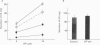Abstract
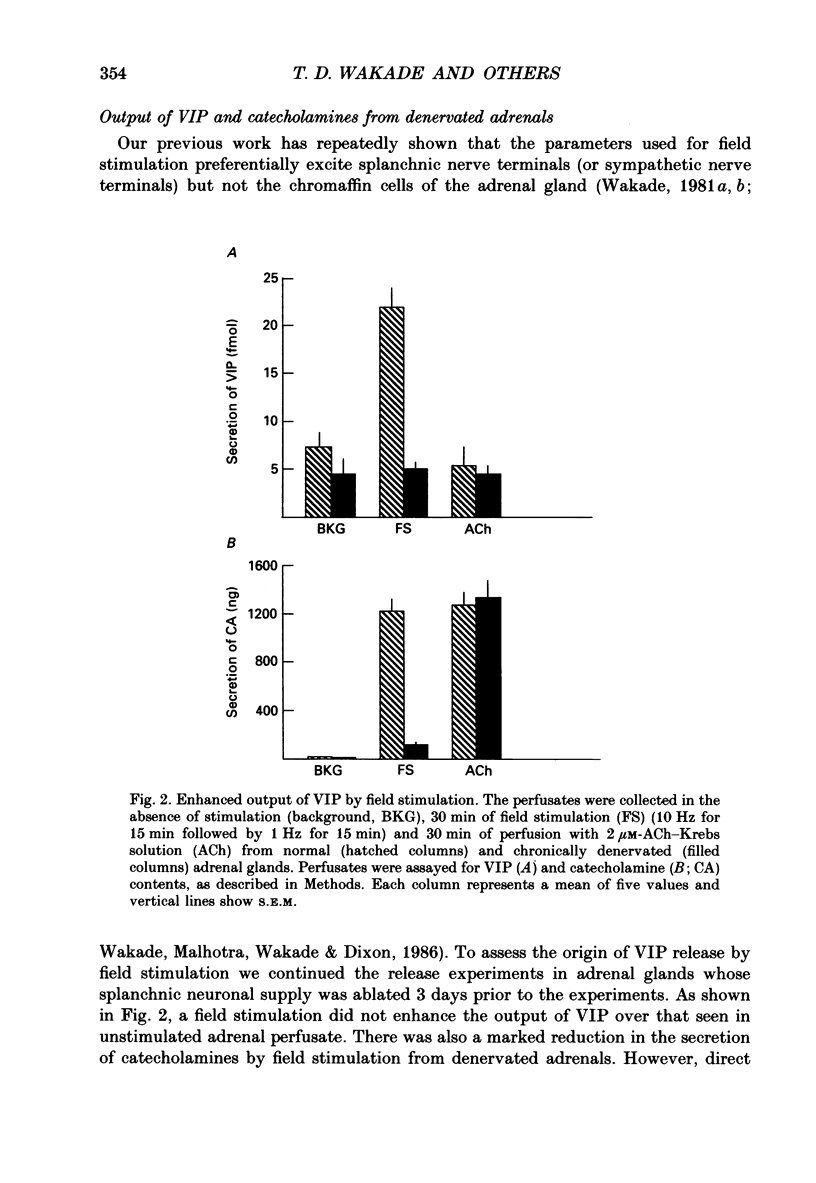
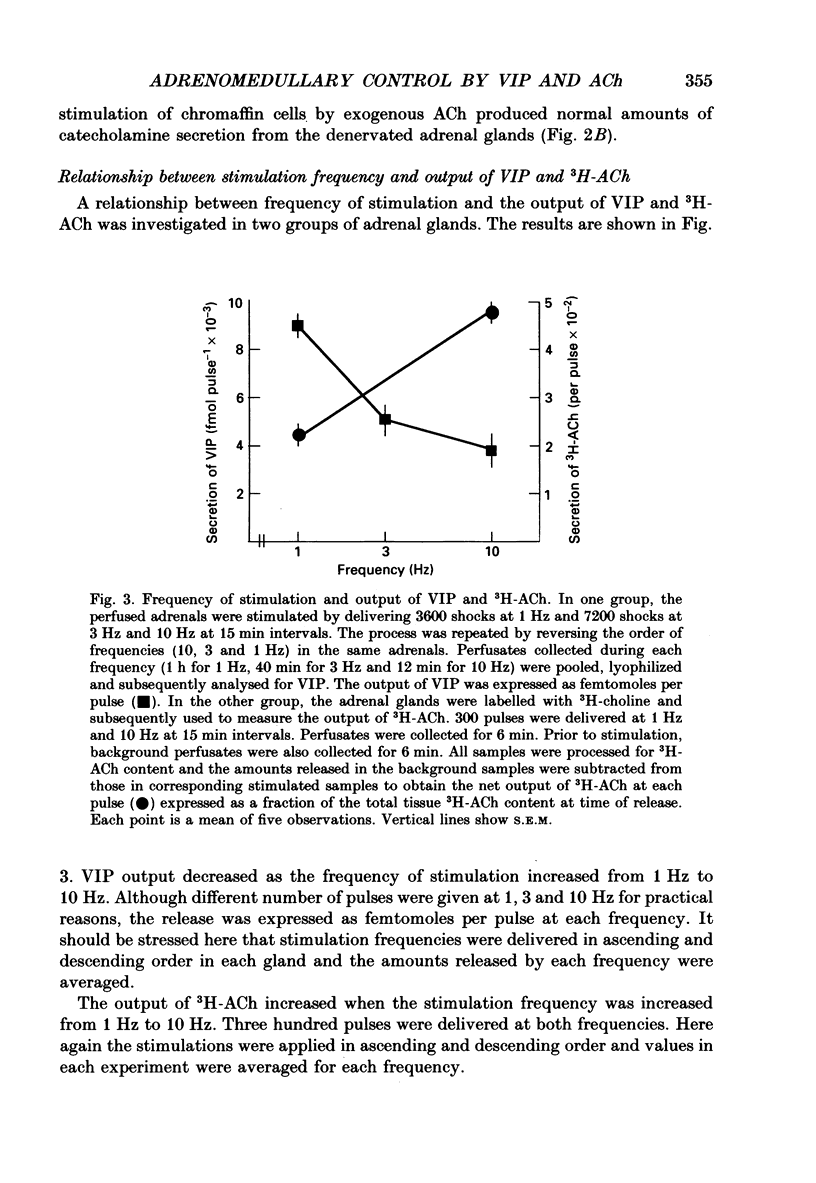
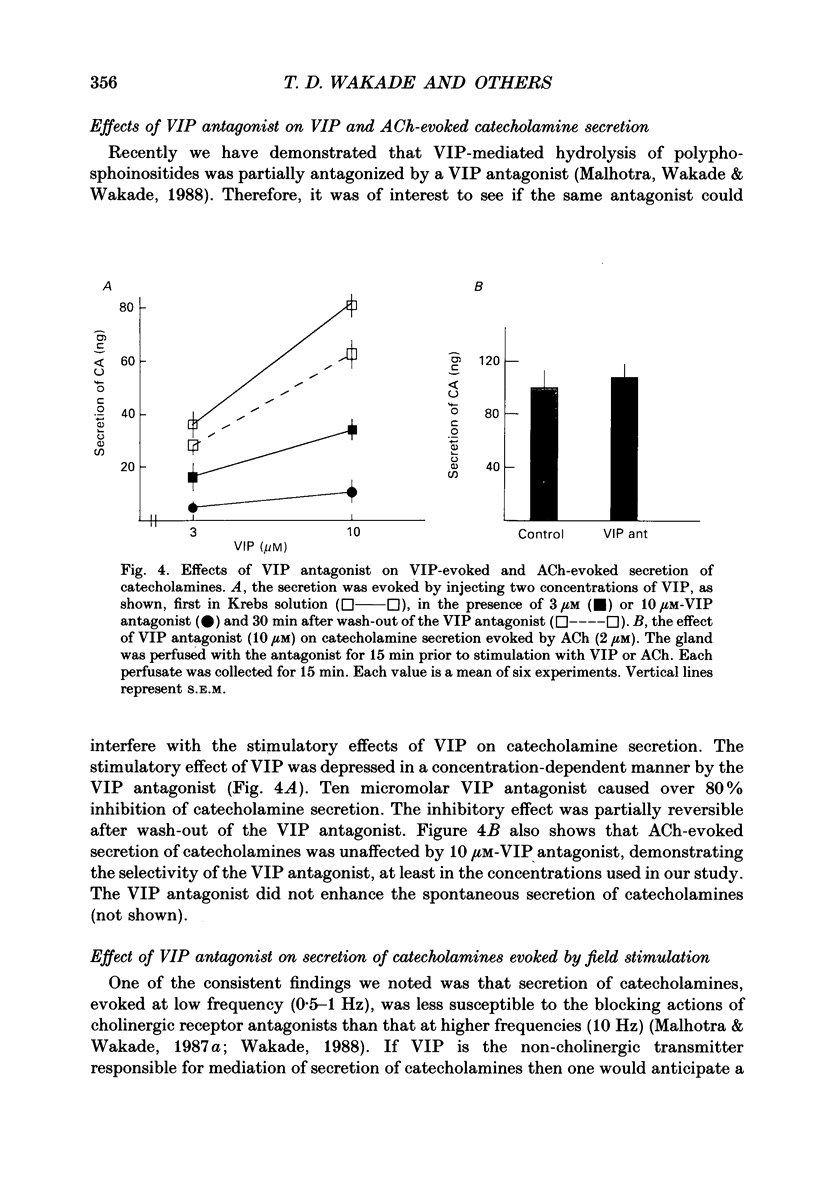
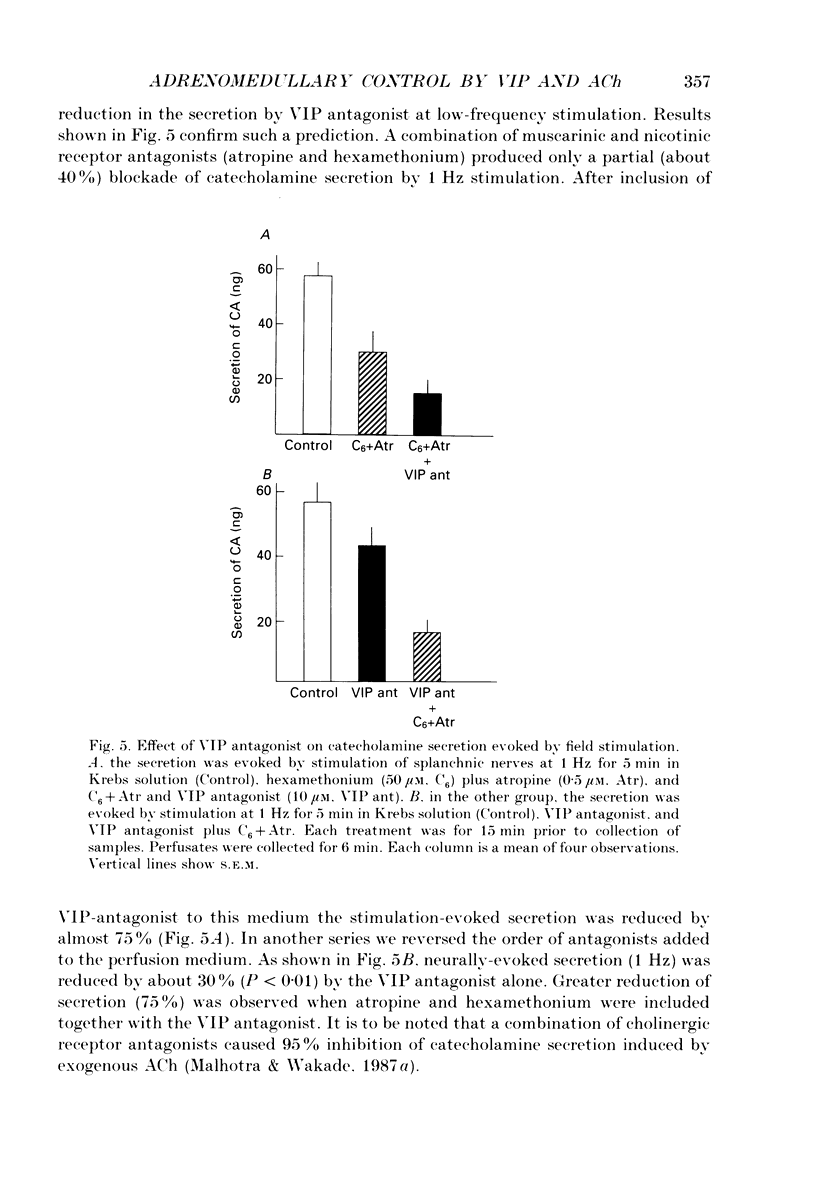
1. The perfused adrenal gland of the rat was used to establish the identity of a non-cholinergic substance involved in splanchnic nerve-mediated secretion of catecholamines. 2. The perfused adrenal medulla was rich in vasoactive intestinal polypeptide (VIP) content (28 pmol g-1 of wet tissue). VIP-immunoreactive nerve fibres were present in the adrenal medulla and the adrenal cortex. 3. Field stimulation (10 Hz for 15 min plus 1 Hz for 15 min) caused a large increase in the output of VIP in the perfusate over the spontaneous release of VIP. Secretion of catecholamines was also greatly elevated by field stimulation. Field stimulation-evoked output of VIP and catecholamines was abolished after chronic denervation of the adrenal glands. 4. Infusion of acetylcholine (ACh) did not increase the output of VIP but caused a robust secretion of catecholamines. 5. The VIP output declined when the stimulation frequency was increased (8.6 x 10(-3) fmol pulse-1 at 1 Hz and 4.0 x 10(-3) fmol pulse-1 at 10 Hz). 6. In contrast, the output of 3H-acetylcholine (3H-ACh, expressed as a fraction of tissue 3H-ACh content) increased from 7.0 x 10(-2) pulse-1 at 1 Hz to 16.3 x 10(-2) pulse-1 at 10 Hz. 7. Secretion of catecholamines evoked by low-frequency stimulation (1 Hz) was reduced by 40% in the presence of cholinergic receptor antagonists (atropine plus hexamethonium). Inclusion of a VIP receptor antagonist ([Ac-Tyr1, D-Phe2]-GRF 1-29 amide) caused about 75% inhibition. 8. The VIP receptor antagonist inhibited VIP-evoked secretion of catecholamines without affecting ACh-evoked secretion. 