Abstract
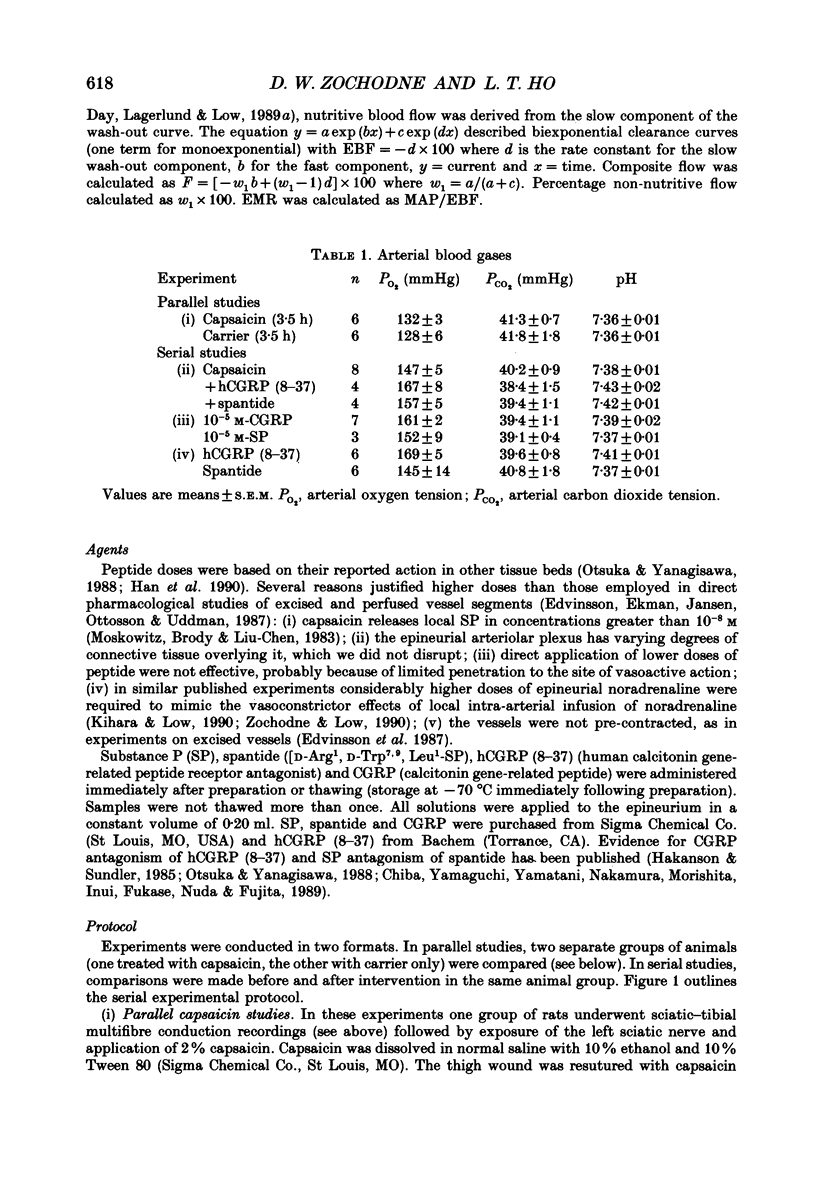
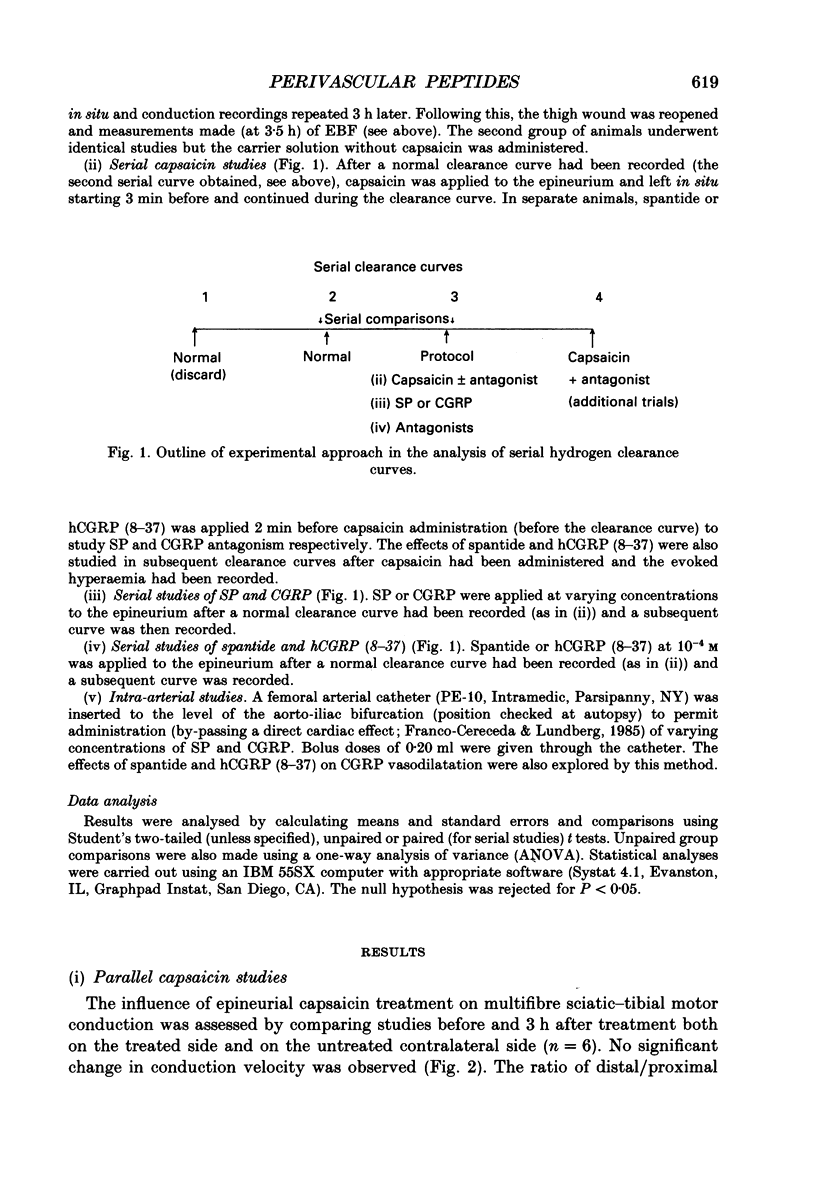
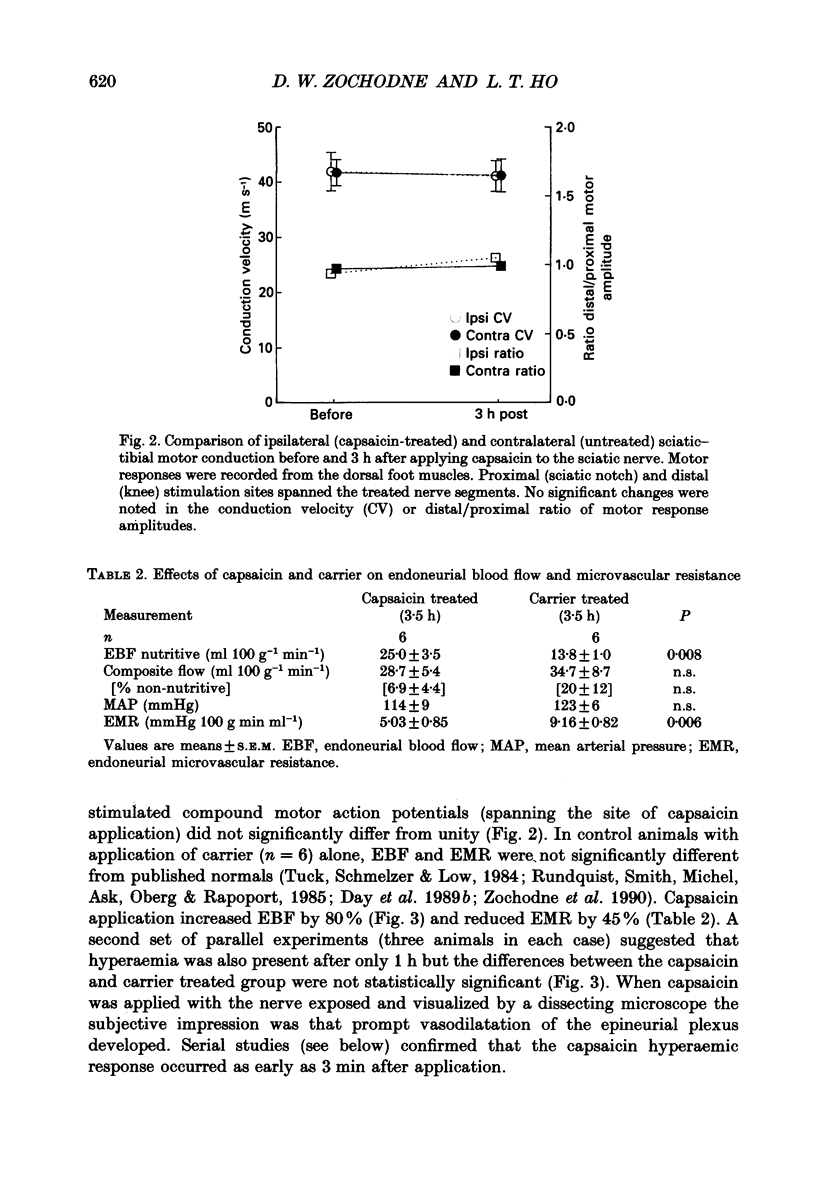
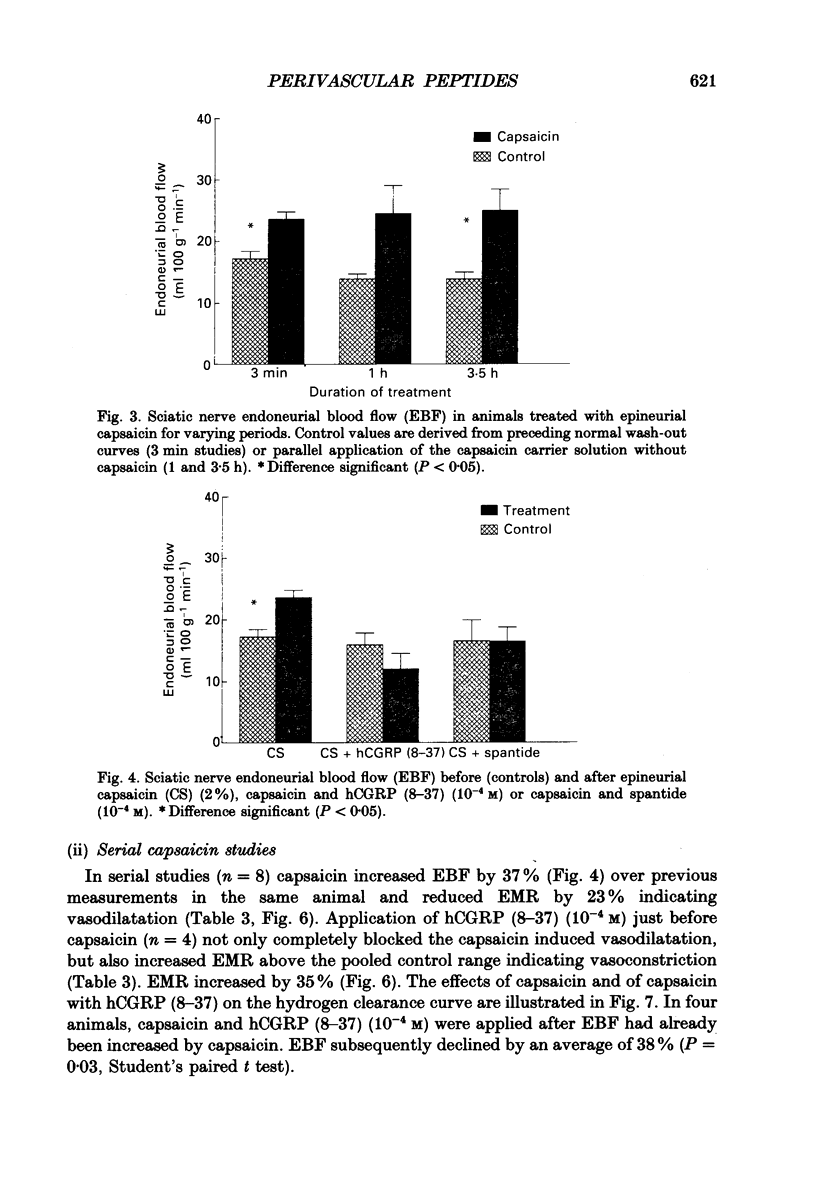
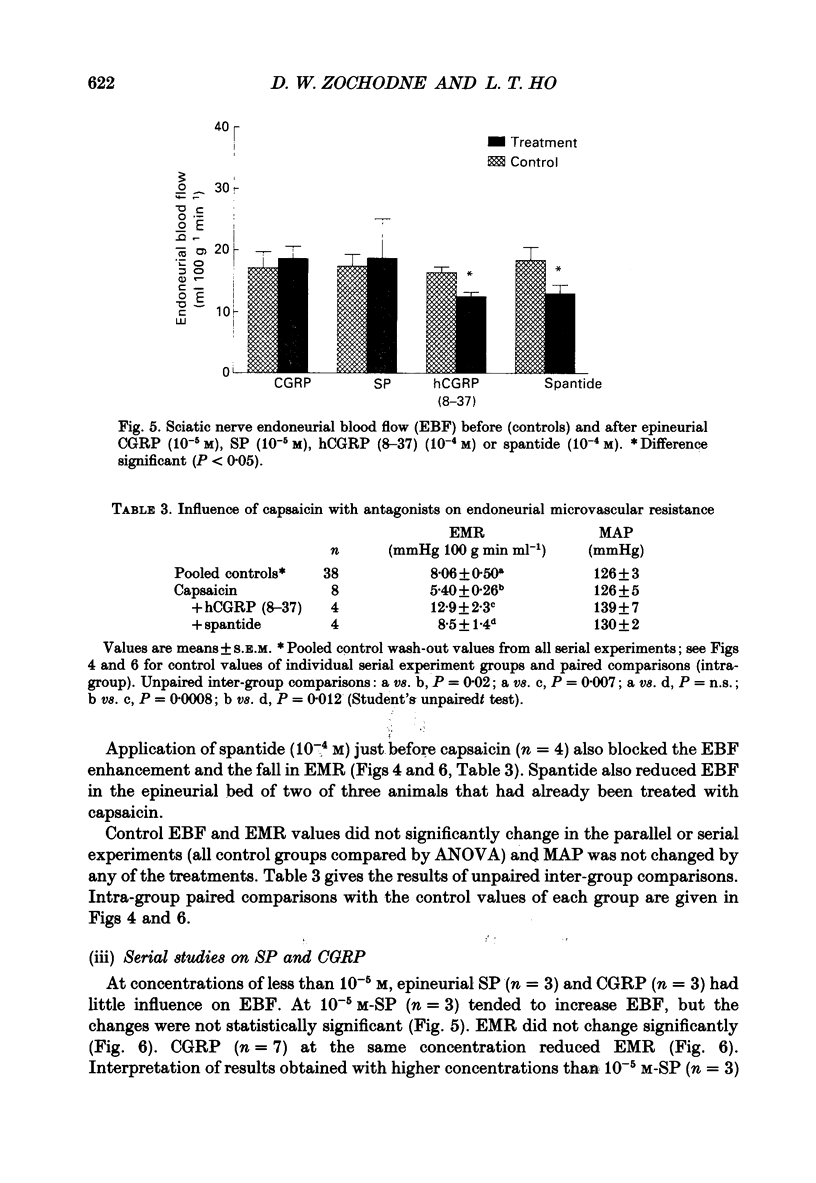
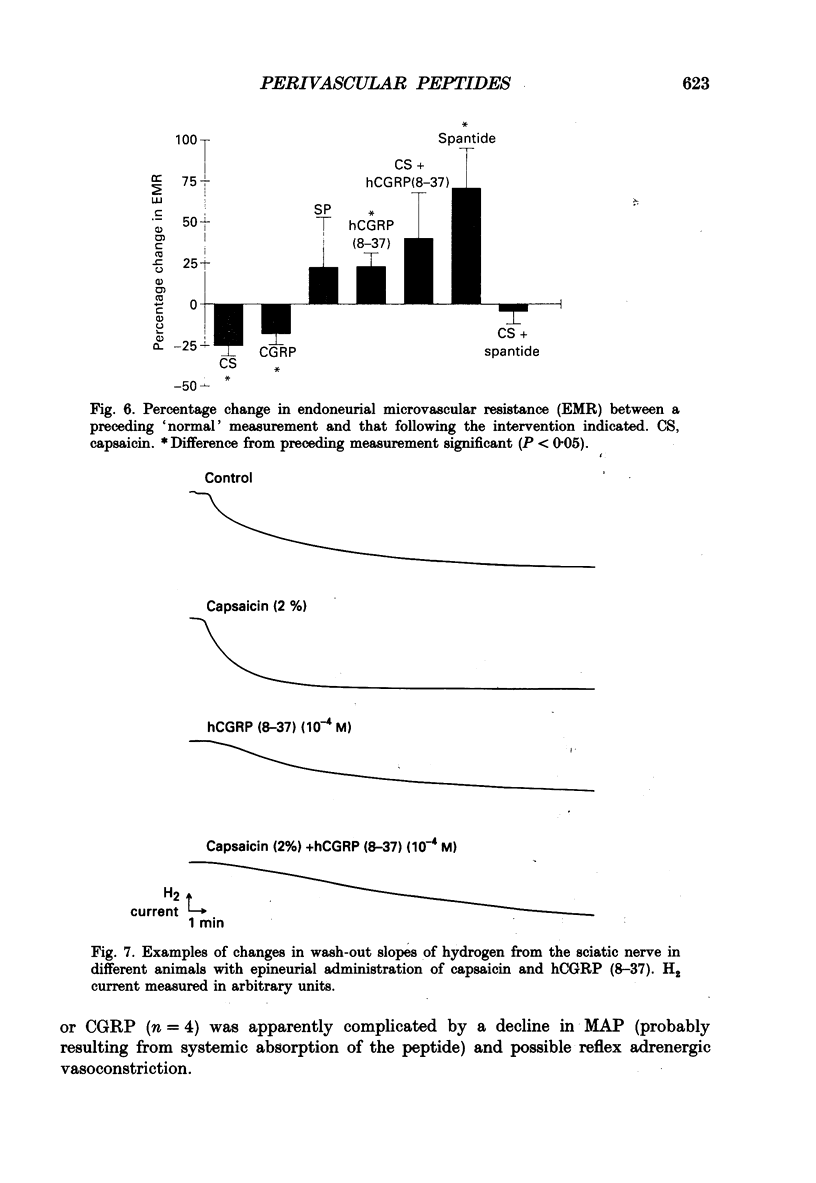
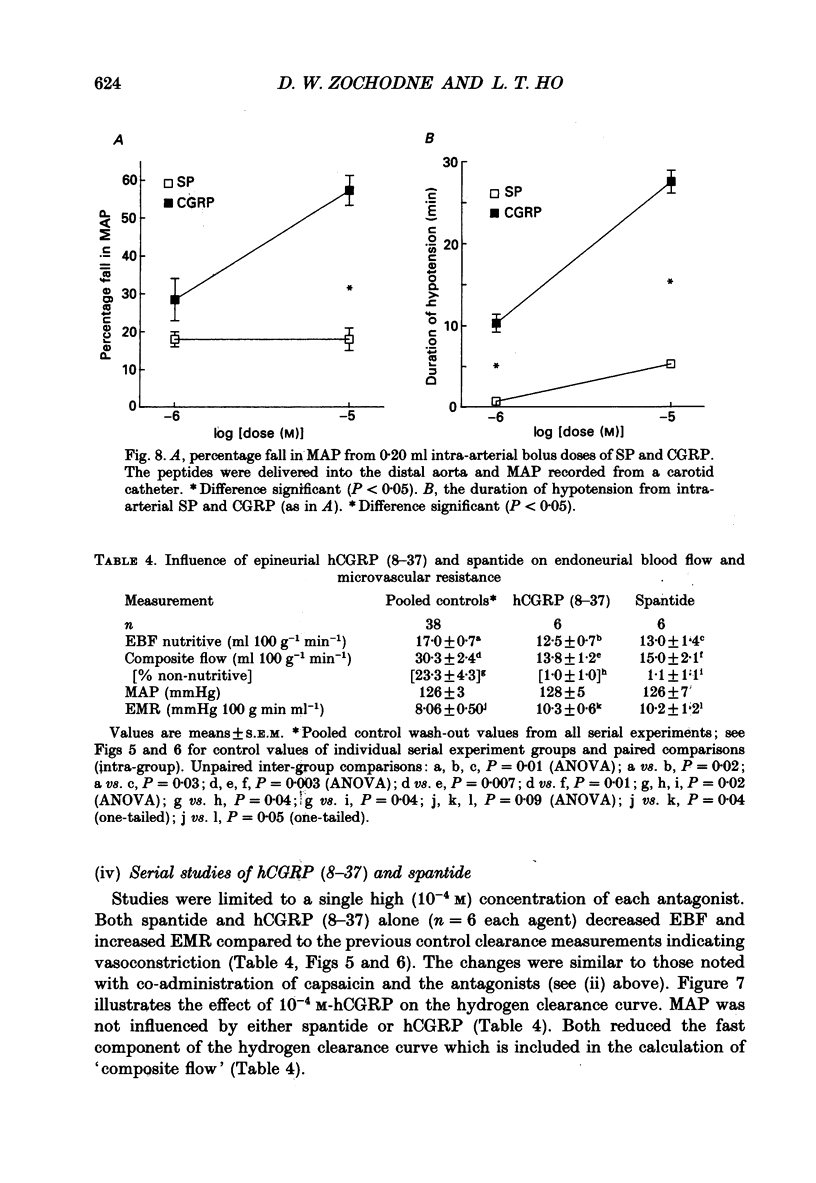
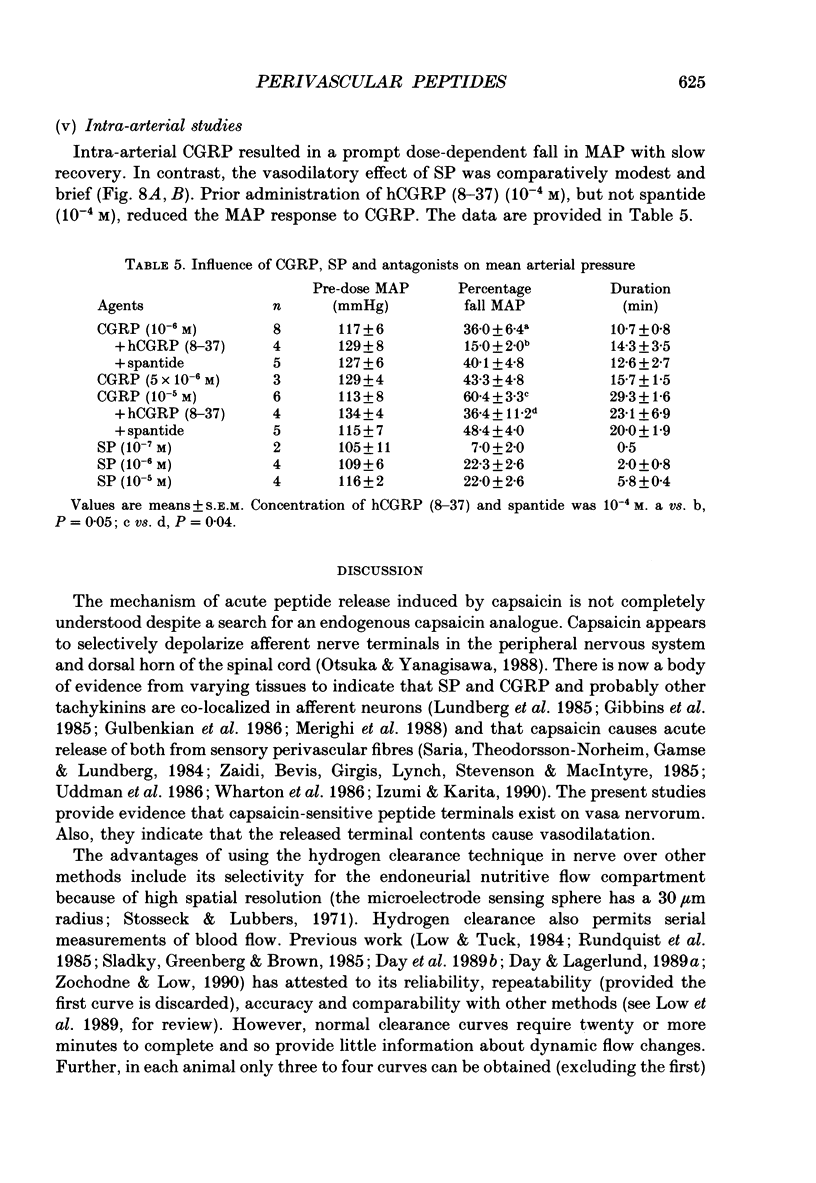
1. A variety of vasoactive peptides has been identified in the axon terminals innervating vasa nervorum but their function is unknown. In mesenteric arterioles, substance P (SP) and calcitonin gene-related peptide (CGRP) have been postulated to have a role in tonic vasodilatation. 2. We explored the effect of epineurial capsaicin, SP, CGRP, spantide (SP antagonist), and hCGRP (8-37) (CGRP antagonist) on blood flow (EBF) and microvascular resistance (EMR) in the endoneurial compartment of the rat sciatic nerve, as measured by hydrogen clearance. 3. Epineurial capsaicin induced a prompt, intense and prolonged increase in EBF and lowering of EMR as compared to epineurial application of the carrier alone in a separate animal group. The hyperaemic response was also confirmed by studying serial clearance curves in individual animals. 4. Multifibre sciatic-tibial motor conduction was not changed by epineurial capsaicin. 5. When co-administered with capsaicin, hCGRP (8-37) completely blocked the hyperaemic response and increased EMR above the pooled control range. Spantide also blocked the capsaicin response. 6. When administered alone, both epineurial hCGRP (8-37) and spantide lowered EBF below and increased EMR above the control measurements in the same animals. 