Abstract
HC-toxin, a cyclic peptide made by the filamentous fungus Cochliobolus carbonum, is an inhibitor of histone deacetylase (HDAC) from many organisms. It was shown earlier that the HDAC activity in crude extracts of C. carbonum is relatively insensitive to HC-toxin as well as to the chemically unrelated HDAC inhibitors trichostatin and D85, whereas the HDAC activity of Aspergillus nidulans is sensitive (G. Brosch et al., Biochemistry 40:12855-12863, 2001). Here we report that HC-toxin-resistant HDAC activity was present in other, but not all, plant-pathogenic Cochliobolus species but not in any of the saprophytic species tested. The HDAC activities of the fungi Alternaria brassicicola and Diheterospora chlamydosporia, which also make HDAC inhibitors, were resistant. The HDAC activities of all C. carbonum isolates tested, except one non-toxin-producing isolate, were resistant. In a cross between a sensitive isolate and a resistant isolate, resistance genetically cosegregated with HC-toxin production. When fractionated by anion-exchange chromatography, extracts of resistant and sensitive isolates and species had two peaks of HDAC activity, one that was fully HC-toxin resistant and a second that was larger and sensitive. The first peak was consistently smaller in extracts of sensitive fungi than in resistant fungi, but the difference appeared to be insufficiently large to explain the differential sensitivities of the crude extracts. Differences in mRNA expression levels of the four known HDAC genes of C. carbonum did not account for the observed differences in HDAC activity profiles. When mixed together, resistant extracts protected extracts of sensitive C. carbonum but did not protect other sensitive Cochlibolus species or Neurospora crassa. Production of this extrinsic protection factor was dependent on TOXE, the transcription factor that regulates the HC-toxin biosynthetic genes. The results suggest that C. carbonum has multiple mechanisms of self-protection against HC-toxin.
Biologically active secondary metabolites often inhibit critical metabolic processes, and therefore, the organisms that produce them must have mechanisms of self-protection. Known self-protection mechanisms of microorganisms include intracellular modification of the compound to a less toxic form, posttranslational modification of the enzyme target site, synthesis of a novel insensitive form of the target site, and secretion pumps and carriers for removing the compound from the cell (10, 17, 21, 25, 32, 42). Plant-pathogenic microorganisms produce toxic secondary metabolites, and a number of these are phytotoxins with established roles in determining host range, virulence, and symptom development (8, 20, 29, 46, 51). The mechanisms by which toxin-producing plant pathogens protect themselves against their own toxic compounds have been elucidated in several cases. Trichothecenes, produced by the fungus Fusarium graminearum, are detoxified by acetylation and also excreted from the cell by a transporter of the major facilitator superfamily (MFS) (4, 31). The fungus Cercospora kikuchii also uses an MFS transporter to protect itself from its toxin, cercosporin, and also has other mechanisms of self-protection (14). The bacterium Pseudomonas syringae pv. phaseolicola synthesizes a resistant form of the target enzyme, ornithine carbamoyltransferase, concomitant with production of phaseolotoxin (8, 35, 39).
HC-toxin, a cyclic tetrapeptide, is a determinant of virulence and host specificity in the interaction between the producing fungus, Cochliobolus carbonum, and its host, maize (49). HC-toxin biosynthesis is controlled by a single Mendelian locus called TOX2. At the molecular level, TOX2 is a large dispersed gene cluster (1, 46). The six known genes of TOX2 are present in multiple copies in all known toxin-producing (Tox2+) strains and are completely lacking in naturally occurring non-toxin-producing (Tox2−) strains.
The maize Hm1 gene controls resistance to C. carbonum and insensitivity to HC-toxin. Hm1 encodes HC-toxin reductase, which detoxifies HC-toxin by reduction of a critical carbonyl group (27, 34). Maize plants that are homozygous recessive for Hm1 are sensitive to HC-toxin and hence susceptible to C. carbonum. Although HC-toxin is selectively active in vivo, it is a general and potent inhibitor of in vitro histone deacetylase (HDAC) activities of many organisms, including yeast, plants, birds, mammals, and protozoa (12, 18). A number of other secondary metabolites, including at least four other cyclic peptides related to HC-toxin, are also potent inhibitors of HDACs (for examples, see references 18, 30, and 43). Most of them are made by filamentous fungi.
