Abstract
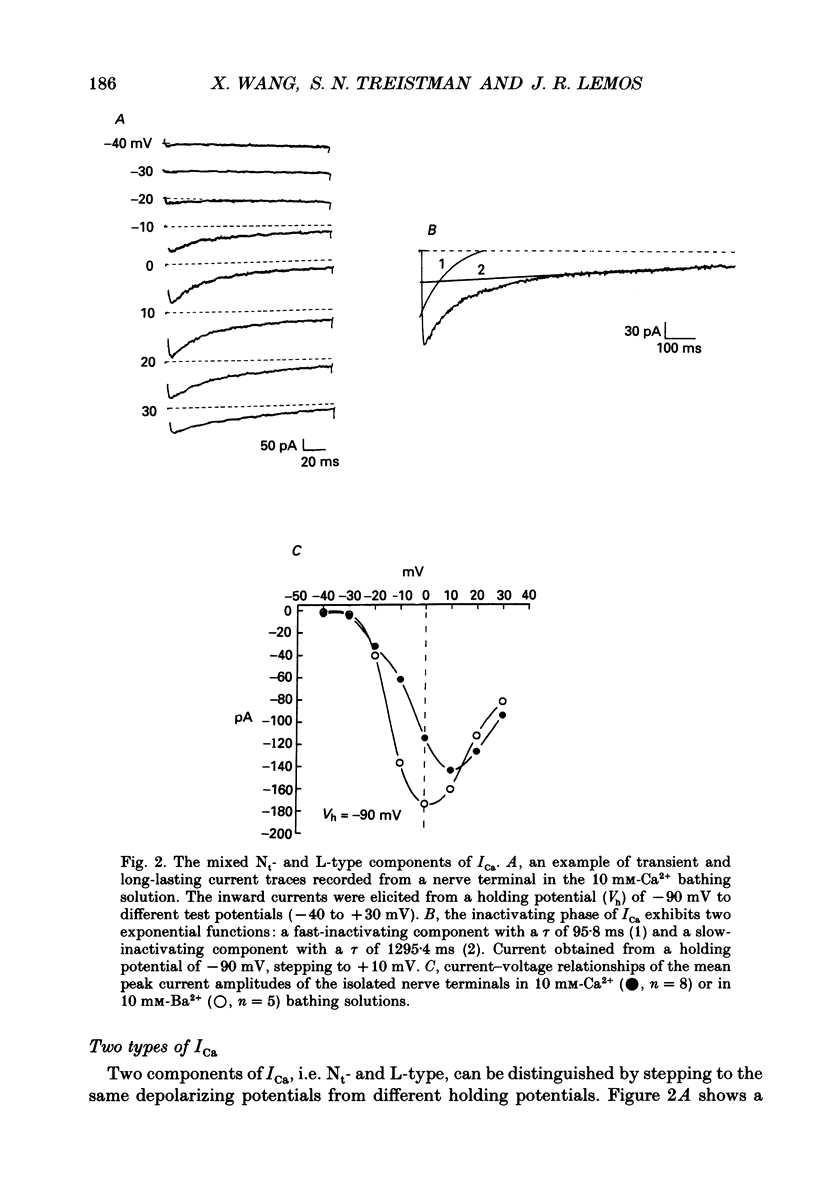
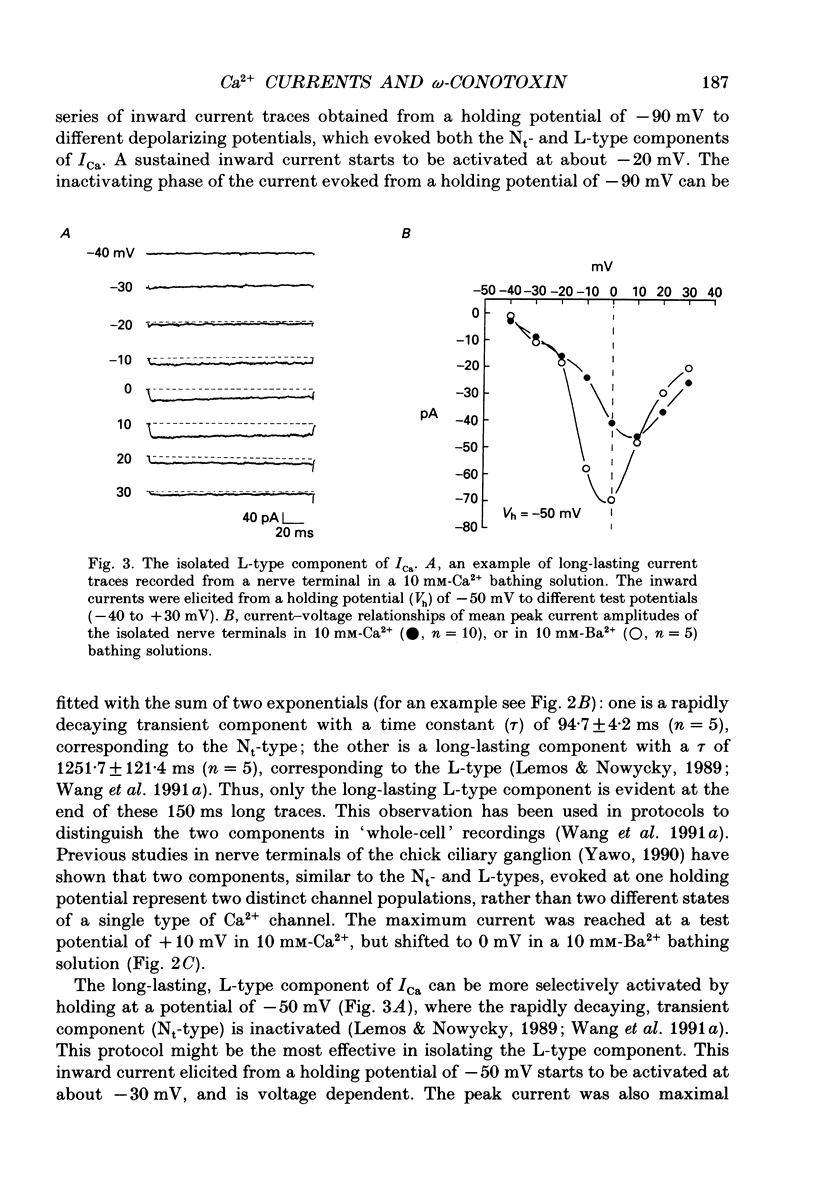
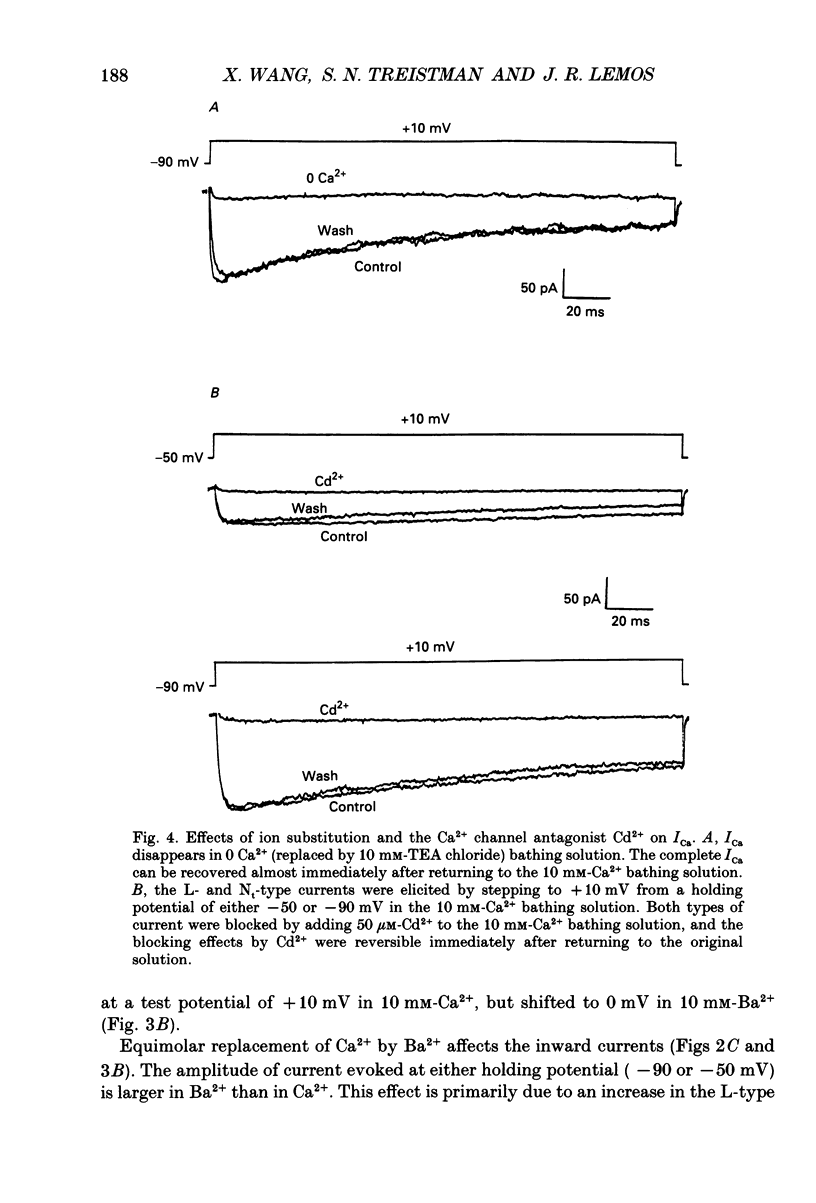
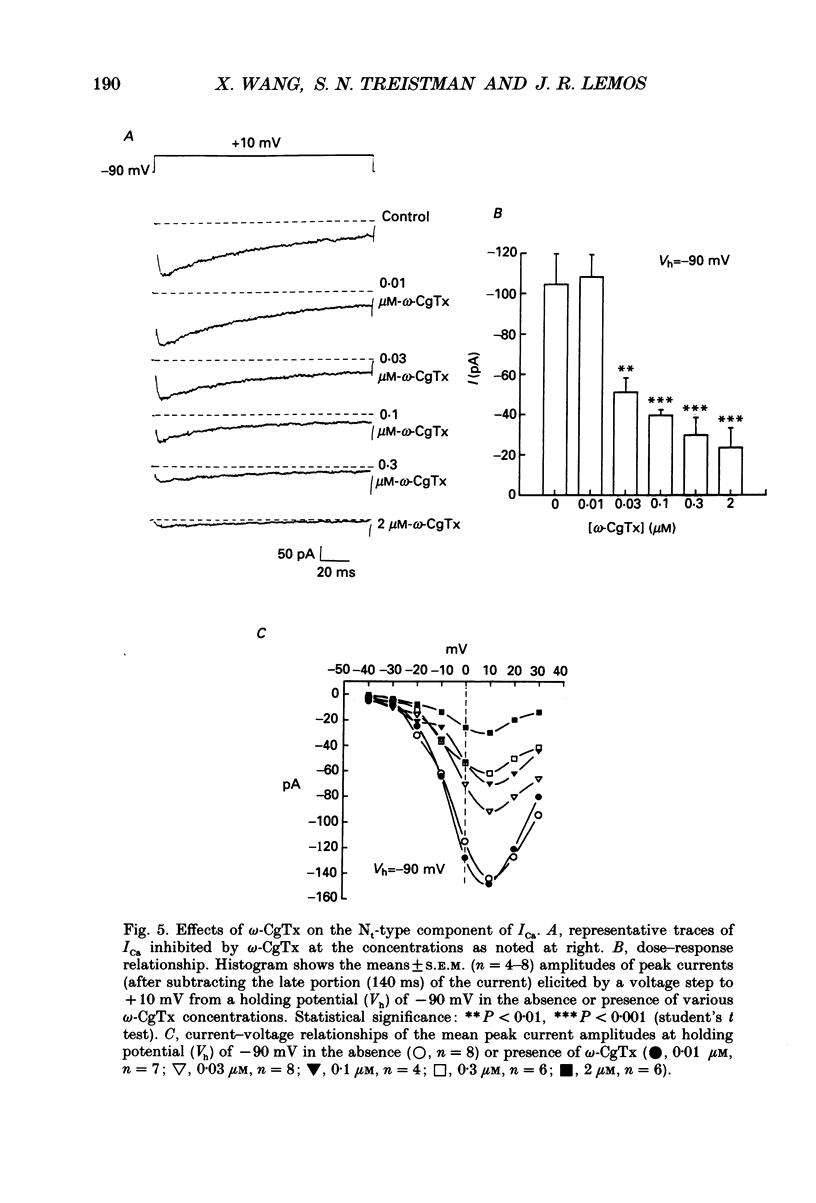
1. The neurohypophysis comprises the nerve terminals of hypothalamic neurosecretory cells, which contain arginine vasopressin (AVP) and oxytocin. The secretory terminals of rat neurohypophyses were acutely dissociated. The macroscopic calcium currents (ICa) of these isolated peptidergic terminals were studied using 'whole-cell' patch-clamp recording techniques. 2. There are two types ('Nt' (where the subscript 't' denotes terminal) and 'L') of high-threshold voltage-activated ICa in the terminals, which can be distinguished by holding at different potentials i.e. -90 and -50 mV. Replacement of Ca2+ in the bathing solution by Ba2+ increased the amplitude of ICa, primarily due to an increase in the L-type component. Both inward currents were eliminated by adding 50 microM-Cd2+ or when in a Ca(2+)-free bathing solution. 3. omega-Conotoxin GVIA (omega-CgTx) has been widely used as a Ca2+ channel blocker. However, whether this toxin can discriminate between different types of Ca2+ channels is still a subject of controversy. We applied omega-CgTx over a wide range of concentrations (0.01-2 microM) to examine its effects on both Nt- and L-type ICa in these terminals. At a concentration of 30 nM, omega-CgTx selectively reduced, by 48%, the amplitude of Nt-type ICa. In contrast, a higher concentration (300 