Abstract
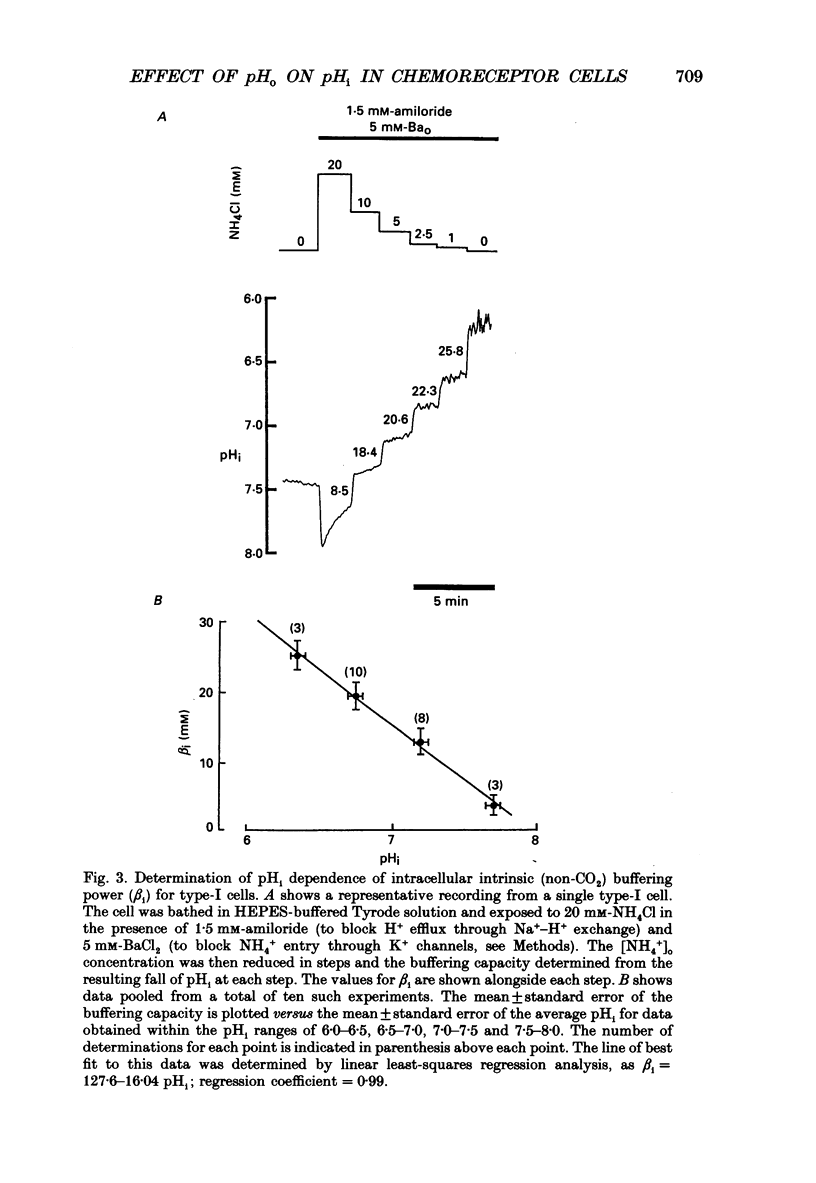
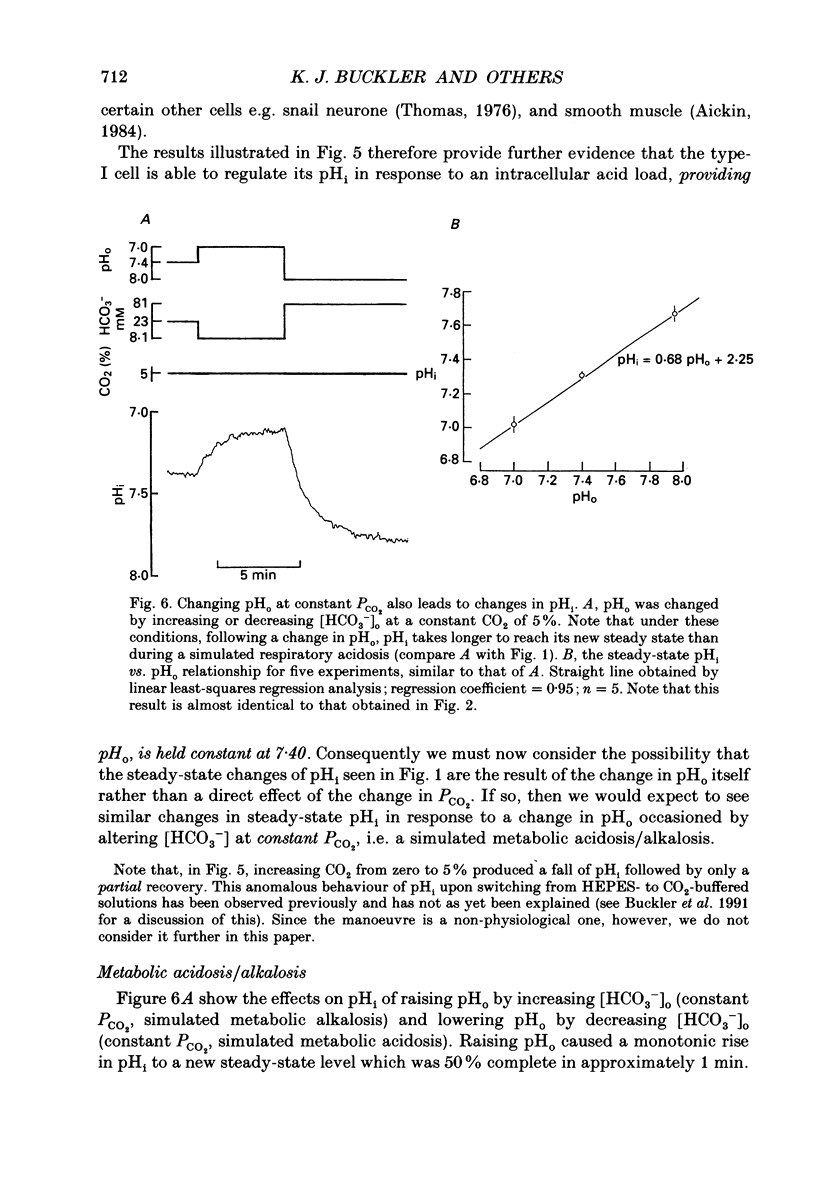
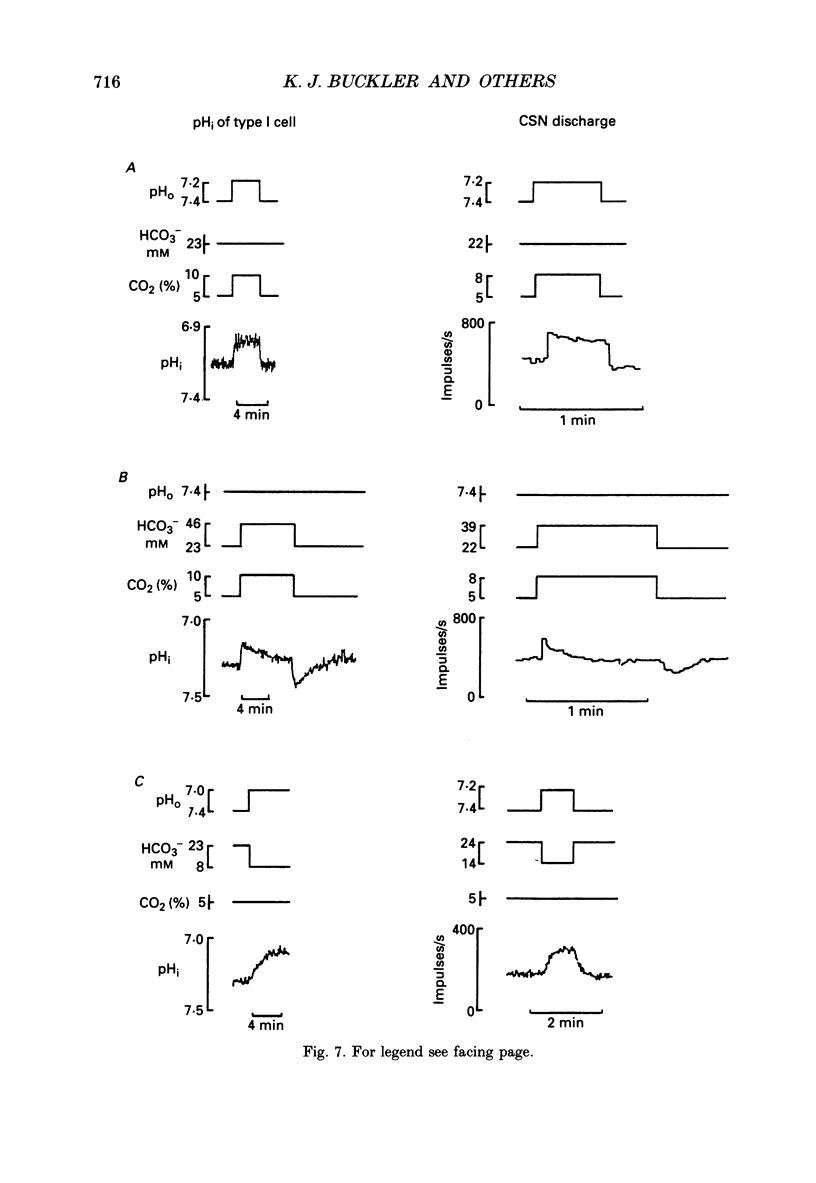
1. The effects of changing PCO2 extracellular pH (pHo) and HCO3- on intracellular pH (pHi) were studied in isolated neonatal rat type-I carotid body cells using the pH-sensitive fluoroprobe, carboxy-SNARF-1. 2. Simulated respiratory acidosis and alkalosis (i.e. changes in PCO2 at constant HCO3-) led to rapid (half-time t0.5 = 3 s) monotonic changes in pHi. The relationship between pHi and pHo under these conditions was linear, steep (0.63 pHi/pHo) and remarkably similar to the response predicted from a passive cell model (i.e. a cell lacking pHi regulation). 3. In order to model the above pHi changes (point 2), it was necessary to determine beta i (intrinsic intracellular buffering power). By using small incremental acid loads in the cell (progressive [NH4+]o removal), beta i was determined as a function of pHi to be: beta i = 127.6-16.04 pHi. 4. Changes in PCO2 at constant pHo (i.e. simultaneously changing HCO3-) caused rapid transient changes in pHi but did not significantly affect steady-state pHi over the range 1-10% CO2. 5. When PCO2 was held constant (5%), changing HCO3- and thus pHo (i.e. a simulated metabolic acidosis/alkalosis) led to much slower changes in pHi (t0.5 approximately 1 min). Steady-state pHi showed an almost identical dependence on pHo (slope 0.68) to that found for simulated respiratory acidosis/alkalosis. Therefore, over the range of pHo, PCO2 and [HCO3-]o tested, steady-state pHi appeared to be a unique function of pHo and independent of PCO2 and [HCO3-]o. 6. The effects on pHi of respiratory acidosis, metabolic acidosis and increases of PCO2 at constant pHo (present work) were compared with previously published work on the ability of similar manoeuvres to increase the carotid sinus nerve (CSN) discharge rate. The two sets of data showed several striking similarities: (i) in both cases, the response to a respiratory acidosis was rapid in onset, maintained and reversible; (ii) in both cases, the speed of response to a metabolic acidosis was significantly slower than in (i) but, again, it was maintained and reversible; (iii) in both cases, increases in PCO2 at constant pHo elicited a rapid response but one which was only transient with no change in the steady-state value. 