Reversible histone acetylation, catalyzed by histone acetylases and HDACs, has emerged in recent years as a major process integrating chromatin structure and gene regulation (26). Deacetylation of histones at particular promoters is usually associated with repression of transcription, and acetylation is associated with gene induction. HDAC inhibitors cause altered gene expression, induction of apoptosis, developmental arrest, and detransformation of oncogene-transformed cells, but they are usually not directly cytotoxic (5, 24, 33, 48, 50). HDAC inhibitors are being actively investigated as anticancer agents (28).
Because C. carbonum, like all eukaryotes, has histones and HDACs, it presumably must have one or more mechanisms to protect itself against its own HDAC inhibitor. One possible protection mechanism is rapid efflux of HC-toxin out of the cytoplasm by the product of TOXA, a putative MFS drug transporter (38). TOXA is tightly linked to HTS1, which encodes the nonribosomal peptide synthetase involved in HC-toxin biosynthesis, and is coregulated with the other genes involved in HC-toxin biosynthesis (1, 37). The inability to recover TOXA mutants of Tox2+ isolates, combined with its genetic association with the other HC-toxin biosynthetic genes, suggests that TOXA is an essential gene when the fungus is making HC-toxin, and therefore has a role in self-protection (38). On the other hand, growth of strains lacking TOXA is not significantly more sensitive to exogenous HC-toxin than growth of strains with TOXA, arguing that TOXA does not serve to protect C. carbonum against HC-toxin (38). Therefore, it seemed possible that C. carbonum has one or more additional self-protection mechanisms instead of or in addition to TOXA. Such additional self-protection mechanisms might act on or be associated with the HDACs themselves rather than the toxin.
It has been reported that, compared with the HDAC activity of other filamentous fungi such as Aspergillus nidulans, the HDAC activity of C. carbonum is insensitive to HC-toxin and the chemically unrelated HDAC inhibitors trichostatin and D85 (11). Evidence that C. carbonum has an alternative, insensitive HDAC whose synthesis is correlated with toxin production was presented. In this paper, we describe further studies on the biological significance and mechanism of resistance of the HDACs of C. carbonum to HC-toxin.
MATERIALS AND METHODS
Fungal methods.
Fungal strains and maintenance, DNA and RNA extraction, and nucleic acid blotting and hybridization have been described previously (6, 38, 44). Tox2+ isolates of C. carbonum produce HC-toxin, and Tox2− isolates do not. SB111 (ATCC 90305) and SB114 are standard lab strains, and the 164R strains are the progeny of a cross between SB111 and SB114 (45). Strains 367-2, 372-12, and 243-10 are descendents of SB111 and SB114 by backcrossing; strains 12X8.2 and 129X9.4 are SB111 with both copies of HTS1 disrupted (and are therefore Tox2−) (36). Strains 181, 161, 81-64, 73-4, 1368, and 1309 are independent field isolates of C. carbonum (2, 44). Strain T647 is a targeted null mutant of TOXE in C. carbonum 164R10 (3, 45). Other species of Cochliobolus were obtained from Gillian Turgeon, Cornell University, Ithaca, N.Y. Diheterospora (Verticillium) chlamydosporia was obtained from George Barron, University of Guelph, Guelph, Canada. Wild-type strains of A. nidulans and Neurospora crassa (74-OR23-1A) were obtained from the Fungal Genetics Stock Center, University of Kansas, Kansas City, and Alternaria brassicicola MUCL20297 was obtained from Willem Broekaert, Katholieke Universiteit Leuven, Louvain, Belgium. All fungi were grown in still culture at 21°C in 1-liter Erlenmeyer flasks containing 125 ml of HMT (modified Fries') medium (44).
HDAC extraction and assay.