nM) of omega-CgTx was necessary to inhibit the L-type ICa. 4. omega-CgTx inhibited both Nt- and L-type ICa in a dose-dependent manner, and the half-maximum inhibition (IC50) of the ICa by the toxin was 50 and 513 nM, respectively, which was approximately a tenfold difference. The reduction in both types of currents did not result from any shift in their current-voltage or steady-state inactivation relationships. 5. In contrast, omega-CgTx, at a concentration of 300 nM, had no effect on the tetrodotoxin-sensitive sodium current (INa) of the isolated peptidergic nerve terminals. Furthermore, omega-CgTx did not reduce the long-lasting, non-inactivating ICa in the isolated non-neuronal secretory cells of the pars intermedia (PI) (intermediate lobe of the pituitary). 6. Our studies suggest that omega-CgTx might exert specific blocking effects on both Nt- and L-type Ca2+ channels, but that in the isolated peptidergic nerve terminals, the Nt-type component is more susceptible to this toxin.
Full text
PDF





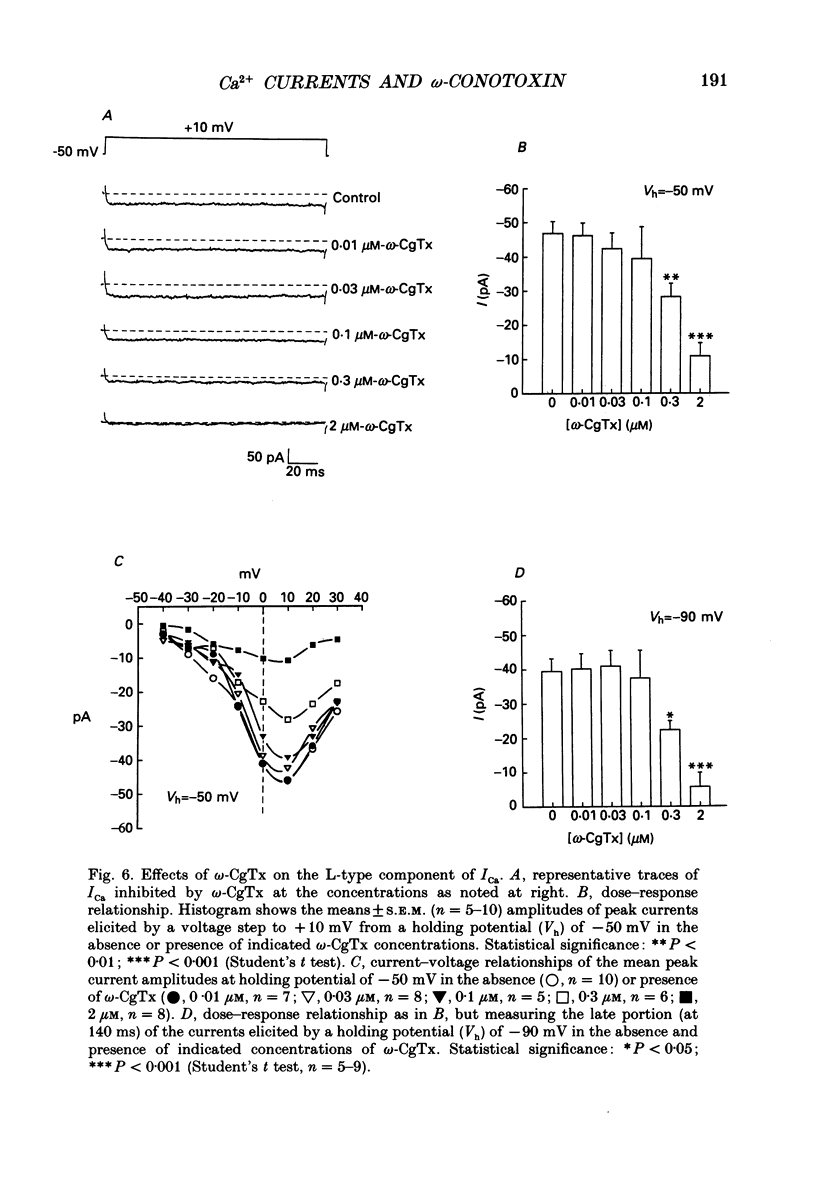
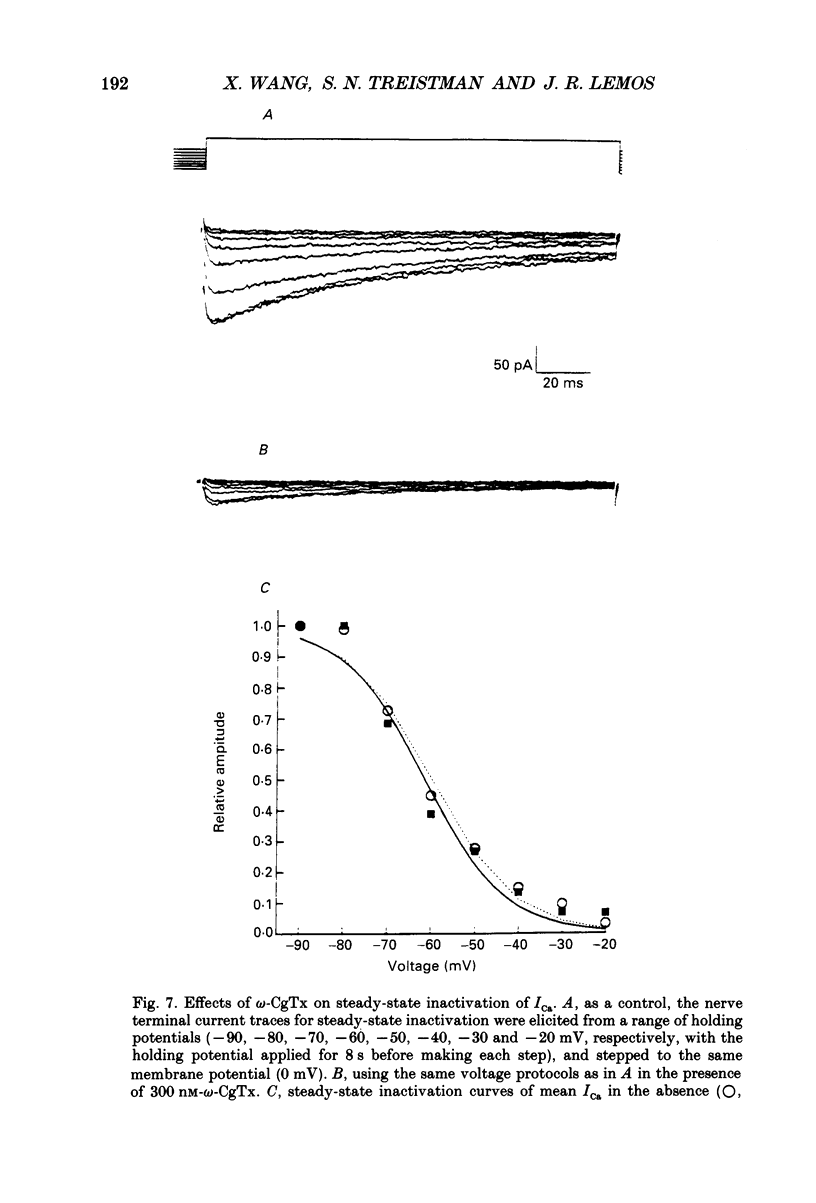






Images in this article
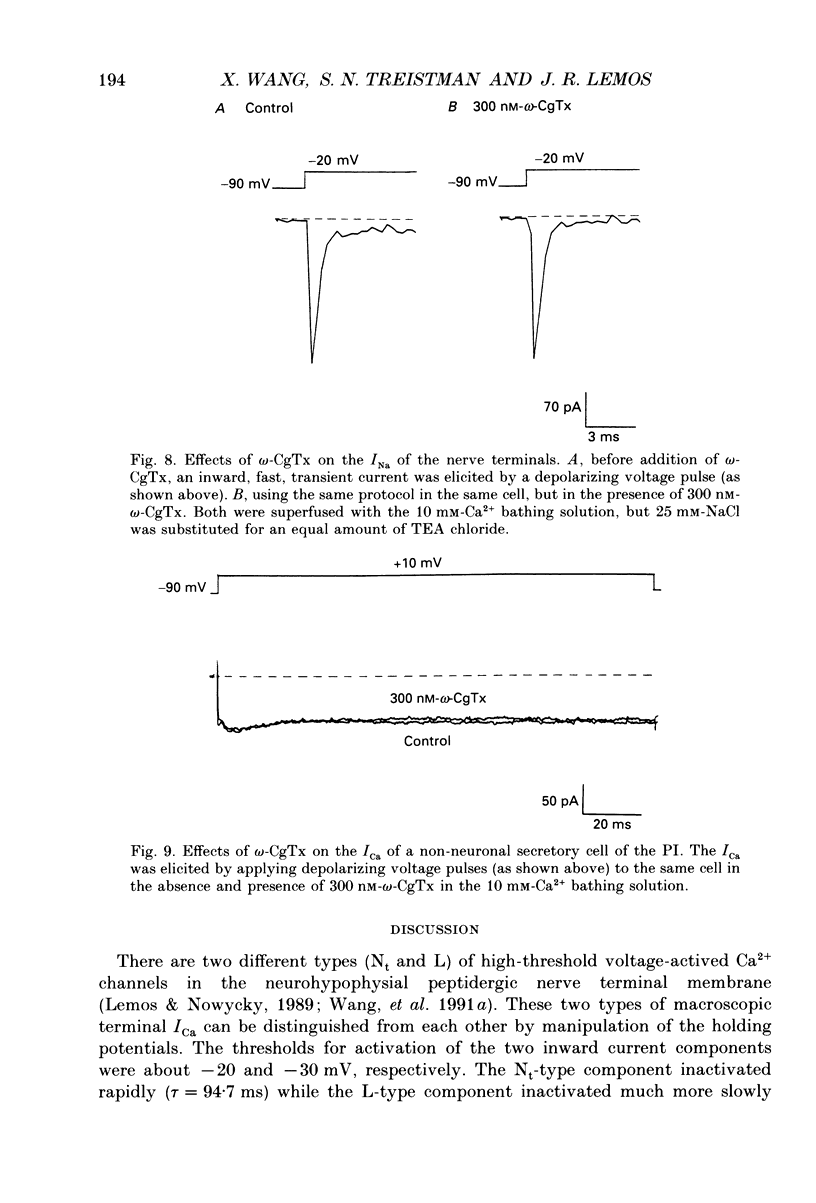
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Harvey A. L. Omega-conotoxin does not block the verapamil-sensitive calcium channels at mouse motor nerve terminals. Neurosci Lett. 1987 Nov 23;82(2):177–180. doi: 10.1016/0304-3940(87)90125-x. [DOI] [PubMed] [Google Scholar]
