Abstract
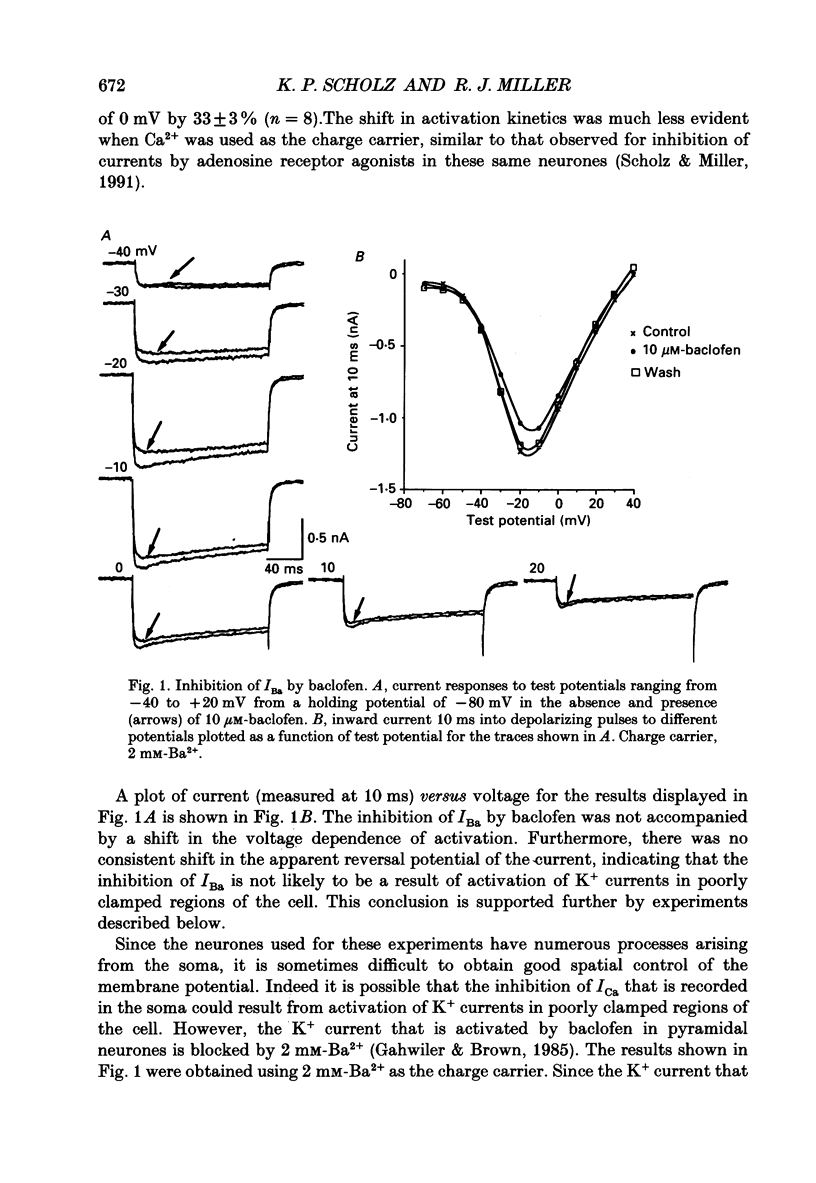
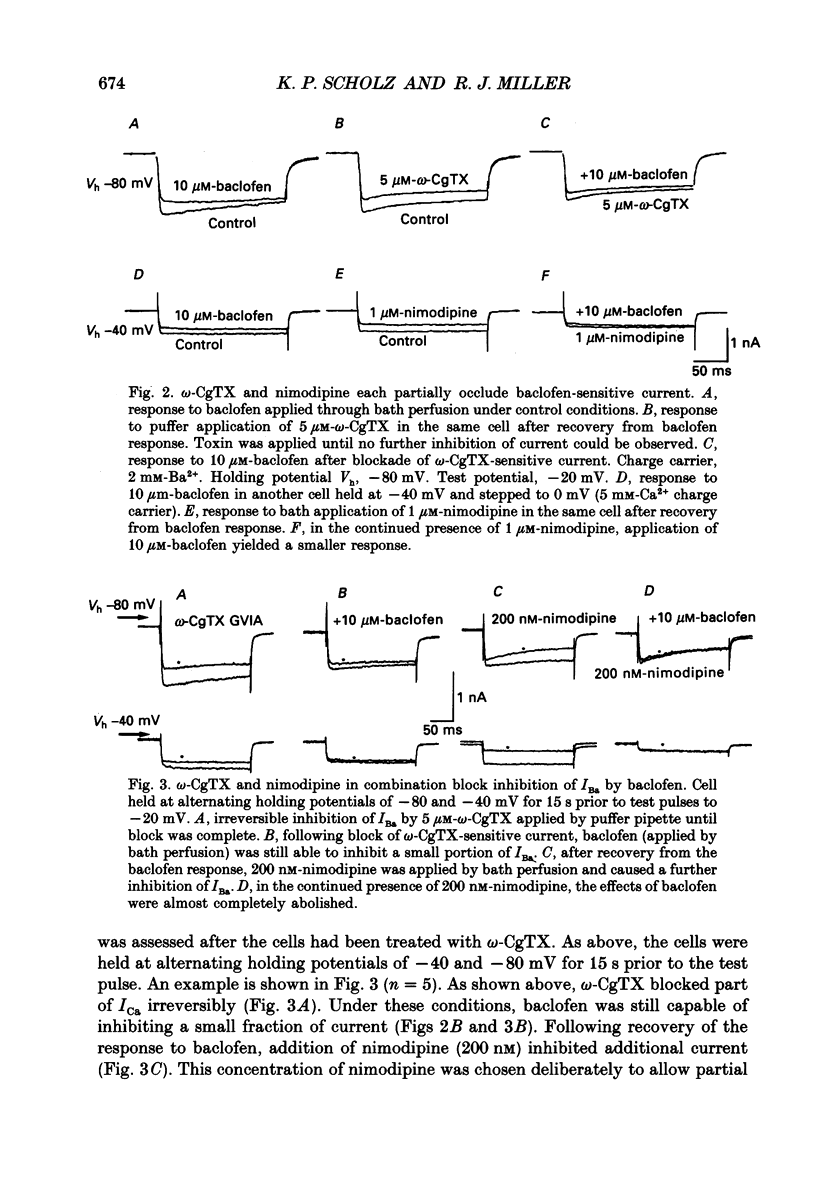
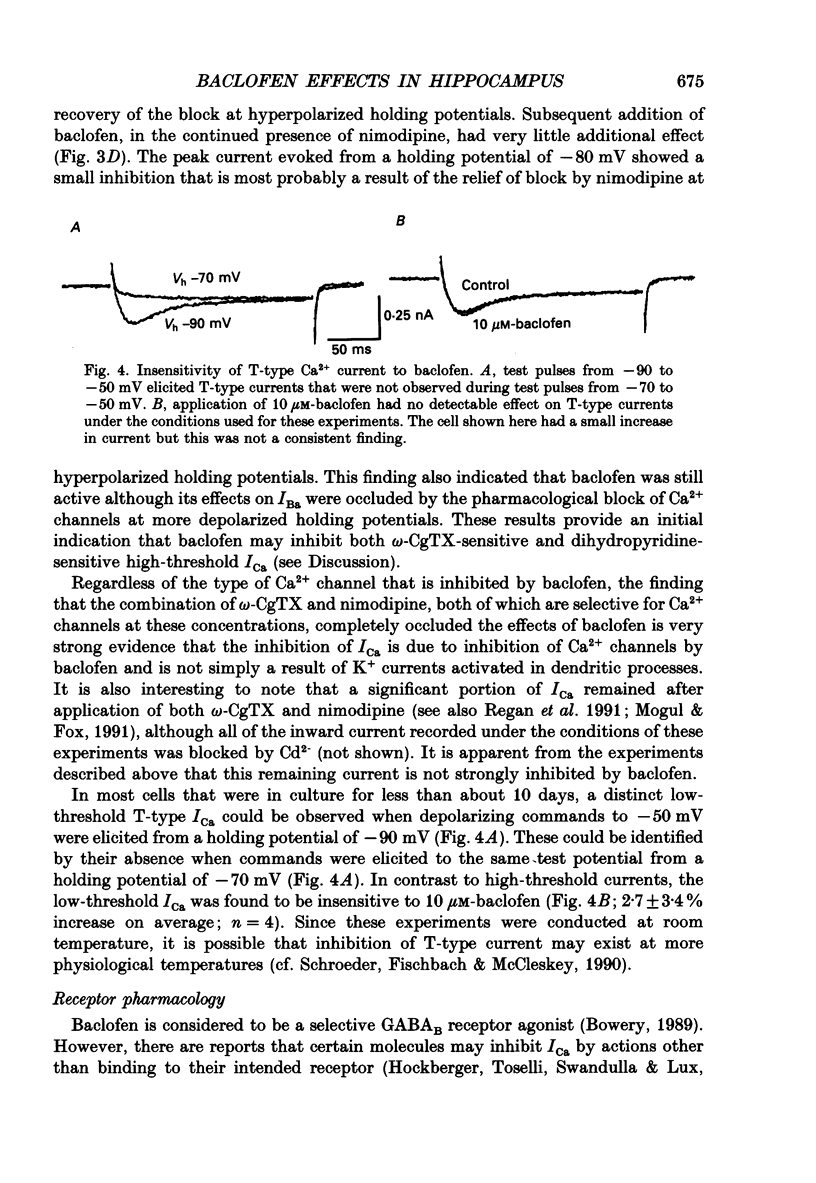
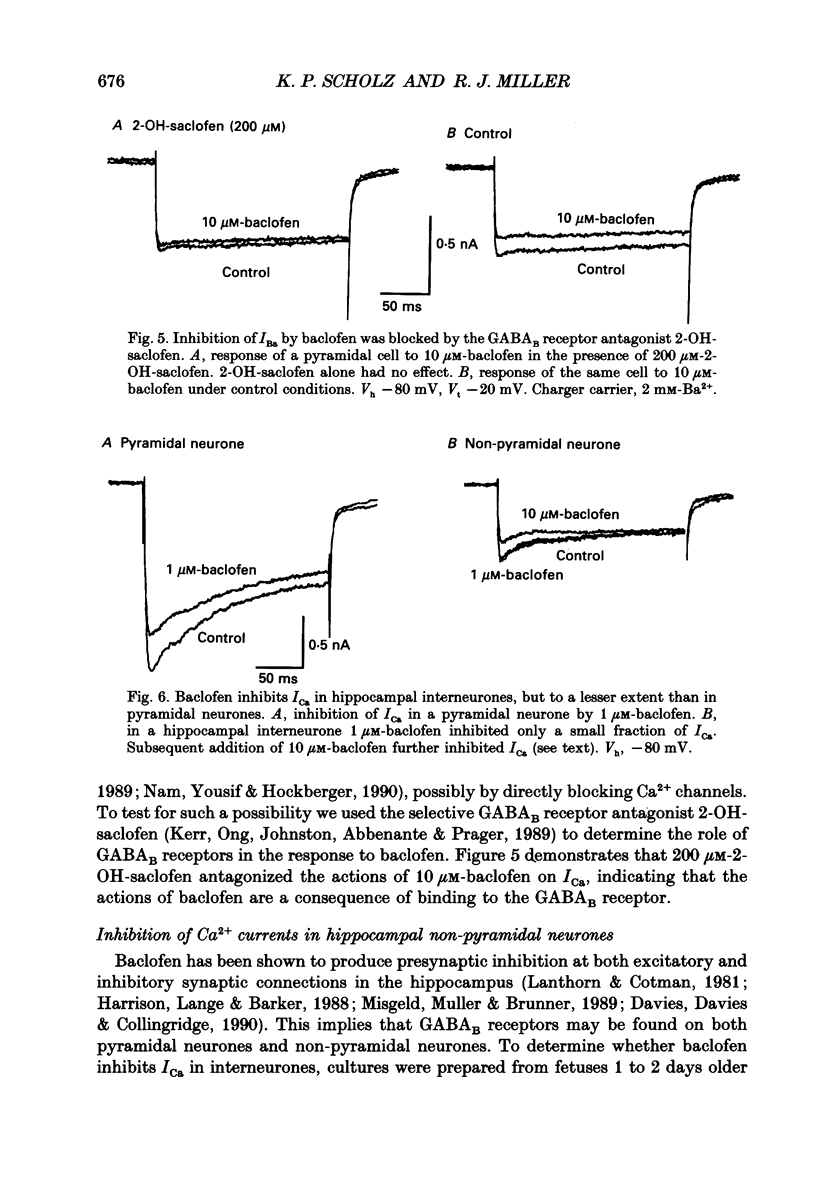
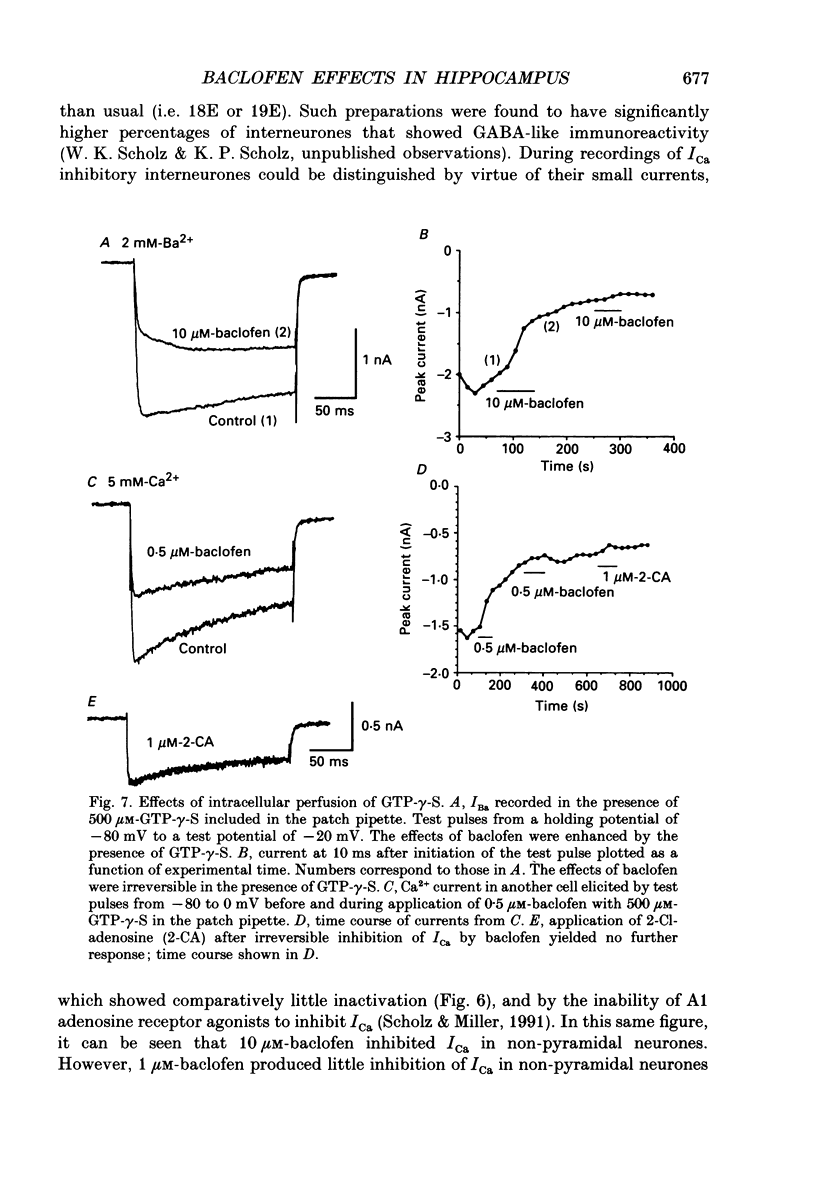
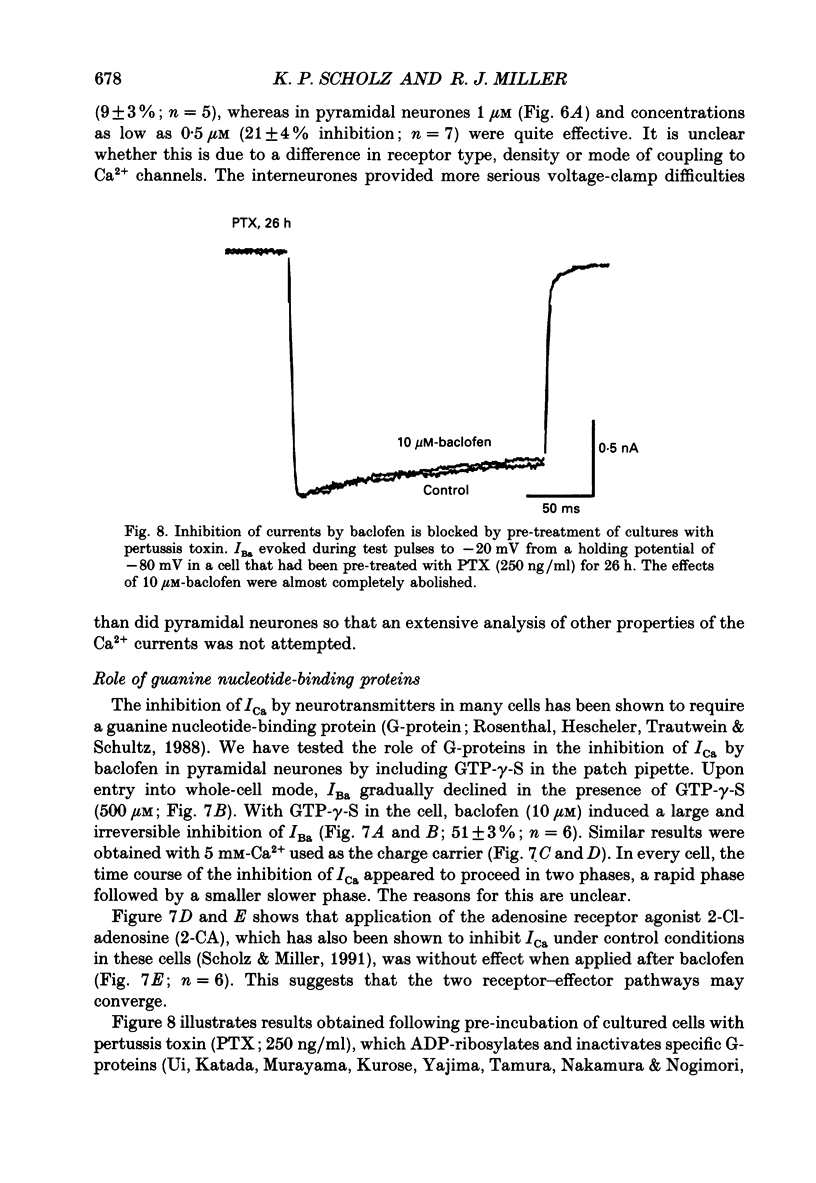
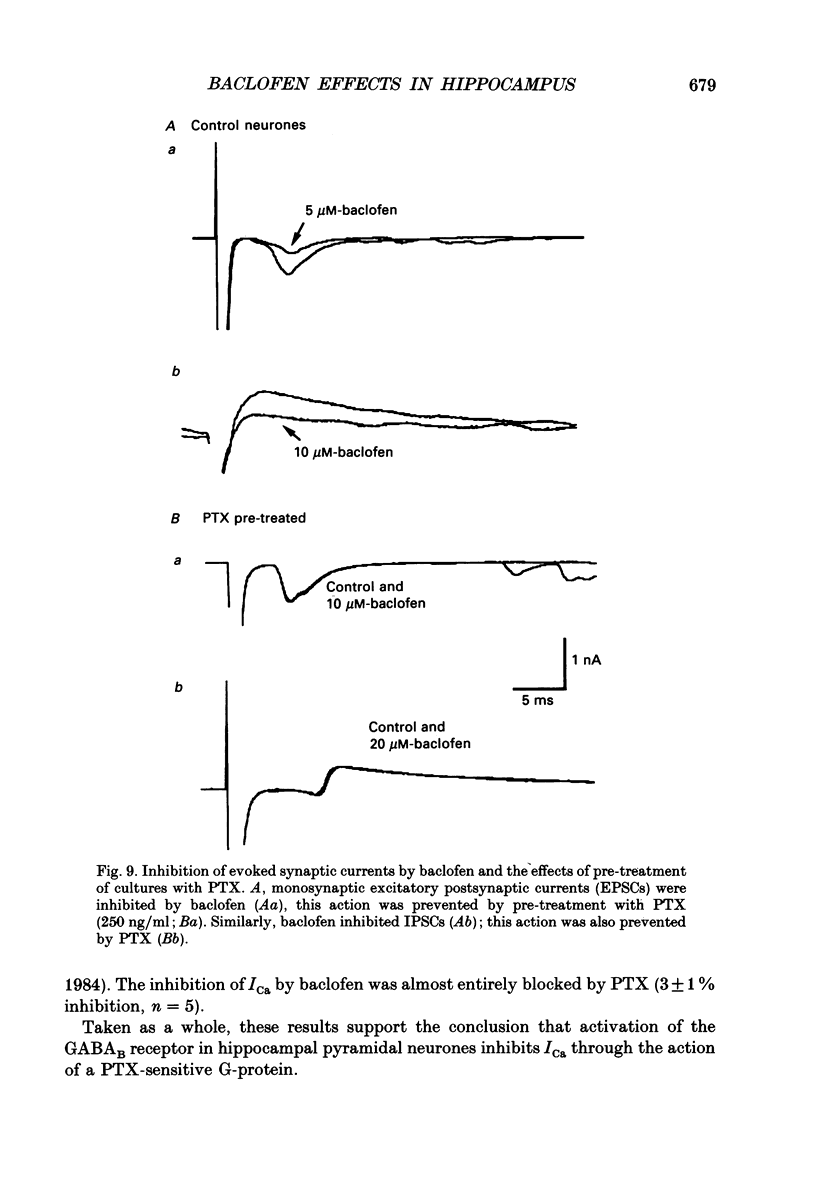
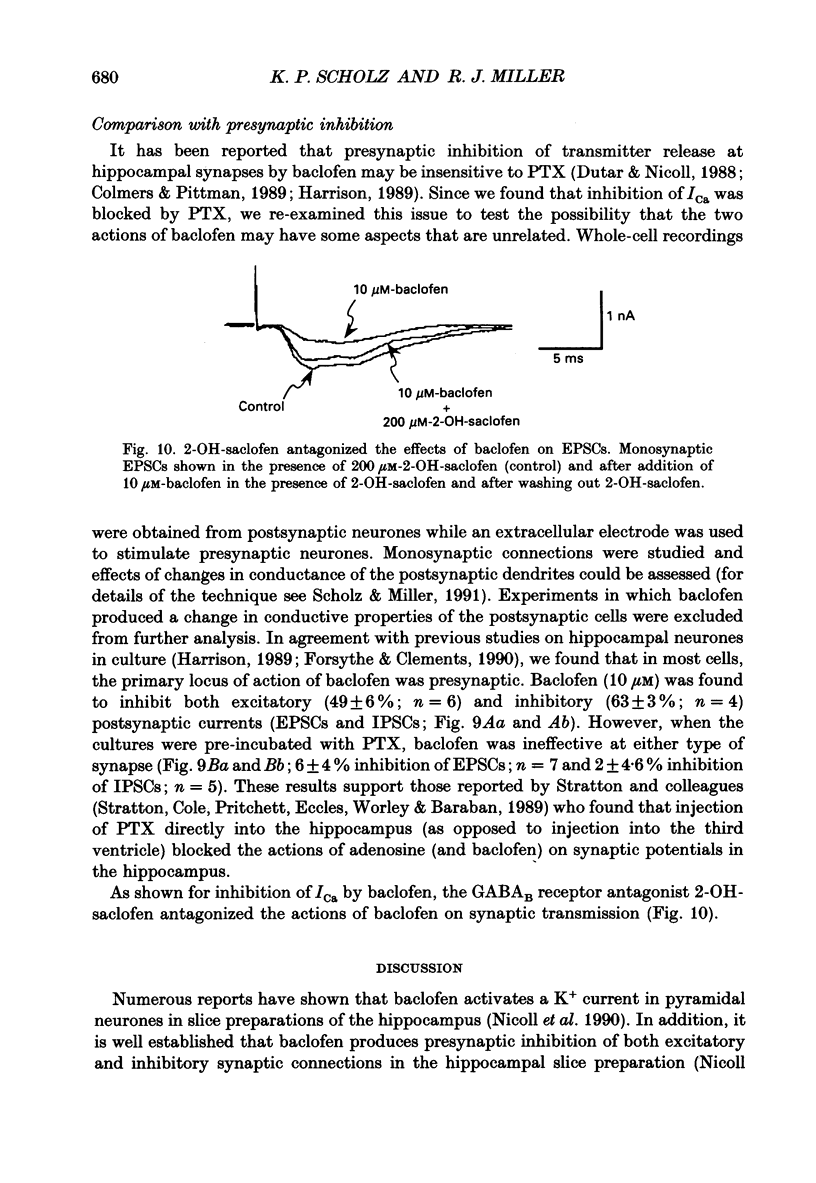
1. The effects of activation of GABAB receptors on Ca2+ currents (ICa) were investigated by application of whole-cell patch-clamp techniques to pyramidal neurones and non-pyramidal interneurones from the rat hippocampus grown in cell culture. 2. (+/-)-Baclofen (10 microM) reduced ICa evoked in pyramidal neurones at 0 mV from a holding potential of -80 mV by 33 +/- 3%. Inhibition could be observed at the peak of ICa with significant inhibition still present after 200 ms at 0 mV. When Ba2+ was used as the charge carrier (IBa) baclofen inhibited 28 +/- 3% of the current at -20 mV from a holding potential of -80 mV. The GABAB receptor antagonist 2-OH-saclofen (50-200 microM) blocked the actions of baclofen. 3. The selective Ca2+ channel blocker, omega-conotoxin fraction GVIA (omega-CgTX), was used to characterize the Ca2+ currents inhibited by baclofen. omega-CgTX (5 microM) blocked 24 +/- 3% of IBa. Following block of the omega-CgTX-sensitive current, baclofen inhibited significantly less current than under control conditions. 