Crude HDAC preparations were made from 0.5 g of lyophilized mycelial mats ground in liquid nitrogen and mixed thoroughly with 4 ml of buffer by gentle vortexing. The buffer contained 15 mM Tris (pH 7.3), 10 mM NaCl, 0.25 mM EDTA, 10% (vol/vol) glycerol, 1 mM 2-mercaptoethanol, and a mixture of protease inhibitors (Complete Mini; Roche, Mannheim, Germany) at a concentration of one tablet per 30 ml of buffer. The samples were centrifuged at 8,000 × g for 10 min, and 3 ml of the supernatant was desalted by passage through a prepacked gel filtration column (Econo-PacI0 DG; Bio-Rad, Hercules, Calif.). The final volume of each crude extract was 4 ml. Assays of crude extracts contained 50 μl of enzyme, 5 μl of [3H]acetate-prelabelled chicken reticulocyte histones (final concentration, 11 μM), and 5 μl of inhibitor or water (11). At the conclusion of the assay incubation (21°C), 35 μl of 1 N HCl was added, and the released [3H]acetate was extracted twice with ethyl acetate. The first extraction involved adding 0.8 ml of ethyl acetate, vortexing and briefly centrifuging the mixture, and removing 0.6 ml of the upper phase. The samples were then reextracted by adding 0.6 ml of ethyl acetate and removing 0.7 ml of the upper phase. The two ethyl acetate extracts were combined and counted in 5 ml of aqueous liquid scintillation cocktail.
For experiments that involved mixing crude extracts of different isolates, the samples were kept on ice for 15 min after mixing prior to the addition of the [3H]histones. Longer or shorter preincubation times did not affect the results. In every mixing experiment assay, the total volume of extract was 50 μl.
HDAC purification.
Anion-exchange chromatography of HDACs was performed on a Waters high-performance liquid chromatograph (HPLC) by using a TSK DEAE-5PW column (TosoHaas, Montgomeryville, Pa.). Typically, 2 ml of desalted crude extract was injected per run. Proteins were eluted with a linear gradient from 10 to 500 mM NaCl in the same buffer used for extraction (omitting the protease inhibitors) in 30 min at a flow rate of 1 ml/min. Fractions of 2 ml were collected.
Chromatographic fractions were assayed as follows. For the first peak of activity eluted from an anion-exchange column, 50 μl of each fraction and 10 μl of [3H]histones (∼70,000 dpm) were incubated for 4 h at 21°C. For the second peak of activity, 20 μl of each fraction, 30 μl of extraction buffer, and 5 μl of [3H]histones were incubated at 21°C for 2 h.
HC-toxin and chlamydocin were purified from the culture filtrates of 21-day cultures of C. carbonum and D. chlamydosporia, respectively, by chloroform extraction and reversed-phase chromatography (47). Trichostatin was purchased from Sigma, St. Louis, Mo. HC-toxin and chlamydocin were typically used at a final concentration of 40 μg/ml, and trichostatin was used at a concentration of 50 ng/ml.
RESULTS
Characterization of HDAC activity in pathogenic and saprophytic fungi.
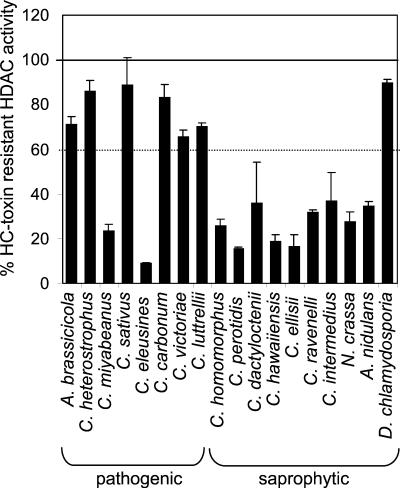
As previously reported (11), in crude extracts of a standard laboratory Tox2+ (HC-toxin producing) strain SB111, HDAC activity was ∼85% resistant at a high concentration (40 μg/ml) of HC-toxin (Fig. 1). For comparison, maize and other HDACs are inhibited by >90% at concentrations of <5 μg/ml (12, 18). Insensitivity to HC-toxin is not a property of all filamentous fungi because the HDAC activities in crude extracts of the saprophytes N. crassa and A. nidulans were inhibited by >70% at the same concentration of HC-toxin (11) (Fig. 1). The concentration necessary for 50% inhibition of N. crassa HDAC activity by HC-toxin was ∼2 μg/ml (data not shown). The HDAC activity of C. carbonum was also resistant to the chemically related compound chlamydocin (tested at 40 μg/ml) and to the unrelated compounds trichostatin (50 ng/ml) and D85, whereas A. nidulans and N. crassa were sensitive to all three (reference 11 and data not shown).
