Abstract
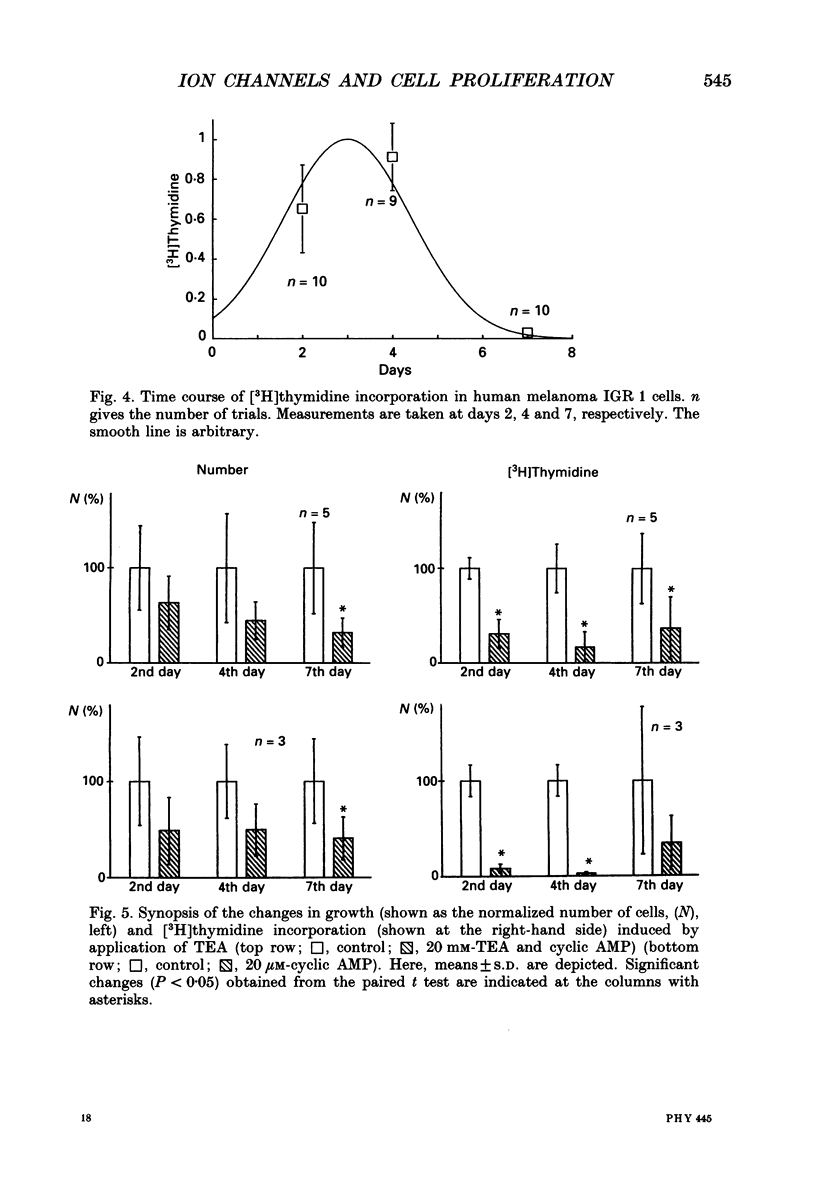
1. Ion channels and their possible relation to cell proliferation have been studied in a human melanoma cell line (IGR 1). Membrane currents were recorded by the patch-clamp technique using the cell-attached, cell-free and whole-cell mode. Cell growth was monitored by counting the number of cells at different days after seeding and [3H]thymidine incorporation. 2. A voltage-dependent 10 pS non-inactivating potassium channel (delayed rectifier) is the most commonly observed ion channel in this type of human cell. The channel is active at the normal resting potential and can be blocked by tetraethylammonium chloride (TEA) and also by a membrane-permeable cyclic adenosine monophosphate (8-(4-chlorophenylthio)adenosine 3',5'-cyclic monophosphate, cyclic AMP). A second type of potassium channel shows properties similar to voltage-dependent A-type potassium channels with complete inactivation. 3. A voltage-independent, non-selective cation channel with a single-channel conductance of approximately 20 pS could be seen in only 8% of the patches. Its properties of modulation are still unknown. 4. The incidence of the 10 pS, non-inactivated potassium channel was maximal at the fourth day after seeding (in 89% of the patches) and was significantly reduced at the seventh day (in 35% of the patches). 5. [3H]thymidine incorporation is maximal at the third day after seeding and is reduced when cells are grown in the presence of TEA or cyclic AMP. This peak of maximal [3H]thymidine incorporation correlated with the incidence of non-inactivated potassium channels. 6. In the presence of TEA or cyclic AMP, growth of the cells is inhibited. We suppose that due to block of potassium channels, most of the melanoma cells are not able to enter the S-phase in the cell division cycle. 7. It is concluded that delayed rectifier potassium channels are involved in the control of melanoma cell proliferation. A similar finding has been reported for K+ channels in T-lymphocytes and human breast carcinoma cells. It is suggested that potassium channels may be involved in controlling the driving force for a calcium influx thereby interacting with Ca(2+)-dependent cell cycle control proteins.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo L. G., Weight F. F. Characterization of membrane currents in dissociated adult rat pineal cells. J Physiol. 1988 Nov;405:397–419. doi: 10.1113/jphysiol.1988.sp017339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert C., Lagrange C., Rorsman H., Rosengren E. Catechols in primary and metastatic human malignant melanoma cells in monolayer culture. Eur J Cancer. 1976 Jun;12(6):441–445. doi: 10.1016/0014-2964(76)90033-5. [DOI] [PubMed] [Google Scholar]
- Aubert C., Rougé F., Galindo J. R. Differentiation and tumorigenicity of human malignant melanocytes in relation to their culture conditions. J Natl Cancer Inst. 1984 Jan;72(1):3–12. doi: 10.1093/jnci/72.1.3. [DOI] [PubMed] [Google Scholar]