7. The close correlation between the effects of changing pHo, PCO2 and [HCO3-]o on pHi and on CSN discharge suggests that a change in type-I cell pHi is the first step in the chemoreception of blood pH by the carotid body.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C. Direct measurement of intracellular pH and buffering power in smooth muscle cells of guinea-pig vas deferens. J Physiol. 1984 Apr;349:571–585. doi: 10.1113/jphysiol.1984.sp015174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Baud C., Barish M. E. Changes in membrane hydrogen and sodium conductances during progesterone-induced maturation of Ambystoma oocytes. Dev Biol. 1984 Oct;105(2):423–434. doi: 10.1016/0012-1606(84)90299-9. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R., Eisner D. A., O'Neill S. C., Valdeolmillos M. Measurements of intracellular Ca2+ in dissociated type I cells of the rabbit carotid body. J Physiol. 1989 Sep;416:421–434. doi: 10.1113/jphysiol.1989.sp017769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Responses of type I cells dissociated from the rabbit carotid body to hypoxia. J Physiol. 1990 Sep;428:39–59. doi: 10.1113/jphysiol.1990.sp018199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J., Sampson S. R. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970 May;208(1):121–131. doi: 10.1113/jphysiol.1970.sp009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Vaughan-Jones R. D. Continuous direct measurement of intracellular chloride and pH in frog skeletal muscle. J Physiol. 1977 Sep;270(3):801–833. doi: 10.1113/jphysiol.1977.sp011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C., Vaughan-Jones R. D. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989 Nov;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3-. Am J Physiol. 1988 Dec;255(6 Pt 1):C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990 Oct;417(2):234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D., Peers C., Nye P. C. Intracellular pH and its regulation in isolated type I carotid body cells of the neonatal rat. J Physiol. 1991 May;436:107–129. doi: 10.1113/jphysiol.1991.sp018542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., Thomas R. C. Microelectrode measurement of the intracellular pH of mammalian heart cells. Nature. 1976 Jul 15;262(5565):224–225. doi: 10.1038/262224a0. [DOI] [PubMed] [Google Scholar]
