Abstract
Monogalactosyldiacylglycerol and digalactosyldiacylglycerol are major chloroplast lipids of algae and land plants and are synthesized within the plastid envelope. Here we report that in Toxoplasma gondii and Plasmodium falciparum lysates, radiolabeled UDP-galactose is incorporated into monogalactosylcerebrosides, monogalactosyldiacylglycerol, and digalactosyldiacylglycerol due to distinct enzymological activities. Furthermore, DGDG is immunologically detected in apicomplexans.
Apicomplexan parasites contain a vestigial plastid, the apicoplast, limited by multiple membranes (4, 9, 11, 19). Apicoplast proteins encoded in the nucleus exhibit bipartite N-terminal targeting sequences comprised of a signal peptide and a chloroplast-like transit peptide (1, 6, 17, 18). Therefore, the envelope membranes that are shared between green and nongreen plastids in land plants might be among the multiple membranes limiting the apicoplast (10). A striking feature of the plastid envelope is the synthesis of monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG), which constitute more than 70% of chloroplast lipids of algae and land plants as well as cyanobacteria (3). In this report, we analyzed galactolipid synthesis in Toxoplasma gondii (RH strain grown 72 h in confluent Vero cells, initially infected with 3 × 107 tachyzoites in Dulbecco modified Eagle medium with 2% fetal calf serum) and Plasmodium falciparum (FCBR strain, maintained as previously described [15]).
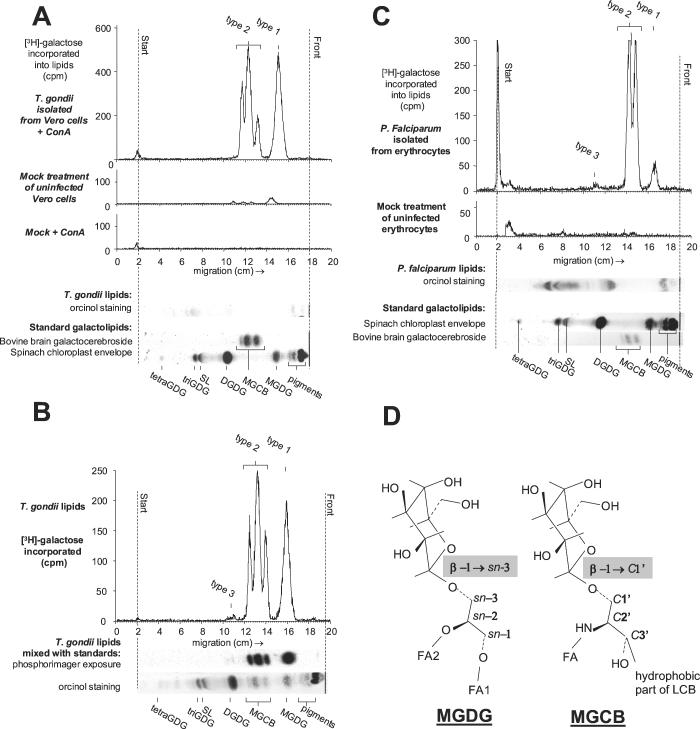
Free T. gondii tachyzoites were released from host cell debris by passing twice through a 25-gauge needle and through a 20-ml glass wool column. T. gondii tachyzoites and P. falciparum cells (50% schizonts in unsynchronized culture) were hypotonically lysed (15). Fresh lysates (2 × 108 T. gondii tachyzoites or 5 × 108 P. falciparum cells) were washed three times in 500 μl of reaction buffer (10 mM MOPS [morpholinepropanesulfonic acid], pH 7.8, 1 mM dithiothreitol, 2% [wt/vol] glycerol, 50 mM KCl) and incubated with 4 μCi of UDP-[3H]galactose (75 μM in 100 μl of reaction buffer). Incubation was stopped by glycolipid extraction (12), and thin-layer chromatography (TLC) on 60-μm-thick silica gel plates resolved with chloroform-methanol-water (65:25:4, vol/vol/vol). Labeled lipids were detected with a TLC analyzer (LB2842 automatic TLC scanner). Complete identification of lipids included glycolipid orcinol staining, comigration with standards, hydrolysis of the polar head groups by green coffee bean α-galactosidase and bovine testes β-galactosidase, and deacylation by mild alkaline hydrolysis (5). We identified three types of radiolabeled galactolipids: type 1 comigrated with MGDG from spinach chloroplasts, type 2 comigrated with monogalactosylcerebrosides (MGCB) from bovine brain, and type 3 comigrated with DGDG from spinach chloroplast (Fig. 1).