4. Addition of the dihydropyridine Ca2+ channel antagonist nimodipine (1 microM) inhibited 18 +/- 5% of ICa at 0 mV from a holding potential of -80 mV and 44 +/- 9% from a holding potential of -40 mV. In addition, nimodipine partially occluded subsequent responses to application of baclofen. 5. In the presence of both 5 microM-omega-CgTX and 200 nM-nimodipine, responses to baclofen were almost completely blocked at depolarized holding potentials where the dihydropyridines are most effective. 6. Inclusion of 500 microM-guanosine 5'-O-(3-thiotriphosphate) (GTP-gamma-S) in the patch pipette enhanced the response to a subsaturating concentration of baclofen and rendered the response irreversible. Subsequent addition of the adenosine receptor agonist 2-Cl-adenosine (2-CA) (1 microM; which also reduces ICa under control conditions) was without effect, suggesting that these two receptor-effector pathways converge. 7. The actions of baclofen on ICa were blocked by pre-treatment of the cultures with pertussis toxin (250 ng/ml). 8. Baclofen also inhibited ICa in non-pyramidal neurones from the hippocampus, but was slightly less effective. 9. Baclofen reduced both excitatory- and inhibitory postsynaptic currents (EPSCs and IPSCs) recorded as a consequence of extracellular stimulation of presynaptic neurones. This action was blocked by 2-OH-saclofen (200 microM) and also by pretreatment of the cultures with pertussis toxin.