Abstract
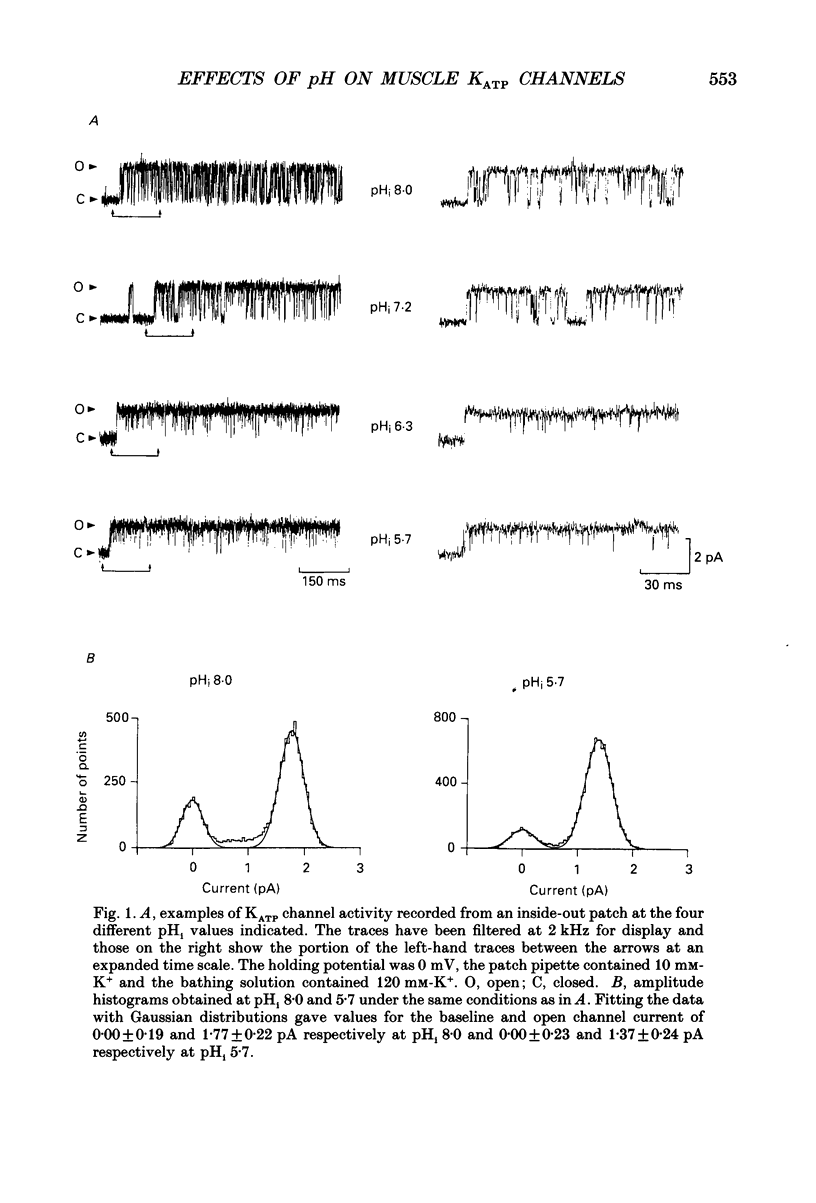
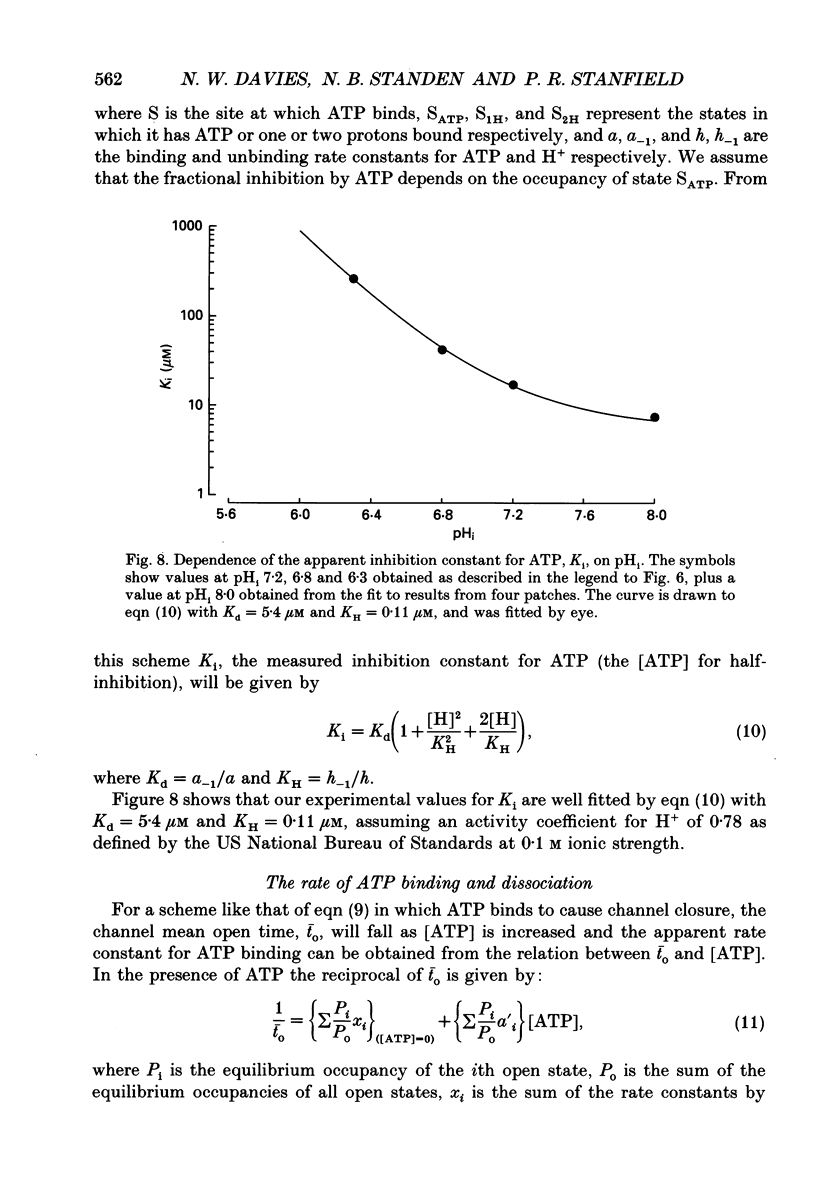
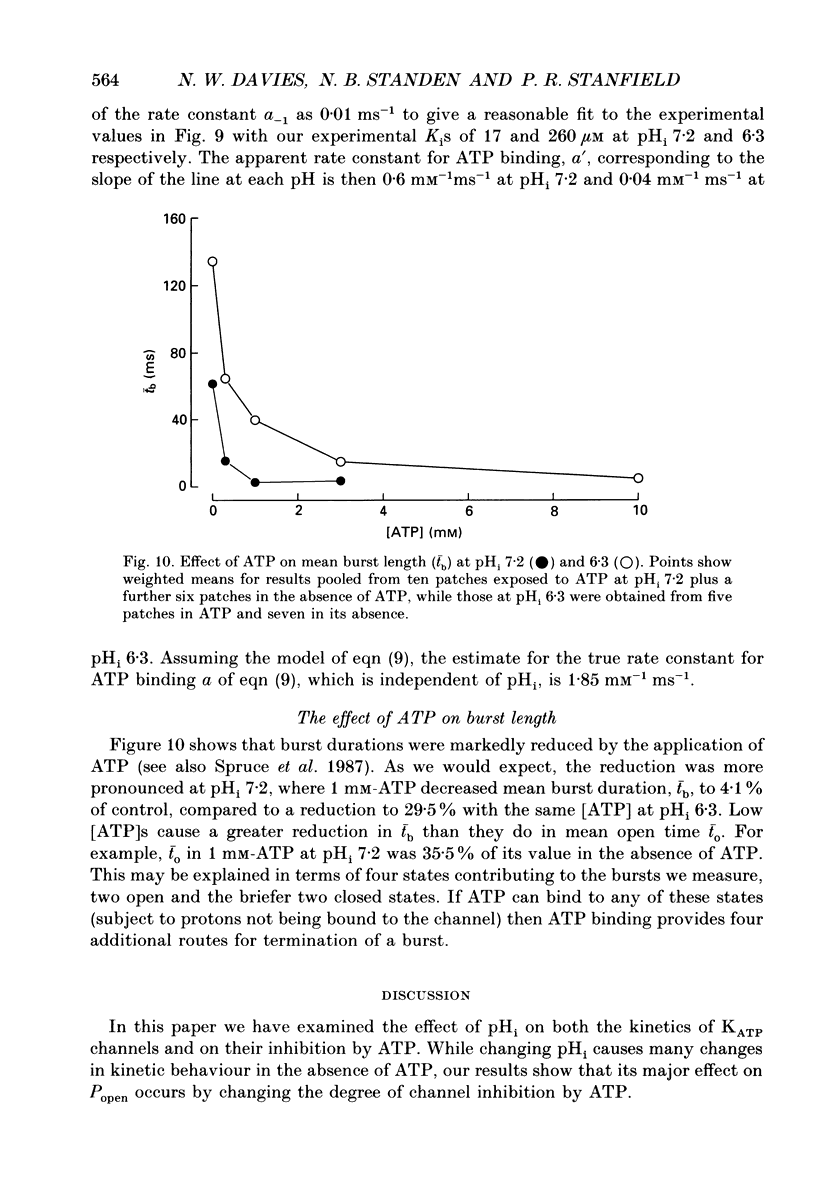
1. We have used patch-clamp methods to study the effects of pH at the cytoplasmic surface of the membrane on ATP-dependent K+ channels (KATP channels) in patches excised from frog (Rana temporaria) skeletal muscle, and to study the kinetics of ATP binding. 2. In the absence of ATP, a reduction in pH led to a slight decrease in single-channel current amplitude, an increase in the number of very brief closings, an increase in the apparent mean open time, and an increase in burst duration. After correction for missed closings, the change in mean open time was slight. Despite these changes in detailed kinetics, the channel open-state probability, Popen, changed little with changes in pH in the absence of ATP. 3. In the presence of ATP, a decrease in internal pH (pHi) reduced the degree of channel inhibition by ATP, shifting the curve relating Popen and [ATP] to higher concentrations of ATP without altering its steepness. The ATP concentration for half-inhibition of channel activity (Ki) was 17 microM at pH 7.2 and 260 microM at pH 6.3. 4. The effect of pH could be modelled by assuming that one or two protons bind to the channel and prevent ATP binding to exert its effect of causing channel closure. The predicted dissociation constants for ATP and H+ respectively were 5.4 and 0.11 microM. 