- Caffrey J. M., Brown A. M., Schneider M. D. Ca2+ and Na+ currents in developing skeletal myoblasts are expressed in a sequential program: reversible suppression by transforming growth factor beta-1, an inhibitor of the myogenic pathway. J Neurosci. 1989 Oct;9(10):3443–3453. doi: 10.1523/JNEUROSCI.09-10-03443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey J. M., Brown A. M., Schneider M. D. Mitogens and oncogenes can block the induction of specific voltage-gated ion channels. Science. 1987 May 1;236(4801):570–573. doi: 10.1126/science.2437651. [DOI] [PubMed] [Google Scholar]
- Chen C. F., Corbley M. J., Roberts T. M., Hess P. Voltage-sensitive calcium channels in normal and transformed 3T3 fibroblasts. Science. 1988 Feb 26;239(4843):1024–1026. doi: 10.1126/science.2449730. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Cahalan M. D. The plasticity of ion channels: parallels between the nervous and immune systems. Trends Neurosci. 1988 May;11(5):214–218. doi: 10.1016/0166-2236(88)90129-4. [DOI] [PubMed] [Google Scholar]
- Lingle C. J., Sombati S., Freeman M. E. Membrane currents in identified lactotrophs of rat anterior pituitary. J Neurosci. 1986 Oct;6(10):2995–3005. doi: 10.1523/JNEUROSCI.06-10-02995.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero M. T., Pappone P. A. Voltage-gated potassium channels in brown fat cells. J Gen Physiol. 1989 Mar;93(3):451–472. doi: 10.1085/jgp.93.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Mauro T. M., Pappone P. A., Isseroff R. R. Extracellular calcium affects the membrane currents of cultured human keratinocytes. J Cell Physiol. 1990 Apr;143(1):13–20. doi: 10.1002/jcp.1041430103. [DOI] [PubMed] [Google Scholar]
- Nilius B., Böhm T., Wohlrab W. Properties of a potassium-selective ion channel in human melanoma cells. Pflugers Arch. 1990 Nov;417(3):269–277. doi: 10.1007/BF00370992. [DOI] [PubMed] [Google Scholar]
- Nilius B. Permeation properties of a non-selective cation channel in human vascular endothelial cells. Pflugers Arch. 1990 Jul;416(5):609–611. doi: 10.1007/BF00382697. [DOI] [PubMed] [Google Scholar]
- Ravesloot J. H., Ypey D. L., Vrijheid-Lammers T., Nijweide P. J. Voltage-activated K+ conductances in freshly isolated embryonic chicken osteoclasts. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6821–6825. doi: 10.1073/pnas.86.17.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A., Moellmann G., Kuklinska E. MSH inhibits growth in a line of amelanotic hamster melanoma cells and induces increases in cyclic AMP levels and tyrosinase activity without inducing melanogenesis. J Cell Sci. 1989 Apr;92(Pt 4):551–559. doi: 10.1242/jcs.92.4.551. [DOI] [PubMed] [Google Scholar]
- Wegman E. A., Young J. A., Cook D. I. A 23-pS Ca2(+)-activated K+ channel in MCF-7 human breast carcinoma cells: an apparent correlation of channel incidence with the rate of cell proliferation. Pflugers Arch. 1991 Feb;417(6):562–570. doi: 10.1007/BF00372952. [DOI] [PubMed] [Google Scholar]
- Whitaker M., Patel R. Calcium and cell cycle control. Development. 1990 Apr;108(4):525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]
- Zahradníková A., Zahradník I., Rýdlová K. Single-channel potassium currents in human melanoma cells. Gen Physiol Biophys. 1988 Feb;7(1):109–112. [PubMed] [Google Scholar]