9. In conclusion, VIP satisfies all the essential criteria to assume the role of a neurotransmitter in the rat adrenal medulla. The contribution of VIP to the secretion of adrenal medullary hormones is more prominent at low rates of neuronal activity whereas ACh is the major contributor at higher activity.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Andersson P. O., Bloom S. R., Edwards A. V., Järhult J. Effects of stimulation of the chorda tympani in bursts on submaxillary responses in the cat. J Physiol. 1982 Jan;322:469–483. doi: 10.1113/jphysiol.1982.sp014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin C., Sasek C. A., Zigmond R. E. Evidence that some preganglionic sympathetic neurons in the rat contain vasoactive intestinal peptide- or peptide histidine isoleucine amide-like immunoreactivities. Neuroscience. 1991;40(1):175–184. doi: 10.1016/0306-4522(91)90183-o. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Jones C. T. The adrenal contribution to the neuroendocrine responses to splanchnic nerve stimulation in conscious calves. J Physiol. 1988 Mar;397:513–526. doi: 10.1113/jphysiol.1988.sp017016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael S. W., Brooks J. C., Malhotra R. K., Wakade T. D., Wakade A. R. Ultrastructural demonstration of exocytosis in the intact rat adrenal medulla. J Electron Microsc Tech. 1989 Aug;12(4):316–322. doi: 10.1002/jemt.1060120404. [DOI] [PubMed] [Google Scholar]
- Cheung C. Y., Holzwarth M. A. Fetal adrenal VIP: distribution and effect on medullary catecholamine secretion. Peptides. 1986 May-Jun;7(3):413–418. doi: 10.1016/0196-9781(86)90007-0. [DOI] [PubMed] [Google Scholar]
- Cooper J. R. A simple method to determine released acetylcholine in the presence of choline. Life Sci. 1989;45(21):2041–2042. doi: 10.1016/0024-3205(89)90578-x. [DOI] [PubMed] [Google Scholar]