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker G. A., Cowan W. M. Further observations on hippocampal neurons in dispersed cell culture. J Comp Neurol. 1979 Oct 1;187(3):469–493. doi: 10.1002/cne.901870302. [DOI] [PubMed] [Google Scholar]
- Banker G. A. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980 Aug 15;209(4458):809–810. doi: 10.1126/science.7403847. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bley K. R., Tsien R. W. Inhibition of Ca2+ and K+ channels in sympathetic neurons by neuropeptides and other ganglionic transmitters. Neuron. 1990 Mar;4(3):379–391. doi: 10.1016/0896-6273(90)90050-p. [DOI] [PubMed] [Google Scholar]
- Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol Sci. 1989 Oct;10(10):401–407. doi: 10.1016/0165-6147(89)90188-0. [DOI] [PubMed] [Google Scholar]
- Colmers W. F., Pittman Q. J. Presynaptic inhibition by neuropeptide Y and baclofen in hippocampus: insensitivity to pertussis toxin treatment. Brain Res. 1989 Sep 25;498(1):99–104. doi: 10.1016/0006-8993(89)90403-4. [DOI] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Presynaptic inhibition at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:543–562. doi: 10.1113/jphysiol.1961.sp006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Calcium channel currents and their inhibition by (-)-baclofen in rat sensory neurones: modulation by guanine nucleotides. J Physiol. 