- Aosaki T., Kasai H. Characterization of two kinds of high-voltage-activated Ca-channel currents in chick sensory neurons. Differential sensitivity to dihydropyridines and omega-conotoxin GVIA. Pflugers Arch. 1989 Jun;414(2):150–156. doi: 10.1007/BF00580957. [DOI] [PubMed] [Google Scholar]
- Barhanin J., Schmid A., Lazdunski M. Properties of structure and interaction of the receptor for omega-conotoxin, a polypeptide active on Ca2+ channels. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1051–1062. doi: 10.1016/0006-291x(88)90736-x. [DOI] [PubMed] [Google Scholar]
- Boland L. M., Dingledine R. Multiple components of both transient and sustained barium currents in a rat dorsal root ganglion cell line. J Physiol. 1990 Jan;420:223–245. doi: 10.1113/jphysiol.1990.sp017909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brethes D., Dayanithi G., Letellier L., Nordmann J. J. Depolarization-induced Ca2+ increase in isolated neurosecretory nerve terminals measured with fura-2. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1439–1443. doi: 10.1073/pnas.84.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Omega-conotoxin blockade distinguishes Ca from Na permeable states in neuronal calcium channels. Pflugers Arch. 1988 Nov;413(1):14–22. doi: 10.1007/BF00581223. [DOI] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. Hormone release from isolated nerve endings of the rat neurohypophysis. J Physiol. 