Abstract
1. Extracellular recordings were made in three monkeys while recording from neurones in the motor cortex (eighty-four cells), ventro-posterior lateralis pars caudalis (VPLc, forty-two cells) and cerebellar thalamus (seventy-seven cells). 2. This experiment was designed to produce active and reflex movements of varying velocities in order to study the relationship between amplitude of velocity and magnitude of neuronal discharge of thalamic neurones. The active movements were voluntary rapid alternating movements (RAMs) of the wrist and the reflex movements were produced by forcibly oscillating the wrist joint between frequencies of 1 and 7 Hz (forced oscillations). 3. This study was also designed to examine cerebellar influences on a reflex path, namely the transcortical reflex loop. Forced oscillations were predicted to provide circumstances where active damping was required to prevent excessive oscillations in the reflex path. Rapid alternating movements of the wrist were predicted to provide circumstances where oscillations at the natural frequency in that reflex path would support and propagate the movements. 4. Forced oscillations from 1 to 7 Hz produced movements of different velocities. VPLc and cerebellar thalamic neurones discharged in relation to the duration of movement in a particular direction, but their discharge levels were unrelated to the magnitude of the velocity. Motor cortex neurones fired in a pattern which was related to the timing but not the magnitude of the acceleration. 5. In forced oscillations of the wrist the resonant frequency was between 3 and 7 Hz. They may be controlled in part by a transcortical reflex. The cerebellar thalamic neurones did not fire before motor cortex neurones. Therefore, it is unlikely that the cerebello-thalamo-cortical pathway is necessary to damp these potentially unstable oscillations by an effect on antagonist-related cortical neurones. 6. Rapid alternating movements (RAMs) of monkeys' wrists were performed in a stereotyped fashion over a narrow range of frequencies with the greatest displacement in joint angle and peak velocity at the natural frequency of 3-5 Hz. 7. During the performance of RAMs, neuronal discharge modulated sinusoidally in the VPLc, cerebellar thalamus and motor cortex. There was no relationship between velocity and neuronal discharge of the cerebellar thalamic and motor cortical neurones but there did appear to be a relationship between velocity and VPLc neuronal discharge. 8. The onset of electromyogram (EMG) discharge changed earlier than neuronal discharge in the motor cortex and thalamus during the performance of RAMs.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



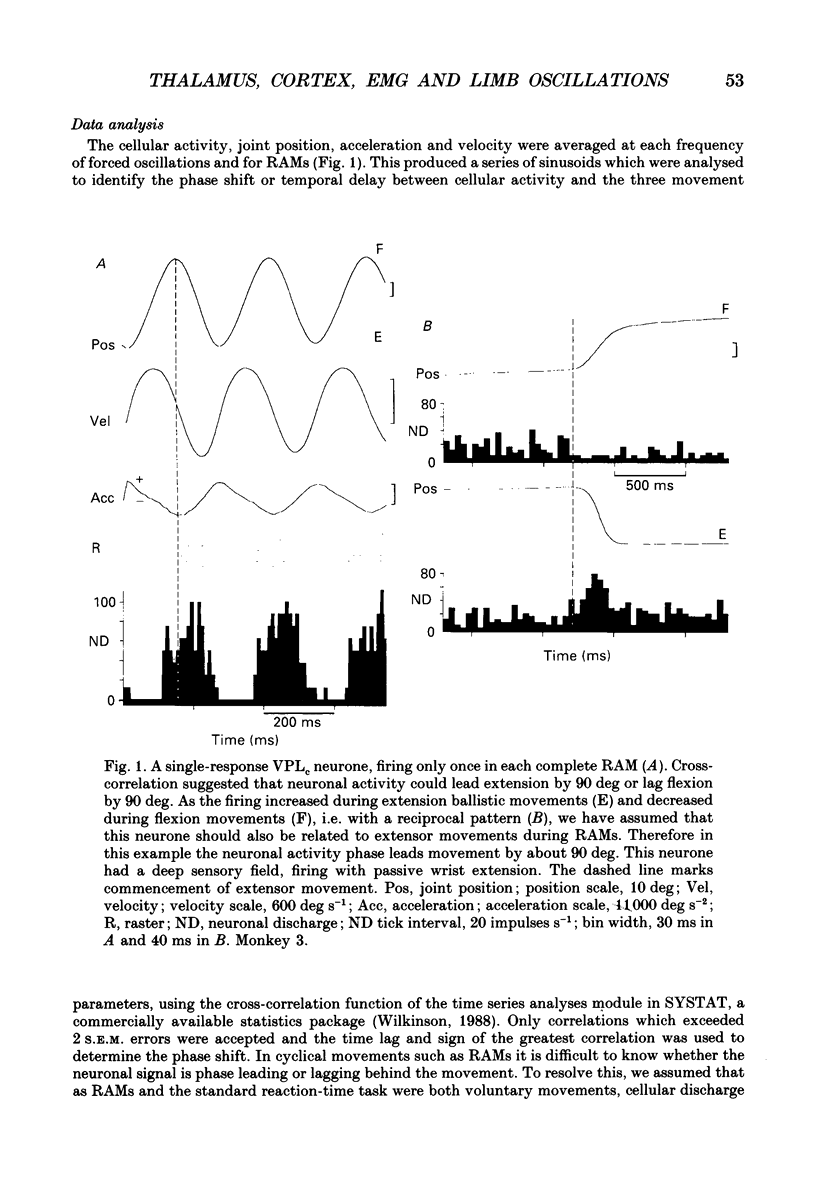




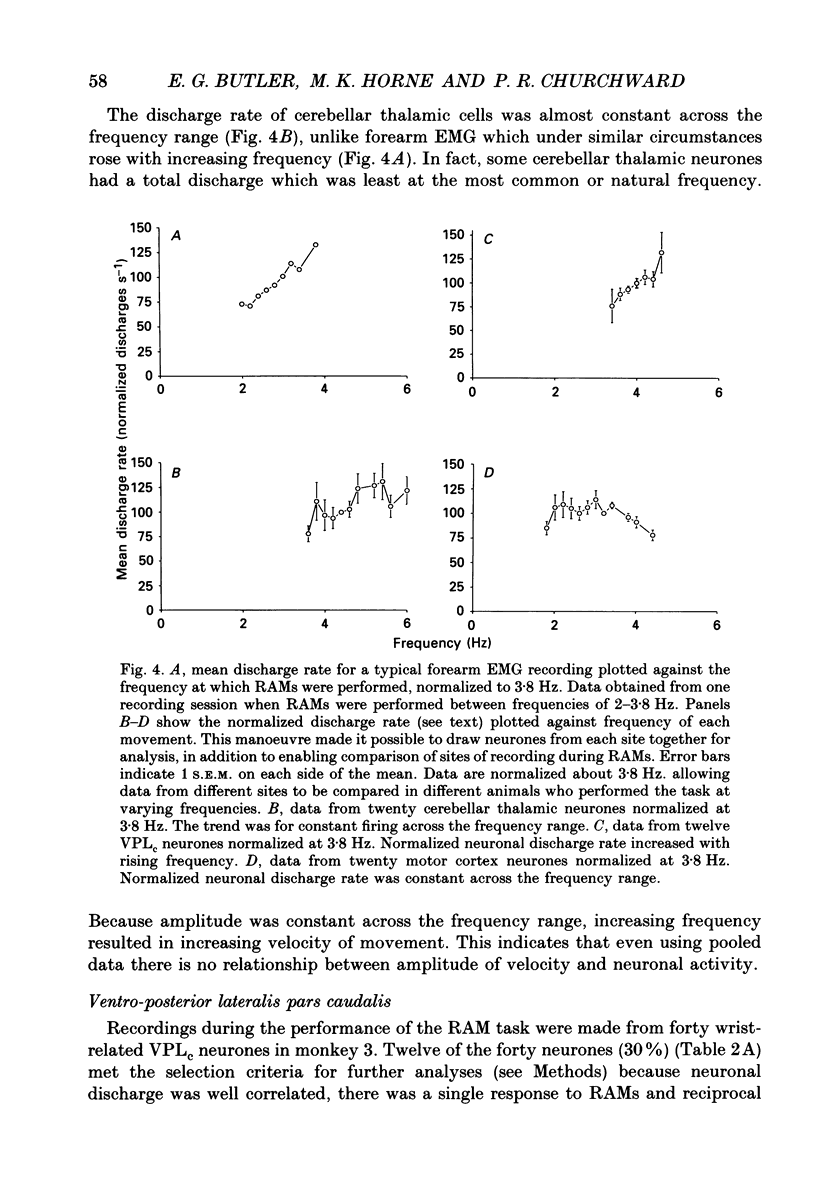

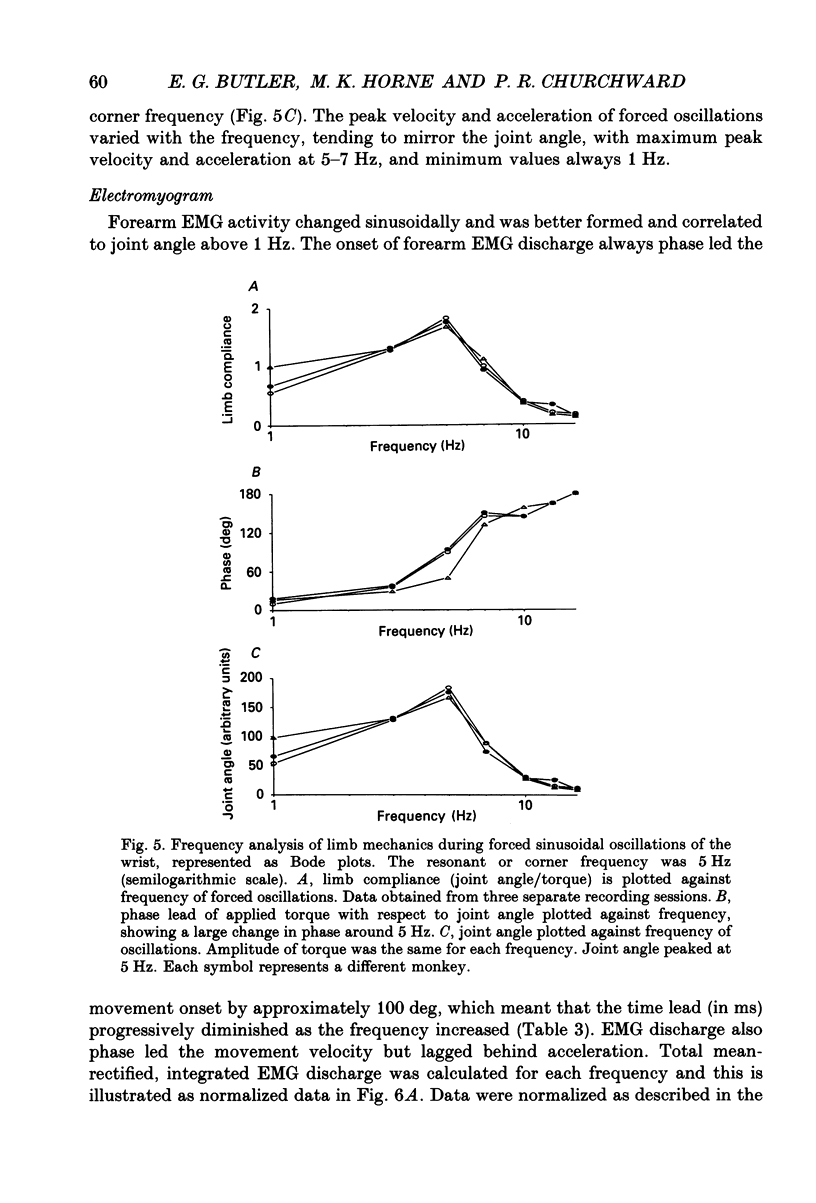