FIG. 1.
Synthesis of galactolipids in T. gondii and P. falciparum. After incubation with tritiated UDP-galactose, lipids were extracted and separated by TLC (chromatograms [A, B, and C]), followed by phosphorimager detection after a 2-week exposure (B). Glycolipids were compared with standard MGCB from bovine brain and MGDG and DGDG from spinach chloroplast envelope membranes. (A) A mock preparation of parasites, using uninfected Vero cells, incorporated galactose (mock treatment of uninfected Vero cells). When we preincubated the membrane suspension with concanavalin A (50 μg for 30 min at 37°C), a lectin with affinity for the rich mannose decoration of mammalian CGalT (UDP-galactose:ceramide galactosyltransferase), aggregates appeared in the suspension and the pattern of incorporation of tritiated UDP-galactose due to the broken Vero cells disappeared (mock + ConA). T. gondii incorporation of galactose was insensitive to concanavalin A treatment (T. gondii isolated from Vero cells + ConA). (C) Erythrocytes uninfected with P. falciparum did not yield significant amounts of labeled glycolipids (mock treatment of uninfected erythrocytes). Standards were either loaded in separate lanes (A and C) or mixed with the parasite sample to demonstrate comigration of the radiolabeled parasite lipids with the galactolipid standards (B). Shown are syntheses of the following: MGDG and MGCB in T. gondii lysates after a 1-h incubation in the absence of Mg2+ (A), digalactolipids in T. gondii lysates after a 1-h incubation in the presence of 5 mM MgCl2 (B), and MGDG, MGCB, and DGDG in P. falciparum after a 1-h incubation in the absence of Mg2+ (C). Counts were integrated for 60 min (A), 30 min (B), and 120 min (C). SL, sulfoquinovosyldiacylglycerol; triGDG, trigalactosyldiacylglycerol; tetraGDG, tetragalactosyldiacylglycerol. (D) Structures of polar portions of MGDG and MGCB. MGDG is made of a glycerol backbone (sn nomenclature of the three carbons) esterified by two fatty acids in the sn-1 and sn-2 positions (FA1 and FA2) and O-galactosylated in the sn-3 position. In MGCB, only the hydrophilic part of the long chain base (LCB) of the ceramide portion is indicated (C1′ to C3′ nomenclature of the first three carbons). The LCB is N-esterified to a fatty acid in C2′, making up the ceramide moiety, and O-galactosylated in the C1′ position.
The types 1 and 2 lipids were equally sensitive to β-galactosidase treatments, indicating that galactose was incorporated with a β-linkage like those in MGDG and MGCB (Fig. 1D). The type 1 lipid was completely deacylated by mild alkaline hydrolysis, demonstrating that its hydrophobic moiety was a diglyceride. Additionally, the type 1 lipid comigrated with spinach chloroplast MGDG in two-dimensional TLC, as described previously (12). These points indicate that type 1 is similar to plant chloroplast MGDG. The type 2 lipid showed three peaks in T. gondii (Fig. 1A and B); relative quantities were stable from one experiment to another. These were not affected by alkaline treatment, showing that their hydrophobic moiety was not a diglyceride. In T. gondii, the type 2 lipids peaks comigrated with the three complex ceramide structures of the bovine brain standard: the upper band contained nonhydroxylated fatty acids, whereas the middle and lower bands contained α-hydroxylated fatty acids, the lowest band being enriched in shorter chains (13). We report therefore MGCB production in P. falciparum and T. gondii and show that the ceramide composition between