1987 May;386:1–17. doi: 10.1113/jphysiol.1987.sp016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988 Sep;1(7):585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Elmslie K. S., Zhou W., Jones S. W. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990 Jul;5(1):75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- Finch D. M., Fisher R. S., Jackson M. B. Miniature excitatory synaptic currents in cultured hippocampal neurons. Brain Res. 1990 Jun 4;518(1-2):257–268. doi: 10.1016/0006-8993(90)90978-k. [DOI] [PubMed] [Google Scholar]
- Fisher R. E., Gray R., Johnston D. Properties and distribution of single voltage-gated calcium channels in adult hippocampal neurons. J Neurophysiol. 1990 Jul;64(1):91–104. doi: 10.1152/jn.1990.64.1.91. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Clements J. D. Presynaptic glutamate receptors depress excitatory monosynaptic transmission between mouse hippocampal neurones. J Physiol. 1990 Oct;429:1–16. doi: 10.1113/jphysiol.1990.sp018240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Lange G. D., Barker J. L. (-)-Baclofen activates presynaptic GABAB receptors on GABAergic inhibitory neurons from embryonic rat hippocampus. Neurosci Lett. 1988 Feb 15;85(1):105–109. doi: 10.1016/0304-3940(88)90437-5. [DOI] [PubMed] [Google Scholar]
