercomplex cells in the cat's striate cortex. Invest Ophthalmol. 1972 May;11(5):355–356. [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald R., Chase R. An improved method for plotting retinal landmarks and focusing the eyes. Vision Res. 1971 Jan;11(1):95–96. doi: 10.1016/0042-6989(71)90207-0. [DOI] [PubMed] [Google Scholar]
- Ferster D. Origin of orientation-selective EPSPs in simple cells of cat visual cortex. J Neurosci. 1987 Jun;7(6):1780–1791. doi: 10.1523/JNEUROSCI.07-06-01780.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Goodwin A. W., Bishop P. O. Spatial summation of responses in receptive fields of single cells in cat striate cortex. Exp Brain Res. 1978 Jun 19;32(2):245–266. doi: 10.1007/BF00239730. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. E., Sillito A. M. A specific subgroup of non-length tuned relay cells in the feline dorsal lateral geniculate nucleus. Exp Brain Res. 1990;82(1):33–39. doi: 10.1007/BF00230835. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kato H., Bishop P. O., Orban G. A. Hypercomplex and simple/complex cell classifications in cat striate cortex. J Neurophysiol. 1978 Sep;41(5):1071–1095. doi: 10.1152/jn.1978.41.5.1071. [DOI] [PubMed] [Google Scholar]
- Murphy P. C., Sillito A. M. Corticofugal feedback influences the generation of length tuning in the visual pathway. Nature. 1987 Oct 22;329(6141):727–729. doi: 10.1038/329727a0. [DOI] [PubMed] [Google Scholar]
- Mustari M. J., Bullier J., Henry G. H. Comparison of response of properties of three types of monosynaptic S-cell in cat striate cortex. J Neurophysiol. 1982 Mar;47(3):439–454. doi: 10.1152/jn.1982.47.3.439. [DOI] [PubMed] [Google Scholar]
- Orban G. A., Kato H., Bishop P. O. Dimensions and properties of end-zone inhibitory areas in receptive fields of hypercomplex cells in cat striate cortex. J Neurophysiol. 1979 May;42(3):833–849. doi: 10.1152/jn.1979.42.3.833. [DOI] [PubMed] [Google Scholar]
- Orban G. A., Kato H., Bishop P. O. End-zone region in receptive fields of hypercomplex and other striate neurons in the cat. J Neurophysiol. 1979 May;42(3):818–832. doi: 10.1152/jn.1979.42.3.818. [DOI] [PubMed] [Google Scholar]
- Rose D. Mechanisms underlying the receptive field properties of neurons in cat visual cortex. Vision Res. 1979;19(5):533–544. doi: 10.1016/0042-6989(79)90138-x. [DOI] [PubMed] [Google Scholar]
- Rose D. Responses of single units in cat visual cortex to moving bars of light as a function of bar length. J Physiol. 1977 Sep;271(1):1–23. doi: 10.1113/jphysiol.1977.sp011987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller P. H., Finlay B. L., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. I. Spatiotemporal organization of receptive fields. J Neurophysiol. 1976 Nov;39(6):1288–1319. doi: 10.1152/jn.1976.39.6.1288. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Oct 24;277(1):63–77. doi: 10.1016/0006-8993(83)90908-3. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Versiani V. The contribution of excitatory and inhibitory inputs to the length preference of hypercomplex cells in layers II and III of the cat's striate cortex. J Physiol. 1977 Dec;273(3):775–790. doi: 10.1113/jphysiol.1977.sp012123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W., Pöppel E., Creutzfeldt O. Inhibitory interaction in the cat's lateral geniculate nucleus. Exp Brain Res. 1972;14(2):210–226. doi: 10.1007/BF00234800. [DOI] [PubMed] [Google Scholar]
- So Y. T., Shapley R. Spatial properties of X and Y cells in the lateral geniculate nucleus of the cat and conduction veolcities of their inputs. Exp Brain Res. 1979 Aug 1;36(3):533–550. doi: 10.1007/BF00238521. [DOI] [PubMed] [Google Scholar]
- Soodak R. E., Shapley R. M., Kaplan E. Linear mechanism of orientation tuning in the retina and lateral geniculate nucleus of the cat. J Neurophysiol. 1987 Aug;58(2):267–275. doi: 10.1152/jn.1987.58.2.267. [DOI] [PubMed] [Google Scholar]
- Vidyasagar T. R., Heide W. Geniculate orientation biases seen with moving sine wave gratings: implications for a model of simple cell afferent connectivity. Exp Brain Res. 1984;57(1):176–200. doi: 10.1007/BF00231146. [DOI] [PubMed] [Google Scholar]
- Vidyasagar T. R., Urbas J. V. Orientation sensitivity of cat LGN neurones with and without inputs from visual cortical areas 17 and 18. Exp Brain Res. 1982;46(2):157–169. doi: 10.1007/BF00237172. [DOI] [PubMed] [Google Scholar]
- Yamane S., Maske R., Bishop P. O. Properties of end-zone inhibition of hypercomplex cells in cat striate cortex. Exp Brain Res. 1985;60(1):200–203. doi: 10.1007/BF00237034. [DOI] [PubMed] [Google Scholar]