these two species differs since the lowest MGCB band was absent in P. falciparum (Fig. 1C). Synthesis of MGDG was two times more sensitive to EDTA inhibition than the MGCB synthetic activity. In addition, only MGDG synthesis was enhanced by Mg2+ whereas only MGCB synthesis was inhibited by Ca2+. An MGDG/MGCB synthesis ratio of 0.48 ± 0.01 was measured in control conditions, decreasing to 0.21 ± 0.06 in the presence of 1 mM EDTA and increasing to 0.8 (0.71 ± 0.08 on average) in the presence of 5 mM Mg2+. These data strongly suggest that an MGDG synthase and a ceramide galactosyltransferase occur in T. gondii. As for plant chloroplast MGDG synthase (8) and mammal endoplasmic reticulum CGalT (16), UDP-glucose was a competitive inhibitor (Table 1), but while large amounts of glucosylcerebrosides were synthesized by T. gondii lysates, monoglucosyldiacylglycerol (MGlcDG) was barely detected. Therefore, the MGDG synthase from T. gondii resembles the land plant MGDG synthase, which is inhibited by UDP-glucose but does not utilize this sugar donor for MGlcDG synthesis (8). In addition, T. gondii MGDG synthesis exhibits enzymological features similar to those of the activity measured in plant chloroplast envelope membranes (8), i.e., an inhibition by EDTA, an enhancement by Mg2+, and an inhibition by Zn2+.
TABLE 1.
Effects of bivalent cations and UDP-glucose on MGDG and MGCB syntheses in T. gondii
| Galactolipid synthesized | [3H]galactose incorporation (% of control)
|
|||||
|---|---|---|---|---|---|---|
| Control | + EDTA, 1 mM | + MgCl2, 5 mM | + CaCl2, 5 mM | + ZnCl2, 1 mM | + UDP-Glc, 10 mM | |
| MGCB | 100 | 57 | 107 | 76 | 66 | NDa |
| MGDG | 100 | 16 | 152 | 108 | 73 | ND |
ND, not detected.
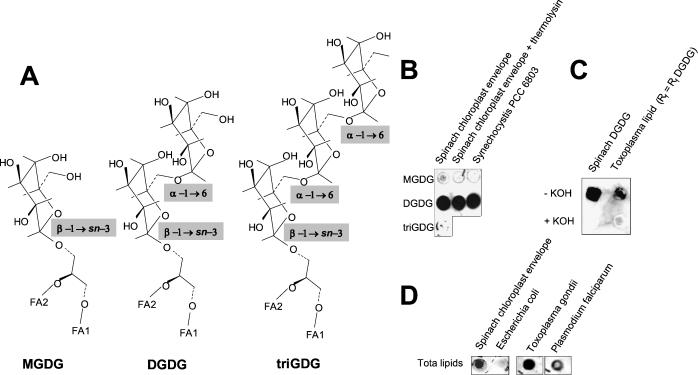
The type 3 lipids were not affected by β-galactosidase treatment and decreased after an α-galactosidase incubation and mild alkaline hydrolysis, like plant DGDG. In T. gondii, the weakly labeled peak 3 (Fig. 1B) was broad and was never completely hydrolyzed by mild alkaline hydrolysis, indicating that the hydrophobic moiety partially contained diacylglycerol. We produced specific polyclonal antibodies by immunizing rabbits with 2.5 mg of pure spinach chloroplast DGDG, and immunoblottings were performed as previously described (5). The antibody reacted with DGDG from spinach chloroplast and from cyanobacteria (Fig. 2B) and did not react with MGDG, trigalactosyldiacylglycerol (triGDG) (Fig. 2A), or lipids from Escherichia coli (Fig. 2D), which are rich in phospho- and glycolipids but devoid of DGDG (2). Attempts to label subcellular structures of T. gondii with the polyclonal anti-DGDG antibody failed. When lipid extracts from T. gondii (2 × 109 tachyzoites) or P. falciparum (109 cells) were analyzed by TLC, no orcinol-stained glycolipid could be detected at the precise level of DGDG (Fig. 1A and C). However, after a blind scraping of the TLC plate at the Rf level of DGDG and blotting on nitrocellulose, the anti-DGDG antibody reacted with the corresponding spots from T. gondii and P. falciparum (Fig. 2C and D). In addition, when the scraped lipids from T. gondii or from spinach chloroplast DGDG were hydrolyzed by mild alkaline treatment and the remaining lipids were reextracted, the anti-DGDG antibody failed to react with any spot (Fig. 2C), confirming that the hydrophobic moiety of the spotted lipid was a diacylglycerol. Together, these data show that at least a portion of the type 3 lipids correspond to a chloroplastic-like DGDG.