- Harrison N. L. On the presynaptic action of baclofen at inhibitory synapses between cultured rat hippocampal neurones. J Physiol. 1990 Mar;422:433–446. doi: 10.1113/jphysiol.1990.sp017993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- Hockberger P., Toselli M., Swandulla D., Lux H. D. A diacylglycerol analogue reduces neuronal calcium currents independently of protein kinase C activation. Nature. 1989 Mar 23;338(6213):340–342. doi: 10.1038/338340a0. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Kream R. M., Spiegel A., Dunlap K. G proteins couple alpha-adrenergic and GABAb receptors to inhibition of peptide secretion from peripheral sensory neurons. J Neurosci. 1989 Feb;9(2):657–666. doi: 10.1523/JNEUROSCI.09-02-00657.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. T., Kunze D. L., Angelides K. J. Localization and mobility of omega-conotoxin-sensitive Ca2+ channels in hippocampal CA1 neurons. Science. 1989 Jun 9;244(4909):1189–1193. doi: 10.1126/science.2543080. [DOI] [PubMed] [Google Scholar]
- Kasai H., Aosaki T., Fukuda J. Presynaptic Ca-antagonist omega-conotoxin irreversibly blocks N-type Ca-channels in chick sensory neurons. Neurosci Res. 1987 Feb;4(3):228–235. doi: 10.1016/0168-0102(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Kasai H., Aosaki T. Modulation of Ca-channel current by an adenosine analog mediated