- Eckenstein F., Baughman R. W. Two types of cholinergic innervation in cortex, one co-localized with vasoactive intestinal polypeptide. Nature. 1984 May 10;309(5964):153–155. doi: 10.1038/309153a0. [DOI] [PubMed] [Google Scholar]
- Edwards A. V., Jones C. T. Adrenal responses to splanchnic nerve stimulation in conscious calves given naloxone. J Physiol. 1989 Nov;418:339–351. doi: 10.1113/jphysiol.1989.sp017844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J., Galbo H., Holst J. J., Schaffalitzky de Muckadell O. B. Influence of the autonomic nervous system on the release of vasoactive intestinal polypeptide from the porcine gastrointestinal tract. J Physiol. 1978 Jul;280:405–422. doi: 10.1113/jphysiol.1978.sp012391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Goyal R. K., Rattan S., Said S. I. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurones. Nature. 1980 Nov 27;288(5789):378–380. doi: 10.1038/288378a0. [DOI] [PubMed] [Google Scholar]
- Green D. E., Fry M. On reagents that convert cytochrome oxidase from an inactive to an active coupling state. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1951–1955. doi: 10.1073/pnas.77.4.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K. E., Hallin R. G., Hongell A., Torebjörk H. E., Wallin B. G. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972 Feb;84(2):164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hallin R. G., Torebjörk H. E. Single unit sympathetic activity in human skin nerves during rest and various manoeuvres. Acta Physiol Scand. 1974 Nov;92(3):303–317. doi: 10.1111/j.1748-1716.1974.tb05749.x. [DOI] [PubMed] [Google Scholar]
- Holzwarth M. A. The distribution of vasoactive intestinal peptide in the rat adrenal cortex and medulla. J Auton Nerv Syst. 1984 Nov;11(3):269–283. doi: 10.1016/0165-1838(84)90041-9. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Lundberg J. M., Schultzberg M., Fahrenkrug J. Immunohistochemical evidence for a local VIP-ergic neuron system in the adrenal gland of the rat. Acta Physiol Scand. 1981 Dec;113(4):575–576. doi: 10.1111/j.1748-1716.1981.tb06944.x. [DOI] [PubMed] [Google Scholar]
