Abstract
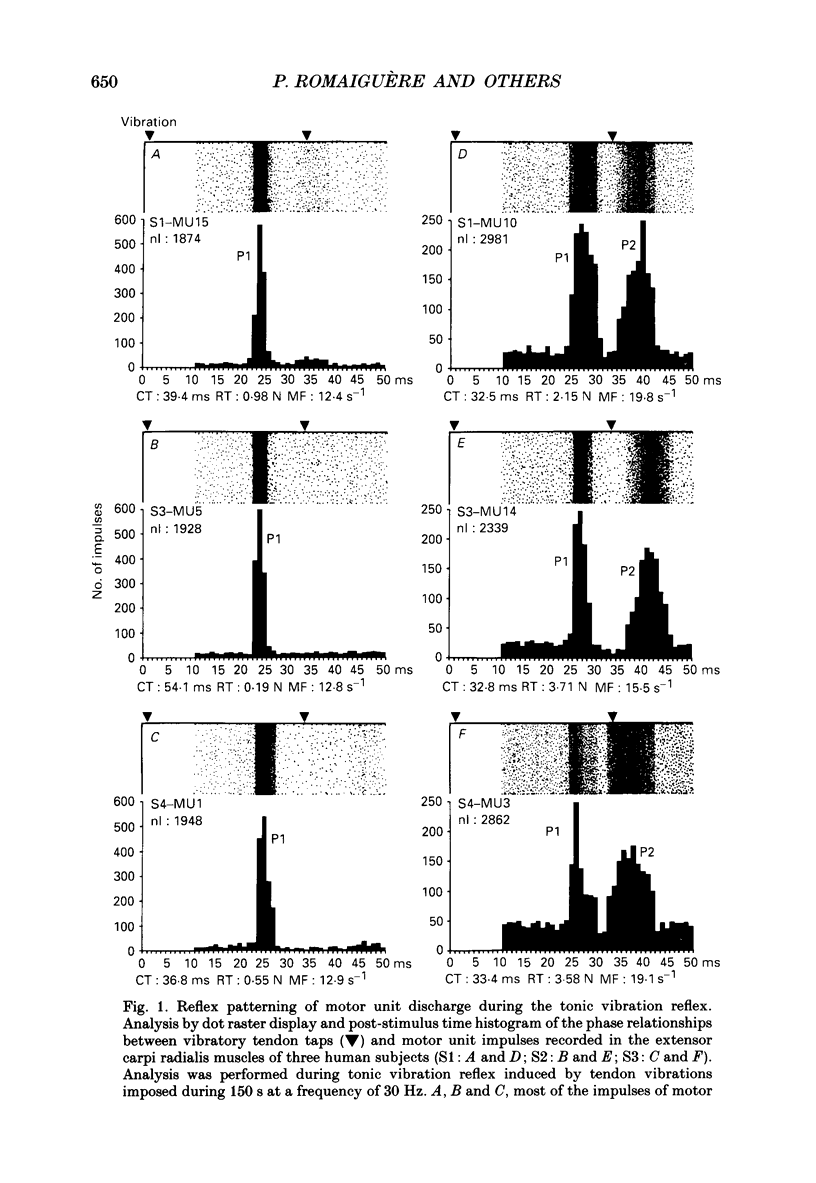
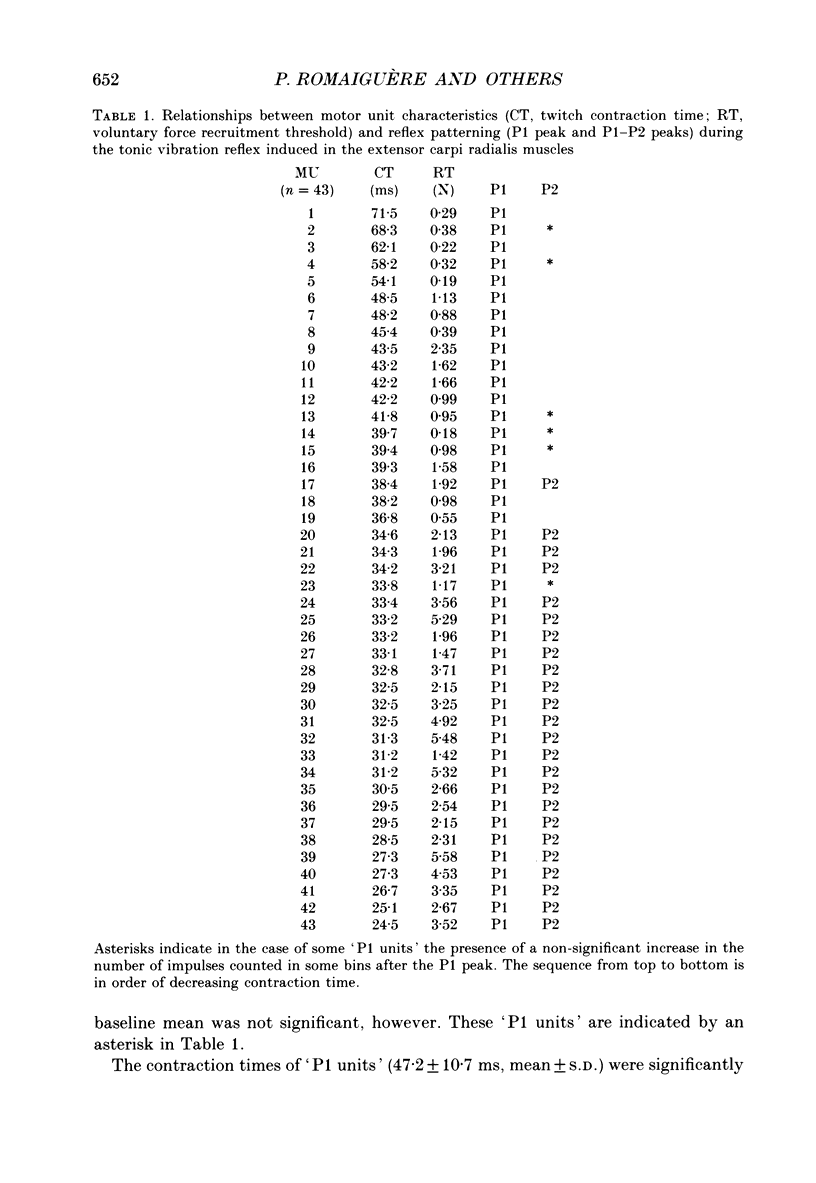
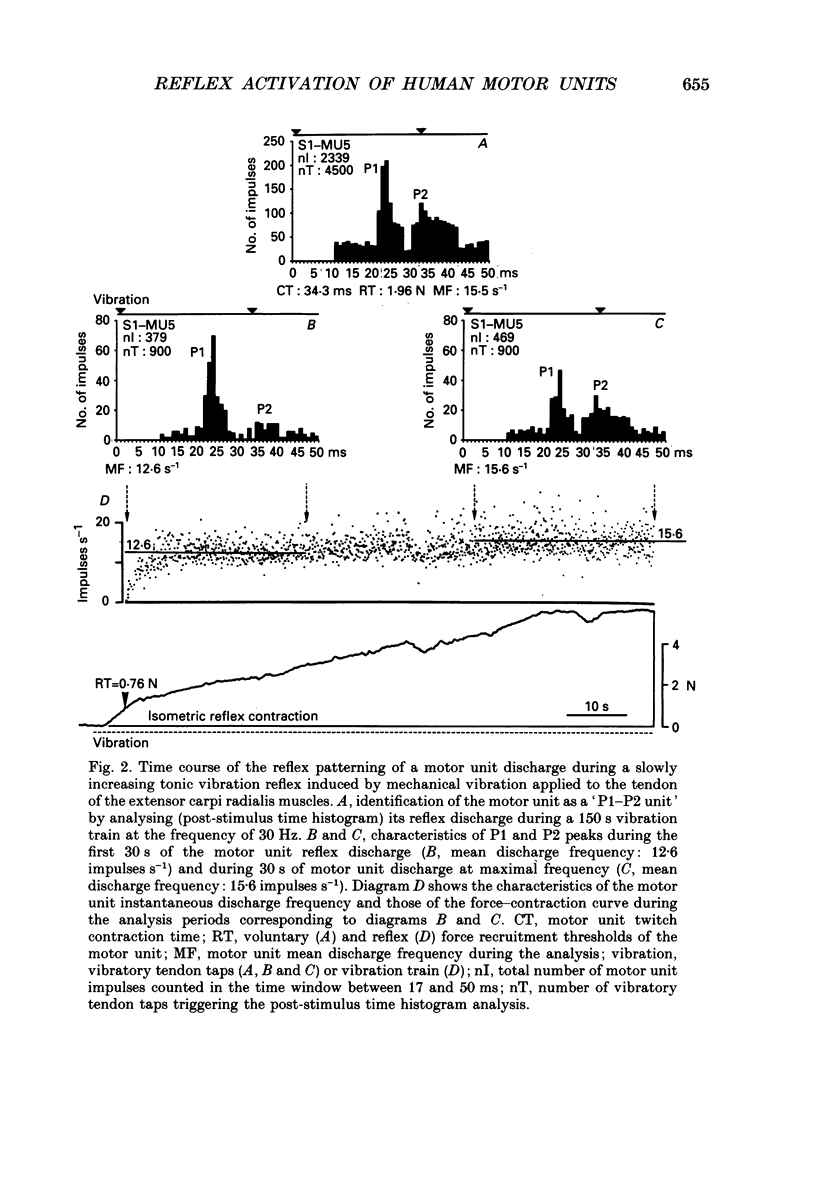
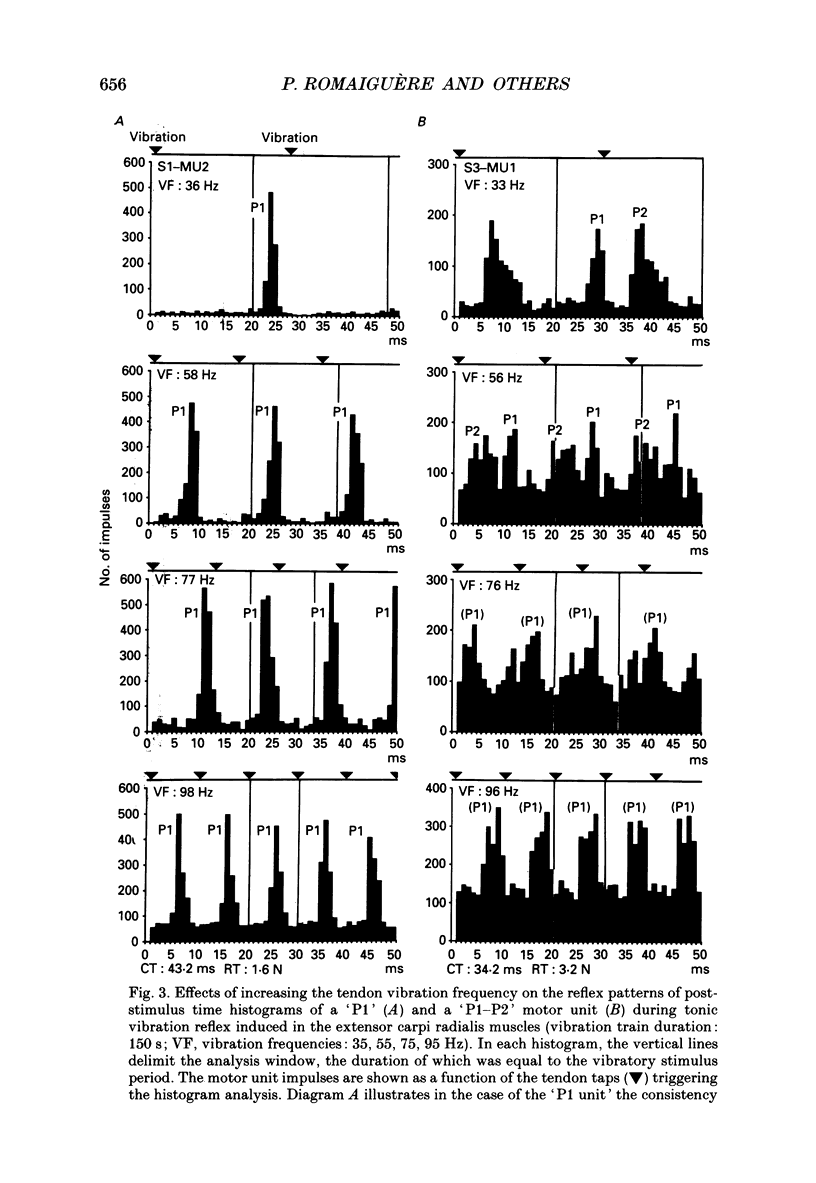
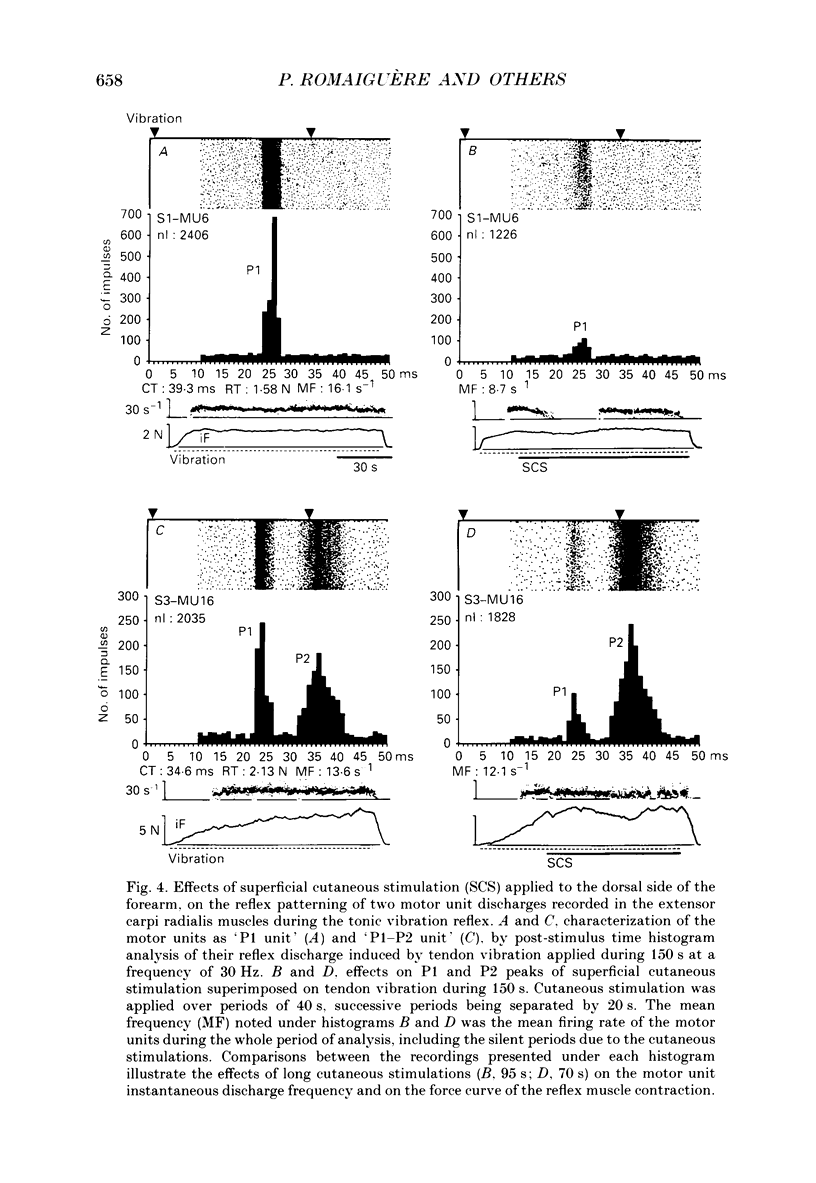
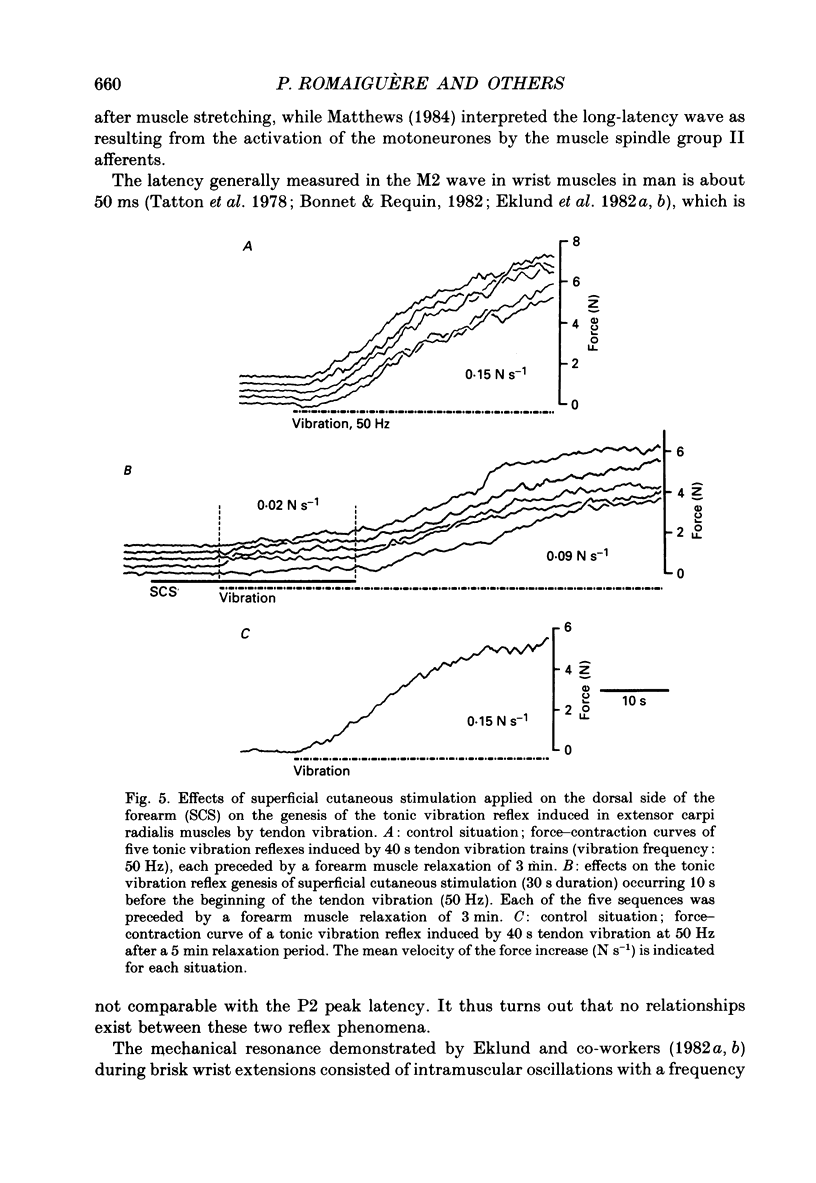
1. Single motor unit activity was recorded in the extensor carpi radialis longus and extensor carpi radialis brevis muscles of five healthy human subjects, using metal microelectrodes. 2. Motor units were characterized on the basis of their twitch contraction times and their force recruitment thresholds during voluntary imposed-ramp contractions. 3. The discharge patterns of forty-three motor units were studied during tonic vibration reflex elicited by prolonged (150 s) trains of vibration (30 Hz) applied to the distal tendons of the muscles. The temporal relationships between the individual small tendon taps of the vibratory stimulus and the motor unit impulses were analysed on dot raster displays and post-stimulus time histograms. 4. After tendon taps, the impulses of motor units with long twitch contraction times (mean +/- S.D., 47.2 +/- 10.7 ms) and low recruitment thresholds (0.88 +/- 0.6 N) formed a single narrow peak (P1) with a latency (22.7 +/- 1.4 ms) which was comparable to that of the tendon jerk in the extensor carpi radialis muscles. These motor units were named 'P1 units'. On the other hand, the response of motor units with shorter twitch