by a GTP-binding protein in chick sensory neurons. Pflugers Arch. 1989 Jun;414(2):145–149. doi: 10.1007/BF00580956. [DOI] [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Johnston G. A., Abbenante J., Prager R. H. 2-Hydroxy-saclofen: an improved antagonist at central and peripheral GABAB receptors. Neurosci Lett. 1988 Sep 23;92(1):92–96. doi: 10.1016/0304-3940(88)90748-3. [DOI] [PubMed] [Google Scholar]
- Lanthorn T. H., Cotman C. W. Baclofen selectively inhibits excitatory synaptic transmission in the hippocampus. Brain Res. 1981 Nov 23;225(1):171–178. doi: 10.1016/0006-8993(81)90326-7. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Receptor-mediated regulation of calcium channels and neurotransmitter release. FASEB J. 1990 Dec;4(15):3291–3299. [PubMed] [Google Scholar]
- Misgeld U., Müller W., Brunner H. Effects of (-)baclofen on inhibitory neurons in the guinea pig hippocampal slice. Pflugers Arch. 1989 Jun;414(2):139–144. doi: 10.1007/BF00580955. [DOI] [PubMed] [Google Scholar]
- Mogul D. J., Fox A. P. Evidence for multiple types of Ca2+ channels in acutely isolated hippocampal CA3 neurones of the guinea-pig. J Physiol. 1991 Feb;433:259–281. doi: 10.1113/jphysiol.1991.sp018425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Regan L. J., Sah D. W., Bean B. P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991 