- Giebisch G., Malnic G., De Mello G. B., De Mello Aires M. Kinetics of luminal acidification in cortical tubules of the rat kidney. J Physiol. 1977 Jun;267(3):571–599. doi: 10.1113/jphysiol.1977.sp011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Delpiano M. A., Acker H., Pietruschka F. Ionic currents on type-I cells of the rabbit carotid body measured by voltage-clamp experiments and the effect of hypoxia. Brain Res. 1989 May 1;486(1):79–88. doi: 10.1016/0006-8993(89)91280-8. [DOI] [PubMed] [Google Scholar]
- Izutsu K. T. Intracellular pH, H ion flux and H ion permeability coefficient in bullfrog toe muscle. J Physiol. 1972 Feb;221(1):15–27. doi: 10.1113/jphysiol.1972.sp009735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. Voltage-dependent intracellular pH in Helix aspersa neurones. J Physiol. 1987 Sep;390:433–452. doi: 10.1113/jphysiol.1987.sp016710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Effect of lowered extracellular pH on Ca2(+)-dependent K+ currents in type I cells from the neonatal rat carotid body. J Physiol. 1990 Mar;422:381–395. doi: 10.1113/jphysiol.1990.sp017990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C., Green F. K. Inhibition of Ca(2+)-activated K+ currents by intracellular acidosis in isolated type I cells of the neonatal rat carotid body. J Physiol. 1991 Jun;437:589–602. doi: 10.1113/jphysiol.1991.sp018613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2(+)-activated K+ current. Neurosci Lett. 1990 Nov 13;119(2):253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- Peers C., O'Donnell J. Potassium currents recorded in type I carotid body cells from the neonatal rat and their modulation by chemoexcitatory agents. Brain Res. 1990 Jul 9;522(2):259–266. doi: 10.1016/0006-8993(90)91470-2. [DOI] [PubMed] [Google Scholar]
- Putnam R. W., Grubbs R. D. Steady-state pHi, buffering power, and effect of CO2 in a smooth muscle-like cell line. Am J Physiol. 1990 Mar;258(3 Pt 1):C461–C469. doi: 10.1152/ajpcell.1990.258.3.C461. [DOI] [PubMed] [Google Scholar]
- Ridderstråle Y., Hanson M. A. Histochemical localization of carbonic anhydrase in the cat carotid body. Ann N Y Acad Sci. 1984;429:398–400. doi: 10.1111/j.1749-6632.1984.tb12363.x. [DOI] [PubMed] [Google Scholar]