FIG. 1.
HC-toxin sensitivity of HDAC activity in crude extracts of filamentous fungi. All fungi were grown for 7 days in still culture. The error bars indicate the standard deviations of the means of four replicates; some error bars were too small to be visible. Each isolate was tested at least three times.
To test whether HDAC insensitivity is a property of all species of Cochliobolus, the HDAC sensitivities of a collection of pathogenic and saprophytic Cochliobolus species of known taxonomic relatedness were tested (9). In general, the HDAC activities of pathogenic species were resistant (<40% inhibition at 40 μg of HC-toxin/ml), whereas those of saprophytic species were sensitive (>70% inhibition). All of the saprophytic species were sensitive, but two pathogenic species, C. miyabeanus and C. eleusines, were also sensitive (Fig. 1). Among the species classified by Berbee et al. (9), C. eleusines is most closely related to C. sativus, which was resistant (Fig. 1). Therefore, there was a good but not absolute correlation between pathogenicity and HDAC resistance to inhibitors. Although some of the pathogenic resistant fungi, notably C. heterostrophus and C. victoriae, are known to produce host-selective toxins, the structures of these toxins are different from HC-toxin and they are almost certainly not HDAC inhibitors. Two fungi that produce other HDAC inhibitors were also tested. A. brassicicola, a pathogen of Brassica species, produces depudecin (32). D. chlamydosporia, a soil saprophyte and pathogen of nematode eggs, produces a cyclic peptide, chlamydocin, related to HC-toxin (13, 48). The HDAC activities in crude extracts of both fungi were >70% resistant to HC-toxin (Fig. 1).
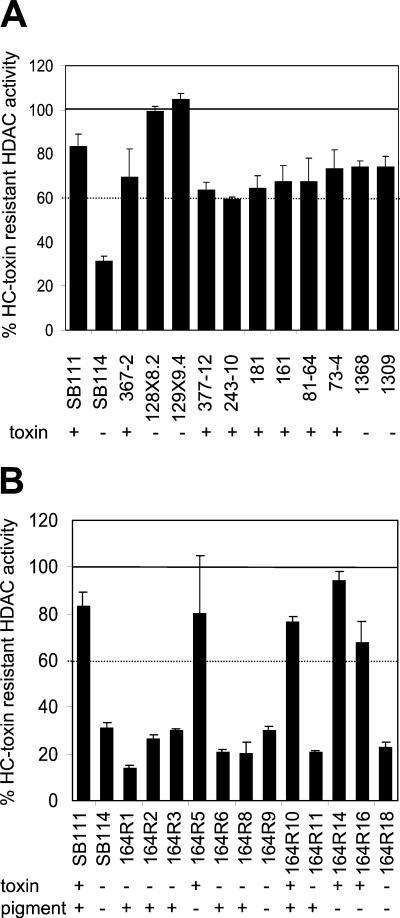
A number of C. carbonum isolates were also tested for HDAC sensitivity to HC-toxin. Isolates SB111, 181, 161, 81-64, and 73-4 are independent Tox2+ field isolates, and isolates 1368 and 1309 are independent Tox2− field isolates (36). Strains 367-2, 372-12, and 243-10 are Tox2+ strains developed in our laboratory from SB111. All of these isolates had resistant HDAC activity, defined by being inhibited <40% by 40 μg of HC-toxin/ml (Fig. 2A). However, SB114, which is an independent Tox2− isolate, was sensitive (Fig. 2A). The HDAC activity of SB114 was also sensitive to chlamydocin and trichostatin (data not shown). This fungal survey indicates that insensitivity to inhibitors is not a universal property of the genus Cochliobolus nor even of C. carbonum itself.
FIG. 2.
HC-toxin sensitivity of HDAC activity in crude extracts of various C. carbonum strains. All fungi were grown for 7 days in still culture. (A) Isolates of C. carbonum. (B) HC-toxin sensitivity in progeny (164R1-18) of a cross between SB111 and SB114. + and − indicate whether the particular strain produces HC-toxin or is pigmented, respectively. The error bars indicate the standard deviations of the means of four replicates.