7. At 10(-5) M epineurial CGRP, but not SP lowered EMR. Vasodilatation from intra-arterial administration of CGRP was much greater and was more prolonged compared with that induced by SP. hCGRP (8-37), but not spantide reduced the intra-arterial response to CGRP. 8. The findings suggest that epineurial peptidergic terminals mediate a vasodilatory response (particularly through CGRP) that increases blood flow in the 'downstream' endoneurial compartment. Physiological peptide release (blocked by SP and CGRP receptor antagonism) may be important in maintaining tonic vasodilatation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appenzeller O., Dhital K. K., Cowen T., Burnstock G. The nerves to blood vessels supplying blood to nerves: the innervation of vasa nervorum. Brain Res. 1984 Jun 25;304(2):383–386. doi: 10.1016/0006-8993(84)90344-5. [DOI] [PubMed] [Google Scholar]
- Barthó L., Holzer P., Leander S., Lembeck F. Evidence for an involvement of substance P, but not cholecystokinin-like peptides, in hexamethonium-resistant intestinal peristalsis. Neuroscience. 1989;28(1):211–217. doi: 10.1016/0306-4522(89)90245-5. [DOI] [PubMed] [Google Scholar]
- Bell M. A., Weddell A. G. A descriptive study of the blood vessels of the sciatic nerve in the rat, man and other mammals. Brain. 1984 Sep;107(Pt 3):871–898. doi: 10.1093/brain/107.3.871. [DOI] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J., Tippins J. R., Morris H. R., MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985 Jan 3;313(5997):54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Burks T. F. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986 Sep;38(3):179–226. [PubMed] [Google Scholar]
- Buck S. H., Shatzer S. A. Agonist and antagonist binding to tachykinin peptide NK-2 receptors. Life Sci. 1988;42(26):2701–2708. doi: 10.1016/0024-3205(88)90246-9. [DOI] [PubMed] [Google Scholar]