- Ip N. Y., Perlman R. L., Zigmond R. E. Acute transsynaptic regulation of tyrosine 3-monooxygenase activity in the rat superior cervical ganglion: evidence for both cholinergic and noncholinergic mechanisms. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2081–2085. doi: 10.1073/pnas.80.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip N. Y., Zigmond R. E. Pattern of presynaptic nerve activity can determine the type of neurotransmitter regulating a postsynaptic event. Nature. 1984 Oct 4;311(5985):472–474. doi: 10.1038/311472a0. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. Further evidence for peptidergic transmission in sympathetic ganglia. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5008–5012. doi: 10.1073/pnas.77.8.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kyoshima K., Olschowka J. A., Jacobowitz D. M. Vasoactive intestinal polypeptide immunoreactive and cholinergic nerves in the whole mount preparation of the major cerebral arteries of the rat. Histochemistry. 1983;79(3):377–381. doi: 10.1007/BF00491773. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundberg J. M., Anggård A., Fahrenkrug J. VIP as a mediator of hexamethonium-sensitive, atropine-resistant vasodilation in the cat tongue. Acta Physiol Scand. 1982 Dec;116(4):387–392. doi: 10.1111/j.1748-1716.1982.tb07156.x. [DOI] [PubMed] [Google Scholar]