5. The rate constants for binding and unbinding of ATP were estimated from the dependence of the mean open time on [ATP] and from the Ki. The apparent rate constants for ATP binding were 0.6 and 0.04 mM-1 ms-1 at pH 7.2 and 6.3 respectively, while the rate constant for unbinding was 0.01 ms-1. In terms of our model the calculated true rate constant for ATP binding was 1.85 mM-1 ms-1. ATP binding also led to a reduction in burst duration. 6. The effect of pH described here differs from findings in cardiac muscle and pancreatic B-cells. The results are discussed in relation to the possible function of KATP channels in skeletal muscle during exercise.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie R. F., Putnam R. W., Roos A. The intracellular pH of frog skeletal muscle: its regulation in isotonic solutions. J Physiol. 1983 Dec;345:175–187. doi: 10.1113/jphysiol.1983.sp014973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford M. L., Sturgess N. C., Trout N. J., Gardner N. J., Hales C. N. Adenosine-5'-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. Pflugers Arch. 1988 Aug;412(3):297–304. doi: 10.1007/BF00582512. [DOI] [PubMed] [Google Scholar]
- CARLSON F. D., SIGER A. The mechanochemistry of muscular contraction. I. The isometric twitch. J Gen Physiol. 1960 Sep;44:33–60. doi: 10.1085/jgp.44.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Davies N. W. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature. 1990 Jan 25;343(6256):375–377. doi: 10.1038/343375a0. [DOI] [PubMed] [Google Scholar]