FIG. 2.
Immunodetection of DGDG in lipid extracts from T. gondii and P. falciparum. (A) Comparison of MGDG, DGDG, and triGDG polar heads. (B) The specificity of the anti-DGDG antibody for the [α-d-galactopyranosyl-(1→6)-O-β-d-galactopyranosyl[ polar head was investigated by dot blot immunostaining (1/100 dilution of anti-DGDG antibody) of 10 μg of lipids purified from spinach chloroplast envelope membranes (first two lanes) or Synechocystis PCC 6803, a cyanobacterium. The anti-DGDG antibody was detected on DGDG spots whereas it did not react with MGDG or triGDG. (C) T. gondii lipids extracted from 2 × 109 free tachyzoites were separated by TLC as shown in Fig. 1. Silica at the level of the DGDG standard was scraped, and lipids were extracted (those with an Rf equal to the Rf of DGDG) and analyzed by dot blot immunostaining. The anti-DGDG antibody reacted with this spot, whereas it did not when the extracted lipids were subjected to mild alkaline hydrolysis and reextracted (+KOH). A similar immunostaining pattern was obtained with a spinach chloroplast DGDG control subjected to identical treatments. The loss of immunostaining after mild KOH hydrolysis showed that the immunostained lipids were deacylated. (D) Dot blot immunostaining with the anti-DGDG antibody of total lipid mixtures from spinach chloroplast envelope membranes (10 μg of lipids), E. coli membranes (10 μg of lipids), T. gondii (total lipid extracts from 2 × 109 tachyzoites), and P. falciparum (total lipid extracts from 109 cells).
In conclusion, the in vitro synthesis of galactolipids in apicomplexans is reported for the first time. We searched T. gondii and P. falciparum databases but found no sequences having a statistically reliable similarity with the primary sequences of ER CGalT, chloroplast envelope MGDG synthase, or DGDG synthase multigenic families. Future prospects therefore include the identification, characterization, and unambiguous subcellular localization of the apicomplexan enzymes responsible for MGCB, MGDG, and DGDG syntheses and the investigation on possible homologies with the corresponding plant enzymes.
Acknowledgments
We thank G. Ajlani, M. F. Cesbron-Delauw, R. Douce, J. F. Dubremetz, J. Kimmel, and C. Mercier.
This work was supported by grants from the Conseil Régional Rhône-Alpes, the Ministries of foreign affairs of France and Germany, the Conselho nacional de desenvolvimento científico e tecnológico of Brasil, the Deutsche Forschungsgemeinschaft, Fonds der Chemischen Industrie, Stiftung, P. E. Kempkes, and the Human Frontier Scientific Program.