Strains 128X8.2 and 128X9.4 are two independent engineered Tox2− mutants of C. carbonum strain SB111 (36). Although these strains have a Tox2+ genetic background, they cannot make HC-toxin as a result of mutation of both copies of HTS1, which encodes the nonribosomal peptide synthetase involved in HC-toxin biosynthesis (36, 41). The HDAC activities of both strains remained resistant to HC-toxin (Fig. 2A), and thus resistance is not induced by HC-toxin.
Genetic linkage of HDAC resistance and HC-toxin production.
To test the genetic relationship between HC-toxin resistance and HC-toxin biosynthesis, the HDAC activities in extracts of the progeny of a cross between SB111 and SB114 were tested for HC-toxin resistance (Fig. 2B). All of the progeny that produced toxin had toxin-resistant HDAC activity, whereas all of the progeny that did not produce HC-toxin had toxin-sensitive HDAC activity. SB114 is albino (nonpigmented), but neither HC-toxin production nor HDAC sensitivity cosegregated with albinism (Fig. 2B). This experiment suggests that HDAC resistance to HC-toxin is genetically linked to TOX2, and therefore the gene or genes controlling resistance might be part of the TOX2 gene cluster. However, this conclusion is tentative for several reasons. First, the progeny number was small, so the apparent linkage might have been fortuitous. Second, the results might have been skewed by the failure to recover any Tox2+ sensitive progeny owing to lethality. Third, C. carbonum, like many other plant-pathogenic fungi, has a high incidence of chromosome polymorphisms, which tends to suppress crossing over (2).
Effect of age on HDAC resistance.
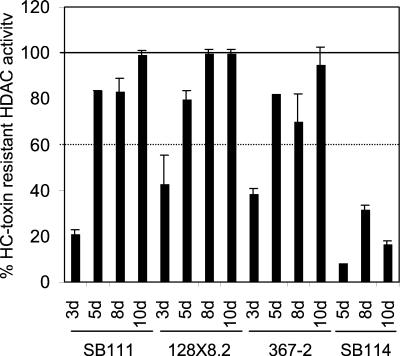
It was reported earlier that the HDAC activity in crude extracts of resistant C. carbonum isolates such as SB111 is sensitive when grown in shake culture instead of still culture (11). However, fungi grown under these two conditions are morphologically and chronologically distinct, e.g., still cultures are normally grown for 7 days, whereas shake cultures are grown for 2 to 3 days (11). To examine the effect of age on HDAC resistance, several isolates were grown in still culture for different lengths of time and HDAC activity and response to HC-toxin were measured in crude extracts. As shown in Fig. 3, resistant isolates were sensitive at 3 days, became resistant at 5 days, and remained resistant through at least 10 days. The sensitive isolate SB114 remained sensitive at all ages (Fig. 3). Thus, age is a critical parameter in regard to HDAC resistance to HC-toxin.
FIG. 3.
Effect of culture age on HC-toxin sensitivity of HDAC activity in crude extracts of C. carbonum. The error bars indicate the standard deviations of the means of four replicates; some error bars were too small to be visible. d, days.
Production of HC-toxin did not influence the age-dependent development of resistance because two engineered Tox2− isolates, 128X8.2 and 128X9.4, behaved similarly to the parent Tox2+ strain SB111 (Fig. 3). Total HDAC activity on either a fresh weight or protein basis was not significantly altered by age in any strain (data not shown).
Chromatographic analysis of C. carbonum HDAC activities.