1987 Sep;390:55–70. doi: 10.1113/jphysiol.1987.sp016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L. J., Johnson D. S., Olivera B. M. Characterization of the omega-conotoxin target. Evidence for tissue-specific heterogeneity in calcium channel types. Biochemistry. 1987 Feb 10;26(3):820–824. doi: 10.1021/bi00377a024. [DOI] [PubMed] [Google Scholar]
- Cruz L. J., Olivera B. M. Calcium channel antagonists. Omega-conotoxin defines a new high affinity site. J Biol Chem. 1986 May 15;261(14):6230–6233. [PubMed] [Google Scholar]
- Dayanithi G., Martin-Moutot N., Barlier S., Colin D. A., Kretz-Zaepfel M., Couraud F., Nordmann J. J. The calcium channel antagonist omega-conotoxin inhibits secretion from peptidergic nerve terminals. Biochem Biophys Res Commun. 1988 Oct 14;156(1):255–262. doi: 10.1016/s0006-291x(88)80833-7. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Dunlap K., Kream R. M. Characterization of the electrically evoked release of substance P from dorsal root ganglion neurons: methods and dihydropyridine sensitivity. J Neurosci. 1988 Feb;8(2):463–471. doi: 10.1523/JNEUROSCI.08-02-00463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Aosaki T., Fukuda J. Presynaptic Ca-antagonist omega-conotoxin irreversibly blocks N-type Ca-channels in chick sensory neurons. Neurosci Res. 1987 Feb;4(3):228–235. doi: 10.1016/0168-0102(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Kerr L. M., Yoshikami D. A venom peptide with a novel presynaptic blocking action. Nature. 1984 Mar 15;308(5956):282–284. doi: 10.1038/308282a0. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nordmann J. J., Cooke I. M., Stuenkel E. L. Single channels and ionic currents in peptidergic nerve terminals. 1986 Jan 30-Feb 5Nature. 319(6052):410–412. doi: 10.1038/319410a0. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nordmann J. J. Ionic channels and hormone release from peptidergic nerve terminals. J Exp Biol. 1986 Sep;124:53–72. doi: 10.1242/jeb.124.1.53. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nowycky M. C. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989 May;2(5):1419–1426. doi: 10.1016/0896-6273(89)90187-6. [DOI] [PubMed] [Google Scholar]
- Martin-Moutot N., Seagar M., Couraud F. Subtypes of voltage-sensitive calcium channels in cultured rat brain neurons. Neurosci Lett. 1990 Jul 31;115(2-3):300–306. doi: 10.1016/0304-3940(90)90472-l. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Dyball R. E. Single ion channel activity in peptidergic nerve terminals of the isolated rat neurohypophysis related to stimulation of neural stalk axons. Brain Res. 1986 Sep 24;383(1-2):279–286. doi: 10.1016/0006-8993(86)90026-0. [DOI] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogul D. J., Fox A. P. Evidence for multiple types of Ca2+ channels in acutely isolated hippocampal CA3 neurones of the guinea-pig. J Physiol. 1991 Feb;433:259–281. doi: 10.1113/jphysiol.1991.sp018425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann J. J., Dayanithi G., Lemos J. R. Isolated neurosecretory nerve endings as a tool for studying the mechanism of stimulus-secretion coupling. Biosci Rep. 1987 May;7(5):411–426. doi: 10.1007/BF01362504. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Obaid A. L., Flores R., Salzberg B. M. Calcium channels that are required for secretion from intact nerve terminals of vertebrates are sensitive to omega-conotoxin and relatively insensitive to dihydropyridines. Optical studies with and without voltage-sensitive dyes. J Gen Physiol. 1989 Apr;93(4):715–729. doi: 10.1085/jgp.93.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., McIntosh J. M., Cruz L. J., Luque F. A., Gray W. R. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984 Oct 23;23(22):5087–5090. doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Rivier J., Clark C., Ramilo C. A., Corpuz G. P., Abogadie F. C., Mena E. E., Woodward S. R., Hillyard D. R., Cruz L. J. Diversity of Conus neuropeptides. Science. 1990 Jul 20;249(4966):257–263. doi: 10.1126/science.2165278. [DOI] [PubMed] [Google Scholar]
- Oyama Y., Tsuda Y., Sakakibara S., Akaike N. Synthetic omega-conotoxin: a potent calcium channel blocking neurotoxin. Brain Res. 1987 Oct 20;424(1):58–64. doi: 10.1016/0006-8993(87)91192-9. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Regan L. J., Sah D. W., Bean B. P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991 Feb;6(2):269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Wagner J. A., Snyder S. H., Thayer S. A., Olivera B. M., Miller R. J. Brain voltage-sensitive calcium channel subtypes differentiated by omega-conotoxin fraction GVIA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8804–8807. doi: 10.1073/pnas.83.22.