- Chahl L. A. Effects of substance P antagonists on the atropine-sensitive and atropine-resistant responses of guinea-pig ileum to substance P. Neurosci Lett. 1985 Mar 22;55(1):35–40. doi: 10.1016/0304-3940(85)90308-8. [DOI] [PubMed] [Google Scholar]
- Chahl L. A. The effects of ruthenium red on the response of guinea-pig ileum to capsaicin. Eur J Pharmacol. 1989 Oct 10;169(2-3):241–247. doi: 10.1016/0014-2999(89)90021-6. [DOI] [PubMed] [Google Scholar]
- Chiba T., Yamaguchi A., Yamatani T., Nakamura A., Morishita T., Inui T., Fukase M., Noda T., Fujita T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8-37). Am J Physiol. 1989 Feb;256(2 Pt 1):E331–E335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- Day T. J., Lagerlund T. D., Low P. A. Analysis of H2 clearance curves used to measure blood flow in rat sciatic nerve. J Physiol. 1989 Jul;414:35–54. doi: 10.1113/jphysiol.1989.sp017675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., Ekman R., Jansen I., Ottosson A., Uddman R. Peptide-containing nerve fibers in human cerebral arteries: immunocytochemistry, radioimmunoassay, and in vitro pharmacology. Ann Neurol. 1987 May;21(5):431–437. doi: 10.1002/ana.410210503. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Gulbenkian S., Wharton J., Jansen I., Polak J. M. Peptide-containing nerves in the rat femoral artery and vein. An immunocytochemical and vasomotor study. Blood Vessels. 1989;26(5):254–271. doi: 10.1159/000158775. [DOI] [PubMed] [Google Scholar]
- Featherstone R. L., Fosbraey P., Morton I. K. A comparison of the effects of three substance P antagonists on tachykinin-stimulated [3H]-acetylcholine release in the guinea-pig ileum. Br J Pharmacol. 1986 Jan;87(1):73–77. doi: 10.1111/j.1476-5381.1986.tb10158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Cereceda A., Lundberg J. M. Calcitonin gene-related peptide (CGRP) and capsaicin-induced stimulation of heart contractile rate and force. Naunyn Schmiedebergs Arch Pharmacol. 1985 Nov;331(2-3):146–151. doi: 10.1007/BF00634231. [DOI] [PubMed] [Google Scholar]