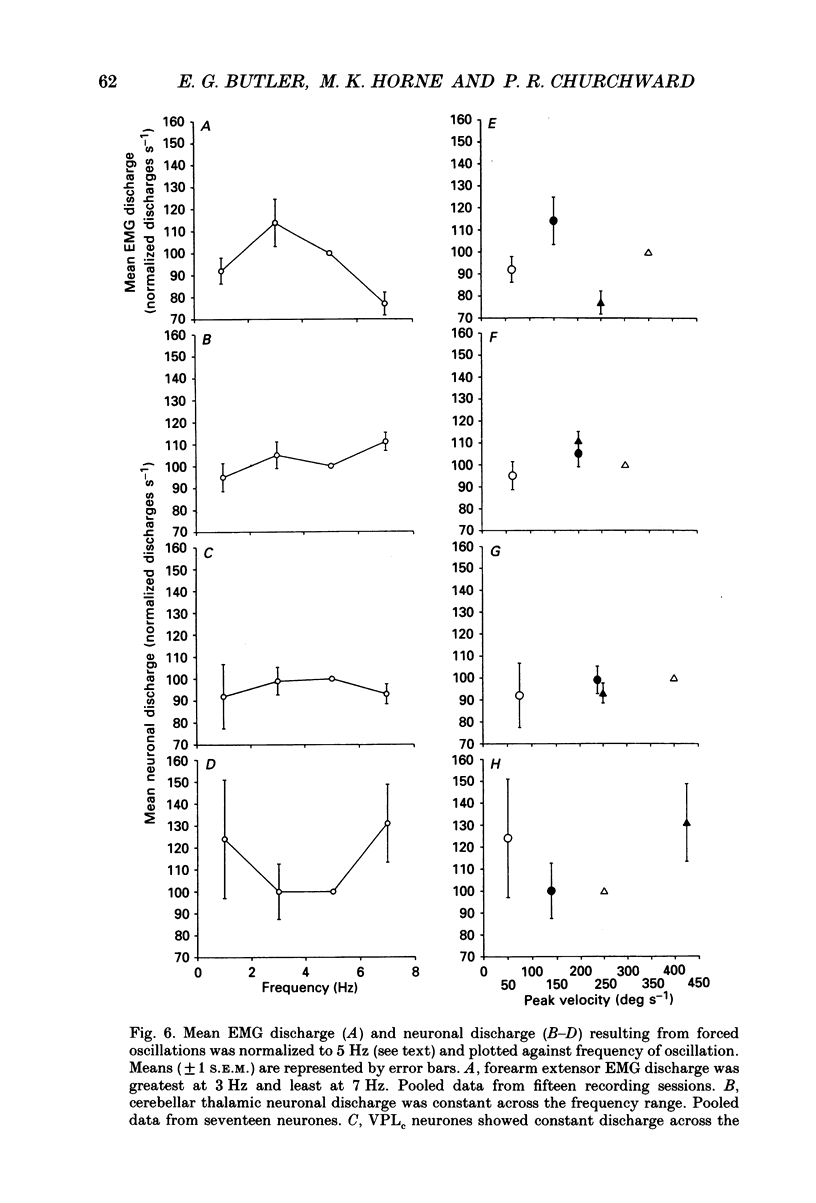






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler E. G., Horne M. K., Hawkins N. J. The activity of monkey thalamic and motor cortical neurones in a skilled, ballistic movement. J Physiol. 1992 Jan;445:25–48. doi: 10.1113/jphysiol.1992.sp018910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler E. G., Horne M. K., Rawson J. A. Sensory characteristics of monkey thalamic and motor cortex neurones. J Physiol. 1992 Jan;445:1–24. doi: 10.1113/jphysiol.1992.sp018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney P. D., Fetz E. E. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol. 1984 Apr;349:249–272. doi: 10.1113/jphysiol.1984.sp015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad B., Matsunami K., Meyer-Lohmann J., Wiesendanger M., Brooks V. B. Cortical load compensation during voluntary elbow movements. Brain Res. 1974 May 17;71(2-3):507–514. doi: 10.1016/0006-8993(74)90994-9. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980 Oct;44(4):751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Flament D., Hore J. Relations of motor cortex neural discharge to kinematics of passive and active elbow movements in the monkey. J Neurophysiol. 1988 Oct;60(4):1268–1284. doi: 10.1152/jn.1988.60.4.1268. [DOI] [PubMed] [Google Scholar]
- Gilman S., Carr D., Hollenberg J. Kinematic effects of deafferentation and cerebellar ablation. Brain. 1976 Jun;99(2):311–330. doi: 10.1093/brain/99.2.311. [DOI] [PubMed] [Google Scholar]