Feb;6(2):269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Rosenthal W., Hescheler J., Trautwein W., Schultz G. Control of voltage-dependent Ca2+ channels by G protein-coupled receptors. FASEB J. 1988 Sep;2(12):2784–2790. doi: 10.1096/fasebj.2.12.2457531. [DOI] [PubMed] [Google Scholar]
- Scholz K. P., Miller R. J. Analysis of adenosine actions on Ca2+ currents and synaptic transmission in cultured rat hippocampal pyramidal neurones. J Physiol. 1991 Apr;435:373–393. doi: 10.1113/jphysiol.1991.sp018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. E., Fischbach P. S., McCleskey E. W. T-type calcium channels: heterogeneous expression in rat sensory neurons and selective modulation by phorbol esters. J Neurosci. 1990 Mar;10(3):947–951. doi: 10.1523/JNEUROSCI.10-03-00947.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. E., Fischbach P. S., Zheng D., McCleskey E. W. Activation of mu opioid receptors inhibits transient high- and low-threshold Ca2+ currents, but spares a sustained current. Neuron. 1991 Jan;6(1):13–20. doi: 10.1016/0896-6273(91)90117-i. [DOI] [PubMed] [Google Scholar]
- Snutch T. P., Leonard J. P., Gilbert M. M., Lester H. A., Davidson N. Rat brain expresses a heterogeneous family of calcium channels. Proc Natl Acad Sci U S A. 1990 May;87(9):3391–3395. doi: 10.1073/pnas.87.9.