- Rigual R., Gonzalez E., Gonzalez C., Fidone S. Synthesis and release of catecholamines by the cat carotid body in vitro: effects of hypoxic stimulation. Brain Res. 1986 May 21;374(1):101–109. doi: 10.1016/0006-8993(86)90398-7. [DOI] [PubMed] [Google Scholar]
- Rigual R., Iñiguez C., Carreres J., Gonzalez C. Carbonic anhydrase in the carotid body and the carotid sinus nerve. Histochemistry. 1985;82(6):577–580. doi: 10.1007/BF00489979. [DOI] [PubMed] [Google Scholar]
- Rigual R., López-López J. R., Gonzalez C. Release of dopamine and chemoreceptor discharge induced by low pH and high PCO2 stimulation of the cat carotid body. J Physiol. 1991 Feb;433:519–531. doi: 10.1113/jphysiol.1991.sp018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher A., Obeso A., Gonzalez C., Herreros B. Ionic mechanisms for the transduction of acidic stimuli in rabbit carotid body glomus cells. J Physiol. 1991 Feb;433:533–548. doi: 10.1113/jphysiol.1991.sp018442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A., Nurse C. A. Chloride channels in cultured glomus cells of the rat carotid body. Am J Physiol. 1989 Aug;257(2 Pt 1):C174–C181. doi: 10.1152/ajpcell.1989.257.2.C174. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol. 1984 Sep;354:3P–22P. doi: 10.1113/jphysiol.1984.sp015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkovsky A. M., Richards C. D. Na+/H+ exchange is the major mechanism of pH regulation in cultured sympathetic neurons: measurements in single cell bodies and neurites using a fluorescent pH indicator. Neuroscience. 1987 Sep;22(3):1093–1102. doi: 10.1016/0306-4522(87)92984-8. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. An investigation of chloride-bicarbonate exchange in the sheep cardiac Purkinje fibre. J Physiol. 1986 Oct;379:377–406. doi: 10.1113/jphysiol.1986.sp016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. Extracellular H+ inactivation of Na(+)-H+ exchange in the sheep cardiac Purkinje fibre. J Physiol. 1990 Sep;428:441–466. doi: 10.1113/jphysiol.1990.sp018221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. pH dependence of intrinsic H+ buffering power in the sheep cardiac Purkinje fibre. J Physiol. 1990 Jun;425:429–448. doi: 10.1113/jphysiol.1990.sp018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S. Smooth muscle intracellular pH: measurement, regulation, and function. Am J Physiol. 1988 Feb;254(2 Pt 1):C213–C225. doi: 10.1152/ajpcell.1988.254.2.C213. [DOI] [PubMed] [Google Scholar]