contraction times (31.1 +/- 3.3 ms) and higher recruitment thresholds (3.21 +/- 1.3 N) showed two peaks: a short latency (23.4 +/- 1.3 ms) P1 peak similar to the previous one and a P2 peak occurring 9.4 +/- 1.2 ms later. These motor units were named 'P1-P2 units'. 5. When the reflex contraction increased slowly, the P1 peaks of 'P1-P2 units' were clearly predominant at the beginning of the contraction, during the rising phase of the motor unit discharge frequency, while the P2 peaks became predominant when the units had reached their maximal discharge frequency. 6. Increasing the tendon vibration frequency (35, 55, 75, 95 Hz) did not modify the 'P1 unit' discharge pattern. Due to interference between vibration period and peak latencies, increasing the vibration frequency caused the P1 and P2 peaks of 'P1-P2 units' to overlap. 7. Superficial cutaneous stimulation of the dorsal side of the forearm during tendon vibration noticeably decreased the P1 peaks in both types of motor units. In the P2 peaks it could result in either a decrease or an increase but the average effect was a slight increase. 8. When applied 10 s before tendon vibration, cutaneous stimulation considerably suppressed the tonic vibration reflex.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Etholm B., Gordon G. Presynaptic and post-synaptic inhibition elicited in the cat's dorsal column nuclei by mechanical stimulation of skin. J Physiol. 