- Malhotra R. K., Wakade A. R. Non-cholinergic component of rat splanchnic nerves predominates at low neuronal activity and is eliminated by naloxone. J Physiol. 1987 Feb;383:639–652. doi: 10.1113/jphysiol.1987.sp016434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R. K., Wakade A. R. Vasoactive intestinal polypeptide stimulates the secretion of catecholamines from the rat adrenal gland. J Physiol. 1987 Jul;388:285–294. doi: 10.1113/jphysiol.1987.sp016615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R. K., Wakade T. D., Wakade A. R. Cross-communication between acetylcholine and VIP in controlling catecholamine secretion by affecting cAMP, inositol triphosphate, protein kinase C, and calcium in rat adrenal medulla. J Neurosci. 1989 Dec;9(12):4150–4157. doi: 10.1523/JNEUROSCI.09-12-04150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R. K., Wakade T. D., Wakade A. R. Vasoactive intestinal polypeptide and muscarine mobilize intracellular Ca2+ through breakdown of phosphoinositides to induce catecholamine secretion. Role of IP3 in exocytosis. J Biol Chem. 1988 Feb 15;263(5):2123–2126. [PubMed] [Google Scholar]
- Marley P., Livett B. G. Neuropeptides in the autonomic nervous system. CRC Crit Rev Clin Neurobiol. 1985;1(3):201–283. [PubMed] [Google Scholar]
- Matsuzaki Y., Hamasaki Y., Said S. I. Vasoactive intestinal peptide: a possible transmitter of nonadrenergic relaxation of guinea pig airways. Science. 1980 Dec 12;210(4475):1252–1253. doi: 10.1126/science.6254154. [DOI] [PubMed] [Google Scholar]
- Maubert E., Tramu G., Croix D., Beauvillain J. C., Dupouy J. P. Co-localization of vasoactive intestinal polypeptide and neuropeptide Y immunoreactivities in the nerve fibers of the rat adrenal gland. Neurosci Lett. 1990 May 31;113(2):121–126. doi: 10.1016/0304-3940(90)90290-p. [DOI] [PubMed] [Google Scholar]
- Misbahuddin M., Oka M., Nakanishi A., Morita K. Stimulatory effect of vasoactive intestinal polypeptide on catecholamine secretion from isolated guinea pig adrenal chromaffin cells. Neurosci Lett. 1988 Oct 5;92(2):202–206. doi: 10.1016/0304-3940(88)90061-4. [DOI] [PubMed] [Google Scholar]