- Freedman J., Hökfelt T., Post C., Brodin E., Sundström E., Jonsson G., Terenius L., Leander S., Fischer J. A., Verhofstad A. Immunohistochemical and behavioral analysis of spinal lesions induced by a substance P antagonist and protection by thyrotropin releasing hormone. Exp Brain Res. 1989;74(2):279–292. doi: 10.1007/BF00248861. [DOI] [PubMed] [Google Scholar]
- Freedman J., Post C., Kåhrström J., Ohlen A., Mollenholt P., Owman C., Alari L., Hökfelt T. Vasoconstrictor effects in spinal cord of the substance P antagonist [D-Arg, D-Trp7,9 Leu11]-substance P (Spantide) and somatostatin and interaction with thyrotropin releasing hormone. Neuroscience. 1988 Oct;27(1):267–278. doi: 10.1016/0306-4522(88)90236-9. [DOI] [PubMed] [Google Scholar]
- Gibbins I. L., Furness J. B., Costa M., MacIntyre I., Hillyard C. J., Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett. 1985 Jun 12;57(2):125–130. doi: 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- Gulbenkian S., Merighi A., Wharton J., Varndell I. M., Polak J. M. Ultrastructural evidence for the coexistence of calcitonin gene-related peptide and substance P in secretory vesicles of peripheral nerves in the guinea pig. J Neurocytol. 1986 Aug;15(4):535–542. doi: 10.1007/BF01611735. [DOI] [PubMed] [Google Scholar]
- HROMADA J. ON THE NERVE SUPPLY OF THE CONNECTIVE TISSUE OF SOME PERIPHERAL NERVOUS SYSTEM COMPONENTS. Acta Anat (Basel) 1963;55:343–351. doi: 10.1159/000142483. [DOI] [PubMed] [Google Scholar]