- Goldberger M. E., Growdon J. H. Pattern of recovery following cerebellar deep nuclear lesions in monkeys. Exp Neurol. 1973 Mar-Apr;39(2):307–322. doi: 10.1016/0014-4886(73)90233-1. [DOI] [PubMed] [Google Scholar]
- Hore J., Vilis T. Loss of set in muscle responses to limb perturbations during cerebellar dysfunction. J Neurophysiol. 1984 Jun;51(6):1137–1148. doi: 10.1152/jn.1984.51.6.1137. [DOI] [PubMed] [Google Scholar]
- Ito M., Shiida T., Yagi N., Yamamoto M. Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res. 1974 Jan 4;65(1):170–174. doi: 10.1016/0006-8993(74)90344-8. [DOI] [PubMed] [Google Scholar]
- Jones G. M., Watt D. G. Observations on the control of stepping and hopping movements in man. J Physiol. 1971 Dec;219(3):709–727. doi: 10.1113/jphysiol.1971.sp009684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay W. A., Murphy J. T. Cerebellar modulation of reflex gain. Prog Neurobiol. 1979;13(4):361–417. doi: 10.1016/0301-0082(79)90004-2. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Rothwell J. C., Day B. L. Long-latency automatic responses to muscle stretch in man: origin and function. Adv Neurol. 1983;39:509–539. [PubMed] [Google Scholar]
- Meyer-Lohmann J., Conrad B., Matsunami K., Brooks V. B. Effects of dentate cooling on precentral unit activity following torque pulse injections into elbow movements. Brain Res. 1975 Aug 29;94(2):237–251. doi: 10.1016/0006-8993(75)90059-1. [DOI] [PubMed] [Google Scholar]