- Davies N. W., Spruce A. E., Standen N. B., Stanfield P. R. Multiple blocking mechanisms of ATP-sensitive potassium channels of frog skeletal muscle by tetraethylammonium ions. J Physiol. 1989 Jun;413:31–48. doi: 10.1113/jphysiol.1989.sp017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. W., Standen N. B., Stanfield P. R. ATP-dependent potassium channels of muscle cells: their properties, regulation, and possible functions. J Bioenerg Biomembr. 1991 Aug;23(4):509–535. doi: 10.1007/BF00785809. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., West-Jordan J. A., Abraham R. J., Edwards R. H., Petersen O. H. The gating of nucleotide-sensitive K+ channels in insulin-secreting cells can be modulated by changes in the ratio ATP4-/ADP3- and by nonhydrolyzable derivatives of both ATP and ADP. J Membr Biol. 1988 Sep;104(2):165–177. doi: 10.1007/BF01870928. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. An inexpensive digital tape recorder suitable for neurophysiological signals. J Neurosci Methods. 1985 Oct;15(1):1–13. doi: 10.1016/0165-0270(85)90057-3. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989 Dec;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- Misler S., Gillis K., Tabcharani J. Modulation of gating of a metabolically regulated, ATP-dependent K+ channel by intracellular pH in B cells of the pancreatic islet. J Membr Biol. 1989 Jul;109(2):135–143. doi: 10.1007/BF01870852. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Niggli E., Lederer W. J. Modulation of ATP-sensitive potassium channel activity by flash-photolysis of 'caged-ATP' in rat heart cells. Pflugers Arch. 1990 Jan;415(4):510–512. doi: 10.1007/BF00373635. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Pan J. W., Hamm J. R., Rothman D. L., Shulman R. G. Intracellular pH in human skeletal muscle by 1H NMR. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7836–7839. doi: 10.1073/pnas.85.21.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin D. Y., Takano M., Noma A. Kinetics of ATP-sensitive K+ channel revealed with oil-gate concentration jump method. Am J Physiol. 1989 Nov;257(5 Pt 2):H1624–H1633. doi: 10.1152/ajpheart.1989.257.5.H1624. [DOI] [PubMed] [Google Scholar]
- Renaud J. M. The effect of lactate on intracellular pH and force recovery of fatigued sartorius muscles of the frog, Rana pipiens. J Physiol. 1989 Sep;416:31–47. doi: 10.1113/jphysiol.1989.sp017747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Studies of the unitary properties of adenosine-5'-triphosphate-regulated potassium channels of frog skeletal muscle. J Physiol. 1987 Jan;382:213–236. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985 Aug 22;316(6030):736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R., Ward T. A., Wilson S. W. A new preparation for recording single-channel currents from skeletal muscle. Proc R Soc Lond B Biol Sci. 1984 Jun 22;221(1225):455–464. doi: 10.1098/rspb.1984.0044. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuringer D., Escande D. Apparent competition between ATP and the potassium channel opener RP 49356 on ATP-sensitive K+ channels of cardiac myocytes. Mol Pharmacol. 1989 Dec;36(6):897–902. [PubMed] [Google Scholar]