- Han S. P., Naes L., Westfall T. C. Inhibition of periarterial nerve stimulation-induced vasodilation of the mesenteric arterial bed by CGRP (8-37) and CGRP receptor desensitization. Biochem Biophys Res Commun. 1990 Apr 30;168(2):786–791. doi: 10.1016/0006-291x(90)92390-l. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Izumi H., Karita K. The effects of capsaicin applied topically to inferior alveolar nerve on antidromic vasodilatation in cat gingiva. Neurosci Lett. 1990 Apr 20;112(1):65–69. doi: 10.1016/0304-3940(90)90323-2. [DOI] [PubMed] [Google Scholar]
- Ju G., Hökfelt T., Brodin E., Fahrenkrug J., Fischer J. A., Frey P., Elde R. P., Brown J. C. Primary sensory neurons of the rat showing calcitonin gene-related peptide immunoreactivity and their relation to substance P-, somatostatin-, galanin-, vasoactive intestinal polypeptide- and cholecystokinin-immunoreactive ganglion cells. Cell Tissue Res. 1987 Feb;247(2):417–431. doi: 10.1007/BF00218323. [DOI] [PubMed] [Google Scholar]
- Kihara M., Low P. A. Regulation of rat nerve blood flow: role of epineurial alpha-receptors. J Physiol. 1990 Mar;422:145–152. doi: 10.1113/jphysiol.1990.sp017977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembeck F. The 1988 Ulf Euler Lecture. Substancce P: from extract to excitement. Acta Physiol Scand. 1988 Aug;133(4):435–454. doi: 10.1111/j.1748-1716.1988.tb08427.x. [DOI] [PubMed] [Google Scholar]
- Low P. A., Lagerlund T. D., McManis P. G. Nerve blood flow and oxygen delivery in normal, diabetic, and ischemic neuropathy. Int Rev Neurobiol. 1989;31:355–438. doi: 10.1016/s0074-7742(08)60283-4. [DOI] [PubMed] [Google Scholar]