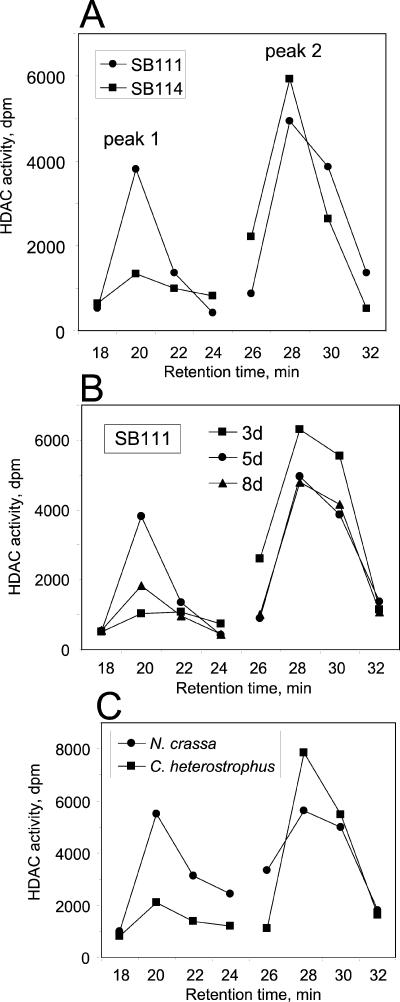
Like other organisms, filamentous fungi have multiple HDACs (7, 11, 22, 23, 40). Potential mechanisms of toxin resistance that could be detected by fractionation of the HDACs of C. carbonum could be a novel HDAC activity, reduced levels of sensitive HDAC activity, higher levels of resistant HDAC activity, or conversion of a normally sensitive HDAC to resistance by posttranslational modification. When fractionated by anion-exchange chromatography, the wild-type strain SB111 exhibited two peaks of HDAC activity (Fig. 4A). The first peak (Fig. 4, peak 1) was almost completely resistant to HC-toxin (93.2% ± 1.4% resistant), whereas the second, broader peak (Fig. 4, peak 2) was almost completely sensitive (2.7% ± 0.9% resistant) (Fig. 4A). All individual fractions comprising peak 1 or peak 2 showed the same degree of resistance or sensitivity, respectively. Note that peak 1 was assayed under conditions of higher substrate and enzyme concentration and longer incubation time, and therefore its activity is much weaker than that of peak 2. The activity in peak 2 was inhibited by salt, and therefore dilution increased its apparent activity. An assay of 20 μl of each 2-ml fraction in a final assay volume of 55 μl gave the highest activity.
FIG. 4.
Anion-exchange HPLC of HDAC activities in C. carbonum and other fungi. In all cases, peak 1 was assayed under conditions which were enhanced compared to those of peak 2 (see Materials and Methods). (A) C. carbonum SB111 compared with SB114. Fungi were grown for 5 days. (B) Effect of culture age on the chromatographic profile of HDAC activity in C. carbonum SB111. (C) HDAC profiles in extracts of N. crassa (sensitive to HC-toxin) and C. heterostrophus (resistant to HC-toxin) (Fig. 1). d, days.
Peak 2 was rather labile and therefore not always present in preparations that had undergone long (i.e., overnight) processing times and that did not include protease inhibitors in the initial extraction buffer. This might explain, in part, why peak 2 was not consistently observed in earlier studies (11). No other HDAC activities were seen in fractions 1 to 17 or >32 (data not shown).
SB114 and SB111 had similar chromatographic profiles of HDAC activity (Fig. 4A), except that peak 1 was always smaller in extracts of SB114. That is, the resistant strain had a higher level of the resistant HDAC activity. Two other toxin-resistant isolates, 367-2 and 128X8.2, had profiles similar to that of SB111, and another toxin-sensitive isolate, 164R1, had a profile similar to that of SB114 (data not shown). Therefore, one possible explanation for the difference in toxin sensitivity of the HDAC activity of different C. carbonum isolates is that the resistant ones have more toxin-resistant HDAC activity. However, integration of the HDAC activities across all chromatographic fractions, taking into consideration the enhanced assay conditions used for peak 1, indicates that peak 1 contributes much less than half of the total HDAC activity. Thus, it seems that the resistance of peak 1 might contribute to, but could not by itself account for, the resistance of crude extracts of SB111 to HC-toxin.
The hypothesis that peak 1 accounts for the resistance of crude extracts is further weakened by analysis of the age dependence of the HDAC chromatographic profile in SB111. Age had relatively little effect on the size of peak 2, but peak 1 was almost undetectable at 3 days, highest at 5 days, and of intermediate size at 8 days (Fig. 4B). Thus, although at day 3 there was a positive correlation between HDAC sensitivity in crude extracts and a small peak 1, this correlation did not hold at other ages because peak 1 decreased at 8 days almost back down to the level found on day 3 (Fig. 4B), even though crude extracts of SB111 were resistant at both 5 and 8 days (Fig. 3).