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnhout I., Hill D. R., Middlemiss D. N. Failure of omega-conotoxin to block L-channels associated with [3H]5-HT release in rat brain slices. Neurosci Lett. 1990 Jul 31;115(2-3):323–328. doi: 10.1016/0304-3940(90)90476-p. [DOI] [PubMed] [Google Scholar]
- Schroeder J. E., Fischbach P. S., Mamo M., McCleskey E. W. Two components of high-threshold Ca2+ current inactivate by different mechanisms. Neuron. 1990 Oct;5(4):445–452. doi: 10.1016/0896-6273(90)90083-r. [DOI] [PubMed] [Google Scholar]
- Scopsi L., Larsson L. I. Increased sensitivity in peroxidase immunocytochemistry. A comparative study of a number of peroxidase visualization methods employing a model system. Histochemistry. 1986;84(3):221–230. doi: 10.1007/BF00495786. [DOI] [PubMed] [Google Scholar]
- Sher E., Pandiella A., Clementi F. Omega-conotoxin binding and effects on calcium channel function in human neuroblastoma and rat pheochromocytoma cell lines. FEBS Lett. 1988 Aug 1;235(1-2):178–182. doi: 10.1016/0014-5793(88)81258-4. [DOI] [PubMed] [Google Scholar]
- Stuenkel E. L. Effects of membrane depolarization on intracellular calcium in single nerve terminals. Brain Res. 1990 Oct 8;529(1-2):96–101. doi: 10.1016/0006-8993(90)90815-s. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Yoshioka T. Differential blocking action of synthetic omega-conotoxin on components of Ca2+ channel current in clonal GH3 cells. Neurosci Lett. 1987 Mar 31;75(2):235–239. doi: 10.1016/0304-3940(87)90303-x. [DOI] [PubMed] [Google Scholar]