1970 Sep;210(2):433–455. doi: 10.1113/jphysiol.1970.sp009219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIANCONI R., van der MEULEN J. The response to vibration of the end organs of mammalian muscle spindles. J Neurophysiol. 1963 Jan;26:177–190. doi: 10.1152/jn.1963.26.1.177. [DOI] [PubMed] [Google Scholar]
- Bonnet M., Requin J. Long loop and spinal reflexes in man during preparation for intended directional hand movements. J Neurosci. 1982 Jan;2(1):90–96. doi: 10.1523/JNEUROSCI.02-01-00090.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976 Oct;261(3):695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol. 1976 Oct;261(3):673–693. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Schiller H. H. Discharge pattern of single motor units in the tonic vibration reflex of human triceps surae. J Neurol Neurosurg Psychiatry. 1976 Aug;39(8):729–741. doi: 10.1136/jnnp.39.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. W., Jones G. M., Kearney R. E., Watt D. G. The 'late' electromyographic response to limb displacement in man. I. Evidence for supraspinal contribution. Electroencephalogr Clin Neurophysiol. 1979 Feb;46(2):173–181. doi: 10.1016/0013-4694(79)90066-x. [DOI] [PubMed] [Google Scholar]
- De Gail P., Lance J. W., Neilson P. D. Differential effects on tonic and phasic reflex mechanisms produced by vibration of muscles in man. J Neurol Neurosurg Psychiatry. 1966 Feb;29(1):1–11. doi: 10.1136/jnnp.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide P. J., Crenna P., Fleron M. H. Cutaneous nerve stimulation and motoneuronal excitability: I, soleus and tibialis anterior excitability after ipsilateral and contralateral sural nerve stimulation. J Neurol Neurosurg Psychiatry. 1981 Aug;44(8):699–707. doi: 10.1136/jnnp.44.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt J. E., Godaux E. Vibration-induced discharge patterns of single motor units in the masseter muscle in man. J Physiol. 1975 Dec;253(2):429–442. doi: 10.1113/jphysiol.1975.sp011198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund G., Hagbarth K. E., Hägglund J. V., Wallin E. U. Mechanical oscillations contributing to the segmentation of the reflex electromyogram response to stretching human muscles. J Physiol. 1982 May;326:65–77. doi: 10.1113/jphysiol.1982.sp014177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund G., Hagbarth K. E., Hägglund J. V., Wallin E. U. The 'late' reflex responses to muscle stretch: the 'resonance hypothesis' versus the 'long-loop hypothesis'. J Physiol. 1982 May;326:79–90. doi: 10.1113/jphysiol.1982.sp014178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Trott J. R. Autogenetic reflex action on to gamma motoneurones by stretch of triceps surae in the decerebrated cat. J Physiol. 1978 Mar;276:49–66. doi: 10.1113/jphysiol.1978.sp012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D. Gamma control of dynamic properties of muscle spindles. J Neurophysiol. 1956 Jul;19(4):356–366. doi: 10.1152/jn.1956.19.4.356. [DOI] [PubMed] [Google Scholar]