- Low P. A., Tuck R. R. Effects of changes of blood pressure, respiratory acidosis and hypoxia on blood flow in the sciatic nerve of the rat. J Physiol. 1984 Feb;347:513–524. doi: 10.1113/jphysiol.1984.sp015079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Franco-Cereceda A., Hua X., Hökfelt T., Fischer J. A. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur J Pharmacol. 1985 Feb 5;108(3):315–319. doi: 10.1016/0014-2999(85)90456-x. [DOI] [PubMed] [Google Scholar]
- Merighi A., Polak J. M., Gibson S. J., Gulbenkian S., Valentino K. L., Peirone S. M. Ultrastructural studies on calcitonin gene-related peptide-, tachykinins- and somatostatin-immunoreactive neurones in rat dorsal root ganglia: evidence for the colocalization of different peptides in single secretory granules. Cell Tissue Res. 1988 Oct;254(1):101–109. doi: 10.1007/BF00220022. [DOI] [PubMed] [Google Scholar]
- Moskowitz M. A., Brody M., Liu-Chen L. Y. In vitro release of immunoreactive substance P from putative afferent nerve endings in bovine pia arachnoid. Neuroscience. 1983 Aug;9(4):809–814. doi: 10.1016/0306-4522(83)90269-5. [DOI] [PubMed] [Google Scholar]
- Nussbaumer J. C., Yanagisawa M., Otsuka M. Pharmacological properties of a C-fibre response evoked by saphenous nerve stimulation in an isolated spinal cord-nerve preparation of the newborn rat. Br J Pharmacol. 1989 Oct;98(2):373–382. doi: 10.1111/j.1476-5381.1989.tb12607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlén A., Lindbom L., Staines W., Hökfelt T., Cuello A. C., Fischer J. A., Hedqvist P. Substance P and calcitonin gene-related peptide: immunohistochemical localisation and microvascular effects in rabbit skeletal muscle. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jul;336(1):87–93. doi: 10.1007/BF00177756. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Yanagisawa M. Effect of a tachykinin antagonist on a nociceptive reflex in the isolated spinal cord-tail preparation of the newborn rat. J Physiol. 