N. crassa and C. heterostrophus showed the same pattern of two chromatographic peaks of HDAC activity (Fig. 4C). As in C. carbonum, the first peak in both cases was >95% resistant to HC-toxin and the second peak was >95% sensitive (data not shown). Although crude extracts of N. crassa were sensitive to HC-toxin (Fig. 1), they had a large peak 1 relative to peak 2 (Fig. 4C). Conversely, although C. heterostrophus had a relatively small peak 1 (Fig. 4C), crude extracts were resistant (Fig. 1). Taken together, the chromatographic analyses argue, first, that there are quantitative but not qualitative differences in the chromatographic activity profiles of resistant and sensitive HDAC preparations and, second, that peak 1 could contribute some, but not all, of the apparent toxin resistance of the crude extracts.
Molecular analysis of HDAC-encoding genes.
As a further investigation into the possibility that resistance is the result of qualitative differences among the HDACs of toxin-resistant and toxin-sensitive isolates, we examined the structure and expression of the C. carbonum HDAC genes. N. crassa, A. nidulans, and C. carbonum have HDACs related to yeast RPD3, HDA1, HOS2, and HOS3, but apparently do not have a homolog of yeast HOS1 (7, 22, 23). This is based on several attempts to find such a gene by using PCR primers based on HOS1, and on BLAST searching with all of the known fungal HDAC genes against the completed genomes of N. crassa (http://www-genome.wi.mit.edu), A. nidulans (http://microbial.cereon.com), C. heterostrophus (http://www.nadii.com), and the basidiomycete Phanerochaete chrysosporium (http://www.jgi.doe.gov/programs/whiterot.htm).
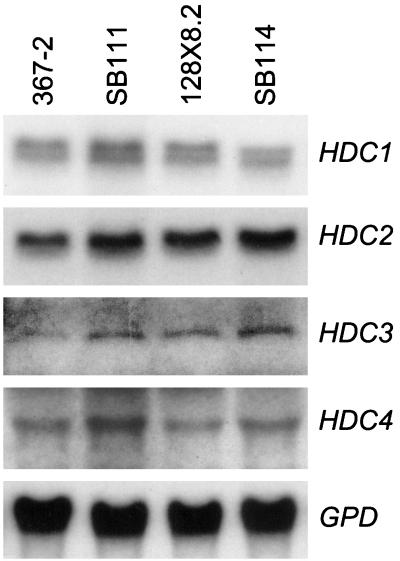
DNA blot analysis of the four HDAC genes of C. carbonum indicated that they are all single-copy genes and that there are no restriction enzyme polymorphisms between isolates 367-2, SB111, 128X8.2, and SB114 (data not shown). The four C. carbonum genes hybridize strongly to single-copy genes in C. heterostrophus (data not shown). RNA blot analysis indicated that all four genes are expressed at similar levels in all four C. carbonum strains (Fig. 5). Therefore, at this level of analysis there is no evidence for a qualitative difference among the HDACs that could account for inhibitor resistance and sensitivity. This analysis cannot exclude the possibility that there is a difference between the HDAC genes below the level of detection of these experiments, e.g., one or more point mutations.
FIG. 5.
RNA blot analysis of expression of HDC1, HDC2, HDC3, and HDC4 in four strains of C. carbonum. HDC1 mRNA consistently appears as a doublet (7). Hybridization probes were prepared with cloned pieces of HDAC genes from C. carbonum (GenBank accession numbers AF349677 and AF306507) (7; S. Graessle, S. Wegener, and J. D. Walton, unpublished results). GPD is the C. carbonum gene encoding glyceraldehyde-3-phosphate dehydrogenase, which was included as a loading control.
Cross-protection of sensitive HDAC by resistant extracts.