- Garnett R., Stephens J. A. Changes in the recruitment threshold of motor units produced by cutaneous stimulation in man. J Physiol. 1981 Feb;311:463–473. doi: 10.1113/jphysiol.1981.sp013598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godaux E., Desmedt J. E., Demaret P. Vibration of human limb muscles: the alleged phase-locking of motor unit spikes. Brain Res. 1975 Dec 12;100(1):175–177. doi: 10.1016/0006-8993(75)90255-3. [DOI] [PubMed] [Google Scholar]
- HAGBARTH K. E. Excitatory and inhibitory skin areas for flexor and extensor motoneurons. Acta Physiol Scand Suppl. 1952;26(94):1–58. [PubMed] [Google Scholar]
- Hagbarth K. E., Hellsing G., Löfstedt L. TVR and vibration-induced timing of motor impulses in the human jaw elevator muscles. J Neurol Neurosurg Psychiatry. 1976 Aug;39(8):719–728. doi: 10.1136/jnnp.39.8.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K. E., Vallbo A. B. Discharge characteristics of human muscle afferents during muscle stretch and contraction. Exp Neurol. 1968 Dec;22(4):674–694. doi: 10.1016/0014-4886(68)90156-8. [DOI] [PubMed] [Google Scholar]
- Hirayama K., Homma S., Kanda K., Nakajima Y., Watanabe S. Crossed inhibition as revealed by cross-correlogram between bilateral homonymous motor unit spikes in man. Arch Ital Biol. 1976 Jul;114(3):213–227. [PubMed] [Google Scholar]