1988 Jan;395:255–270. doi: 10.1113/jphysiol.1988.sp016917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechthand E., Hervonen A., Sato S., Rapoport S. I. Distribution of adrenergic innervation of blood vessels in peripheral nerve. Brain Res. 1986 May 21;374(1):185–189. doi: 10.1016/0006-8993(86)90409-9. [DOI] [PubMed] [Google Scholar]
- Rundquist I., Smith Q. R., Michel M. E., Ask P., Oberg P. A., Rapoport S. I. Sciatic nerve blood flow measured by laser Doppler flowmetry and [14C]iodoantipyrine. Am J Physiol. 1985 Mar;248(3 Pt 2):H311–H317. doi: 10.1152/ajpheart.1985.248.3.H311. [DOI] [PubMed] [Google Scholar]
- Saria A., Theodorsson-Norheim E., Gamse R., Lundberg J. M. Release of substance P- and substance K-like immunoreactivities from the isolated perfused guinea-pig lung. Eur J Pharmacol. 1984 Oct 30;106(1):207–208. doi: 10.1016/0014-2999(84)90699-x. [DOI] [PubMed] [Google Scholar]
- Sladky J. T., Greenberg J. H., Brown M. J. Regional perfusion in normal and ischemic rat sciatic nerves. Ann Neurol. 1985 Feb;17(2):191–195. doi: 10.1002/ana.410170215. [DOI] [PubMed] [Google Scholar]
- Sugimoto H., Monafo W. W., Eliasson S. G. Regional sciatic nerve and muscle blood flow in conscious and anesthetized rats. Am J Physiol. 1986 Dec;251(6 Pt 2):H1211–H1216. doi: 10.1152/ajpheart.1986.251.6.H1211. [DOI] [PubMed] [Google Scholar]
- Tuck R. R., Schmelzer J. D., Low P. A. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain. 1984 Sep;107(Pt 3):935–950. doi: 10.1093/brain/107.3.935. [DOI] [PubMed] [Google Scholar]