- Mitchell S. J., Bloom S. R. Measurement of fasting and postprandial plasma VIP in man. Gut. 1978 Nov;19(11):1043–1048. doi: 10.1136/gut.19.11.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade A. R. Facilitation of secretion of catecholamines from rat and guinea-pig adrenal glands in potassium-free medium or after ouabain. J Physiol. 1981;313:481–498. doi: 10.1113/jphysiol.1981.sp013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade A. R., Malhotra R. K., Wakade T. D., Dixon W. R. Simultaneous secretion of catecholamines from the adrenal medulla and of [3H]norepinephrine from sympathetic nerves from a single test preparation: different effects of agents on the secretion. Neuroscience. 1986 Aug;18(4):877–888. doi: 10.1016/0306-4522(86)90106-5. [DOI] [PubMed] [Google Scholar]
- Wakade A. R. Noncholinergic transmitter(s) maintains secretion of catecholamines from rat adrenal medulla for several hours of continuous stimulation of splanchnic neurons. J Neurochem. 1988 Apr;50(4):1302–1308. doi: 10.1111/j.1471-4159.1988.tb10608.x. [DOI] [PubMed] [Google Scholar]
- Wakade A. R. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981;313:463–480. doi: 10.1113/jphysiol.1981.sp013676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan D. C., Livett B. G. Vasoactive intestinal peptide stimulates proenkephalin A mRNA expression in bovine adrenal chromaffin cells. Neurosci Lett. 1989 Jun 19;101(2):218–222. doi: 10.1016/0304-3940(89)90534-x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Saito H., Sano T., Ohuchi T., Ishimura Y., Morita K., Saito S., Oka M. Localization and release of immunoreactive vasoactive intestinal polypeptide in bovine adrenal medulla. Neurosci Lett. 1990 Mar 26;111(1-2):75–79. doi: 10.1016/0304-3940(90)90347-c. [DOI] [PubMed] [Google Scholar]