- Hirayama K., Homma S., Mizote M., Nakajima Y., Watanabe S. Separation of the contributions of voluntary and vibratory activation of motor units in man by cross-correlograms. Jpn J Physiol. 1974 Jun;24(3):293–304. doi: 10.2170/jjphysiol.24.293. [DOI] [PubMed] [Google Scholar]
- Hongo T., Kitazawa S., Ohki Y., Sasaki M., Xi M. C. A physiological and morphological study of premotor interneurones in the cutaneous reflex pathways in cats. Brain Res. 1989 Dec 25;505(1):163–166. doi: 10.1016/0006-8993(89)90131-5. [DOI] [PubMed] [Google Scholar]
- Hongo T., Kitazawa S., Ohki Y., Xi M. C. Functional identification of last-order interneurones of skin reflex pathways in the cat forelimb segments. Brain Res. 1989 Dec 25;505(1):167–170. doi: 10.1016/0006-8993(89)90132-7. [DOI] [PubMed] [Google Scholar]
- Hori Y., Hiraga K., Watanabe S. The effects of thiamylal sodium on the tonic vibration reflex in man. Brain Res. 1989 Sep 18;497(2):291–295. doi: 10.1016/0006-8993(89)90274-6. [DOI] [PubMed] [Google Scholar]
- Kanda K., Burke R. E., Walmsley B. Differential control of fast and slow twitch motor units in the decerebrate cat. Exp Brain Res. 1977 Aug 8;29(1):57–74. doi: 10.1007/BF00236875. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from muscle spindle secondary endings of intercostal and triceps surae muscles in the cat. J Physiol. 1975 Feb;245(2):64P–66P. [PubMed] [Google Scholar]