- Uddman R., Edvinsson L., Ekblad E., Håkanson R., Sundler F. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regul Pept. 1986 Aug;15(1):1–23. doi: 10.1016/0167-0115(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Wharton J., Gulbenkian S., Mulderry P. K., Ghatei M. A., McGregor G. P., Bloom S. R., Polak J. M. Capsaicin induces a depletion of calcitonin gene-related peptide (CGRP)-immunoreactive nerves in the cardiovascular system of the guinea pig and rat. J Auton Nerv Syst. 1986 Aug;16(4):289–309. doi: 10.1016/0165-1838(86)90035-4. [DOI] [PubMed] [Google Scholar]
- Zaidi M., Bevis P. J., Girgis S. I., Lynch C., Stevenson J. C., MacIntyre I. Circulating CGRP comes from the perivascular nerves. Eur J Pharmacol. 1985 Nov 5;117(2):283–284. doi: 10.1016/0014-2999(85)90616-8. [DOI] [PubMed] [Google Scholar]
- Zochodne D. W., Ho L. T. Endoneurial microenvironment and acute nerve crush injury in the rat sciatic nerve. Brain Res. 1990 Dec 3;535(1):43–48. doi: 10.1016/0006-8993(90)91822-x. [DOI] [PubMed] [Google Scholar]
- Zochodne D. W., Ho L. T. Stimulation-induced peripheral nerve hyperemia: mediation by fibers innervating vasa nervorum? Brain Res. 1991 Apr 12;546(1):113–118. doi: 10.1016/0006-8993(91)91165-w. [DOI] [PubMed] [Google Scholar]
- Zochodne D. W., Huang Z. X., Ward K. K., Low P. A. Guanethidine-induced adrenergic sympathectomy augments endoneurial perfusion and lowers endoneurial microvascular resistance. Brain Res. 1990 Jun 11;519(1-2):112–117. doi: 10.1016/0006-8993(90)90067-l. [DOI] [PubMed] [Google Scholar]
- Zochodne D. W., Low P. A. Adrenergic control of nerve blood flow. Exp Neurol. 1990 Sep;109(3):300–307. doi: 10.1016/s0014-4886(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Zochodne D. W., Ward K. K., Low P. A. Guanethidine adrenergic neuropathy: an animal model of selective autonomic neuropathy. Brain Res. 1988 Sep 27;461(1):10–16. doi: 10.1016/0006-8993(88)90720-2. [DOI] [PubMed] [Google Scholar]