REFERENCES
- 1.DeRocher, A., C. B. Hagen, J. E. Froehlich, J. E. Feagin, and M. Parsons. 2000. Analysis of targeting sequences demonstrates that trafficking to the Toxoplasma gondii plastid branches off the secretory system. J. Cell Sci. 113:3969-3977. [DOI] [PubMed] [Google Scholar]
- 2.Dörmann, P., I. Balbo, and C. Benning. 1999. Arabidopsis galactolipid biosynthesis and lipid trafficking mediated by DGD1. Science 284:2181-2184. [DOI] [PubMed] [Google Scholar]
- 3.Douce, R. 1974. Site of biosynthesis of galactolipids in spinach chloroplasts. Science 183:852-853. [DOI] [PubMed] [Google Scholar]
- 4.Feagin, J. E. 1994. The extrachromosomal DNAs of apicomplexan parasites. Annu. Rev. Microbiol. 48:81-104. [DOI] [PubMed] [Google Scholar]
- 5.Gerold, P., and R. T. Schwarz. 2001. Biosynthesis of glycosphingolipids de-novo by the human malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 112:29-37. [DOI] [PubMed] [Google Scholar]
- 6.Jomaa, H., J. Wiesner, S. Sanderbrand, B. Altincicek, C. Weidemeyer, M. Hintz, I. Turbachova, M. Eberl, J. Zeidler, H. K. Lichtenthaler, D. Soldati, and E. Beck. 1999. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285:1573-1576. [DOI] [PubMed] [Google Scholar]
- 7.Jorasch, P., D. C. Warnecke, B. Lindner, U. Zahringer, and E. Heinz. 2000. Novel processive and nonprocessive glycosyltransferases from Staphylococcus aureus and Arabidopsis thaliana synthesize glycoglycerolipids, glycophospholipids, glycosphingolipids and glycosylsterols. Eur. J. Biochem. 267:3770-3783. [DOI] [PubMed] [Google Scholar]
- 8.Joyard, J., and R. Douce. 1987. Galactolipid synthesis, p. 215-274. In P. K. Stumpf (ed.), The biochemistry of plants, vol. 9. Lipids: structure and function. Academic Press, New York, N.Y.
- 9.Köhler, S., C. F. Delwiche, P. W. Denny, L. G. Tilney, P. Webster, R. J. Wilson, J. D. Palmer, and D. Roos. 1997. A plastid of probable green algal origin in Apicomplexan parasites. Science 275:1485-1489. [DOI] [PubMed] [Google Scholar]
- 10.Maréchal, E., and M. F. Cesbron-Delauw. 2001. The apicoplast: a new member of the plastid family. Trends Plant Sci. 6:200-205. [DOI] [PubMed] [Google Scholar]
- 11.McFadden, G. I., M. E. Reith, J. Munholland, and N. Lang-Unnasch. 1996. Plastid in human parasites. Nature 381:482. [DOI] [PubMed] [Google Scholar]
- 12.Miège, C., E. Maréchal, M. Shimojima, K. Awai, M. A. Block, H. Ohta, K.-I. Takamiya, R. Douce, and J. Joyard. 1999. Biochemical and topological properties of Type A MGDG synthase, a spinach chloroplast envelope enzyme catalyzing the synthesis of both prokaryotic and eukaryotic MGDG. Eur. J. Biochem. 265:990-1001. [DOI] [PubMed] [Google Scholar]
- 13.Nakakuma, H., M. Arai, T. Kawaguchi, K. Horikawa, M. Hidaka, K. Sakamoto, M. Iwamori, Y. Nagai, and K. Takatsuki. 1989. Monoclonal antibody to galactosylceramide: discrimination of structural difference in the ceramide moiety. FEBS Lett. 258:230-232. [DOI] [PubMed] [Google Scholar]
- 14.Striepen, B., J. F. Dubremetz, and R. T. Schwarz. 1999. Glucosylation of glycosylphosphatidylinositol membrane anchors: identification of uridine diphosphate-glucose as the direct donor for side chain modification in Toxoplasma gondii using carbohydrate analogues. Biochemistry 38:1478-1487. [DOI] [PubMed] [Google Scholar]
- 15.Trager, W., and J. B. Jensen. 1977. Human parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 16.van der Bijl, P., G. J. Strous, M. Lopes-Cardozo, J. Thomas-Oates, and G. van Meer. 1996. Synthesis of non-hydroxy-galactosylceramides and galactosyldiglycerides by hydroxy-ceramide galactosyltransferase. Biochem. J. 317:589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waller, R. F., P. J. Keeling, R. G. Donald, B. Striepen, E. Handman, N. Lang-Unnasch, A. F. Cowman, G. S. Besra, D. S. Roos, and G. I. McFadden. 1998. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 95:12352-12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waller, R. F., M. B. Reed, A. F. Cowman, and G. I. McFadden. 2000. Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J. 19:1794-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson, R. J., P. W. Denny, P. R. Preiser, K. Rangachari, K. Roberts, A. Roy, A. Whyte, M. Strath, D. J. Moore, P. W. Moore, and D. H. Williamson. 1996. Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 261:155-172. [DOI] [PubMed] [Google Scholar]