- LAPORTE Y., LLOYD D. P. C. Nature and significance of the reflex connections established by large afferent fibers of muscular origin. Am J Physiol. 1952 Jun;169(3):609–621. doi: 10.1152/ajplegacy.1952.169.3.609. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Characteristics of the excitatory pathway from group II muscle afferents to alpha motoneurones. Brain Res. 1975 May 9;88(3):538–542. doi: 10.1016/0006-8993(75)90667-8. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Comments on reflex actions evoked by electrical stimulation of group II muscle afferents. Brain Res. 1977 Feb 25;122(3):551–555. doi: 10.1016/0006-8993(77)90466-8. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Cutaneous facilitation of transmission in reflex pathways from Ib afferents to motoneurones. J Physiol. 1977 Mar;265(3):763–780. doi: 10.1113/jphysiol.1977.sp011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgren K., Pierrot-Deseilligny E. Evidence for non-monosynaptic Ia excitation of human wrist flexor motoneurones, possibly via propriospinal neurones. J Physiol. 1988 Nov;405:747–764. doi: 10.1113/jphysiol.1988.sp017359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgren K., Pierrot-Deseilligny E. Inhibition of neurones transmitting non-monosynaptic Ia excitation to human wrist flexor motoneurones. J Physiol. 1988 Nov;405:765–783. doi: 10.1113/jphysiol.1988.sp017360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Evidence from the use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol. 1984 Mar;348:383–415. doi: 10.1113/jphysiol.1984.sp015116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. The reflex excitation of the soleus muscle of the decerebrate cat caused by vibbration applied to its tendon. J Physiol. 1966 May;184(2):450–472. doi: 10.1113/jphysiol.1966.sp007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll J. P., Vedel J. P., Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res. 1989;76(1):213–222. doi: 10.1007/BF00253639. [DOI] [PubMed] [Google Scholar]
- Romaiguère P., Vedel J. P., Pagni S. Fluctuations in motor unit recruitment threshold during slow isometric contractions of wrist extensor muscles in man. Neurosci Lett. 1989 Aug 14;103(1):50–55. doi: 10.1016/0304-3940(89)90484-9. [DOI] [PubMed] [Google Scholar]
- Romaiguère P., Vedel J. P., Pagni S., Zenatti A. Physiological properties of the motor units of the wrist extensor muscles in man. Exp Brain Res. 1989;78(1):51–61. doi: 10.1007/BF00230686. [DOI] [PubMed] [Google Scholar]
- Tatton W. G., Forner S. D., Gerstein G. L., Chambers W. W., Liu C. N. The effect of postcentral cortical lesions on motor responses to sudden upper limb displacements in monkeys. Brain Res. 1975 Oct 10;96(1):108–113. doi: 10.1016/0006-8993(75)90580-6. [DOI] [PubMed] [Google Scholar]