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K. R., Cole A. J., Pritchett J., Eccles C. U., Worley P. F., Baraban J. M. Intrahippocampal injection of pertussis toxin blocks adenosine suppression of synaptic responses. Brain Res. 1989 Aug 14;494(2):359–364. doi: 10.1016/0006-8993(89)90604-5. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Wakamori M., Akaike N. Hippocampal CA1 pyramidal cells of rats have four voltage-dependent calcium conductances. Neurosci Lett. 1989 Sep 25;104(1-2):229–234. doi: 10.1016/0304-3940(89)90359-5. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the inhibitory action of gamma-amino-butyric acid on neuromuscular transmission in the crayfish. J Physiol. 1966 Mar;183(2):418–432. doi: 10.1113/jphysiol.1966.sp007874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann R. H. Evidence that guanosine triphosphate (GTP)-binding proteins control a synaptic response in brain: effect of pertussis toxin and GTP gamma S on the late inhibitory postsynaptic potential of hippocampal CA3 neurons. J Neurosci. 1988 Dec;8(12):4589–4602. doi: 10.1523/JNEUROSCI.08-12-04589.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Ui M., Katada T., Murayama T., Kurose H., Yajima M., Tamura M., Nakamura T., Nogimori K. Islet-activating protein, pertussis toxin: a specific uncoupler of receptor-mediated inhibition of adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:145–151. [PubMed] [Google Scholar]