- Nashner L. M. Adapting reflexes controlling the human posture. Exp Brain Res. 1976 Aug 27;26(1):59–72. doi: 10.1007/BF00235249. [DOI] [PubMed] [Google Scholar]
- Neilson P. D. Frequency-response characteristics of the tonic stretch reflexes of biceps brachii muscle in intact man. Med Biol Eng. 1972 Jul;10(4):460–472. doi: 10.1007/BF02474194. [DOI] [PubMed] [Google Scholar]
- Neilson P. D. Speed of response or bandwidth of voluntary system controlling elbow position in intact man. Med Biol Eng. 1972 Jul;10(4):450–459. doi: 10.1007/BF02474193. [DOI] [PubMed] [Google Scholar]
- Robinson D. A. Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol. 1976 Sep;39(5):954–969. doi: 10.1152/jn.1976.39.5.954. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Oğuztöreli M. N. Tremor and other oscillations in neuromuscular systems. Biol Cybern. 1976;22(3):147–157. doi: 10.1007/BF00365525. [DOI] [PubMed] [Google Scholar]
- Vilis T., Hore J. Central neural mechanisms contributing to cerebellar tremor produced by limb perturbations. J Neurophysiol. 1980 Feb;43(2):279–291. doi: 10.1152/jn.1980.43.2.279. [DOI] [PubMed] [Google Scholar]
- Vilis T., Hore J. Effects of changes in mechanical state of limb on cerebellar intention tremor. J Neurophysiol. 1977 Sep;40(5):1214–1224. doi: 10.1152/jn.1977.40.5.1214. [DOI] [PubMed] [Google Scholar]