- Thorn P. J., Wang X. M., Lemos J. R. A fast, transient K+ current in neurohypophysial nerve terminals of the rat. J Physiol. 1991 Jan;432:313–326. doi: 10.1113/jphysiol.1991.sp018386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toselli M., Taglietti V. Pharmacological characterization of voltage-dependent calcium currents in rat hippocampal neurons. Neurosci Lett. 1990 Apr 20;112(1):70–75. doi: 10.1016/0304-3940(90)90324-3. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Wang X. M., Lemos J. R., Dayanithi G., Nordmann J. J., Treistman S. N. Ethanol reduces vasopressin release by inhibiting calcium currents in nerve terminals. Brain Res. 1991 Jun 14;551(1-2):338–341. doi: 10.1016/0006-8993(91)90954-t. [DOI] [PubMed] [Google Scholar]
- Wang X. M., Treistman S. N., Lemos J. R. Direct identification of individual vasopressin-containing nerve terminals of the rat neurohypophysis after 'whole-cell' patch-clamp recordings. Neurosci Lett. 1991 Mar 11;124(1):125–128. doi: 10.1016/0304-3940(91)90838-k. [DOI] [PubMed] [Google Scholar]
- Yawo H. Voltage-activated calcium currents in presynaptic nerve terminals of the chicken ciliary ganglion. J Physiol. 1990 Sep;428:199–213. doi: 10.1113/jphysiol.1990.sp018207. [DOI] [PMC free article] [PubMed] [Google Scholar]