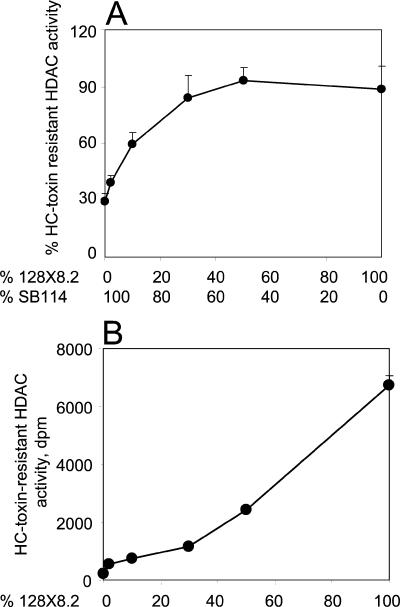
In light of the weak evidence for a mechanism of toxin resistance caused by an assayable property of the HDACs themselves, we investigated whether resistance might be the result of one or more extrinsic factors. When crude extracts of the toxin-sensitive strain SB114 and the toxin-resistant strain 128X8.2 were mixed in various proportions, the sensitivity of the resulting mixture was skewed towards resistance, indicating that resistant extracts had the ability to protect sensitive extracts (Fig. 6A). A reaction mixture containing 10% 128X8.2 plus 90% SB114 had HDAC activity that was ∼60% resistant, and a mixture of 30% 128X8.2 plus 70% SB114 had a level of resistance indistinguishable from 128X8.2 alone (Fig. 6A). Cross-protection was not the result of nonlinearity of apparent resistant activity as a function of the concentration of the 128X8.2 extract because in an assay containing 128X8.2 alone, the total HDAC activity was approximately proportional to the enzyme concentration, and the activity was equally resistant at all concentrations (Fig. 6B). The protective effect was abolished if the 128X8.2 extract was boiled prior to the addition to the SB114 extract (data not shown). An extract of 128X8.2 also protected SB114 against chlamydocin and trichostatin (data not shown). These results indicate that resistant extracts have a heat-labile, and therefore perhaps proteinaceous, factor(s) that can protect sensitive extracts in trans.
FIG. 6.
Cross-protection of sensitive HDAC extracts by resistant extracts. (A) Protection against HC-toxin of HDAC activity in crude extracts of SB114 (sensitive) by extracts of 128X8.2 (resistant). Each assay reaction mixture contained a total of 50 μl of fungal extract. (B) HC-toxin-resistant HDAC activity in extracts of 128X8.2 as a function of enzyme dilution. Each reaction mixture contained a total volume of 50 μl. Percent resistance ranged between 87 and 94% for every dilution.
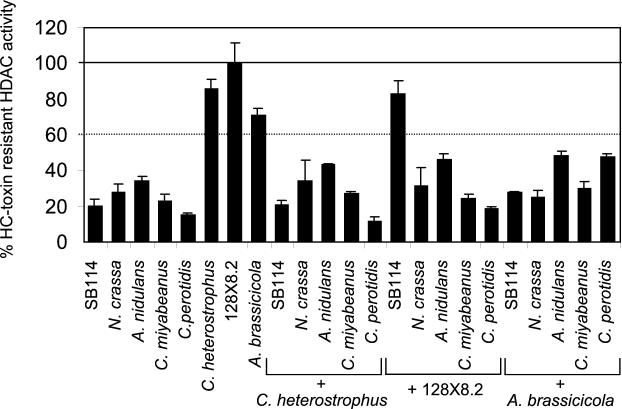
Extracts of SB111 and 367-2 could also protect SB114 extracts, and 164R1 extracts could also be protected by extracts of SB111 (data not shown). However, extracts of 128X8.2 could not protect HDAC activity from any sensitive fungus other than C. carbonum, including N. crassa, A. nidulans, C. miyabeanus, and C. peroditis (Fig. 7). Furthermore, extracts of C. heterostrophus or A. brassicicola, both of which are resistant by themselves (Fig. 1), could not protect any sensitive fungal extract, including C. carbonum SB114 (Fig. 7). Therefore, the cross-protection activity present in C. carbonum is species specific.
FIG. 7.
Response of HDAC activity of sensitive crude extracts of other fungal species to extracts of C. carbonum isolate 128X8.2 (resistant) and response of sensitive C. carbonum HDAC activity to extracts from other resistant species. The first eight bars show the HC-toxin sensitivity of the extracts by themselves. In the other experiments, the assay mixtures contained 80% of an extract of the indicated isolate plus 20% of the isolate indicated by the plus sign. The error bars indicate the standard deviations of the means of four replicates.
Because extracts of resistant C. carbonum did not protect the HDACs of other fungi (Fig. 7), we can exclude metabolic detoxification of HC-toxin by an enzyme present only in resistant extracts as a mechanism of cross-protection. The possibility that cross-protection is due to an enzyme that specifically degrades HC-toxin is also excluded by the fact that cross-protection worked against the chemically unrelated compound trichostatin as well as against HC-toxin.
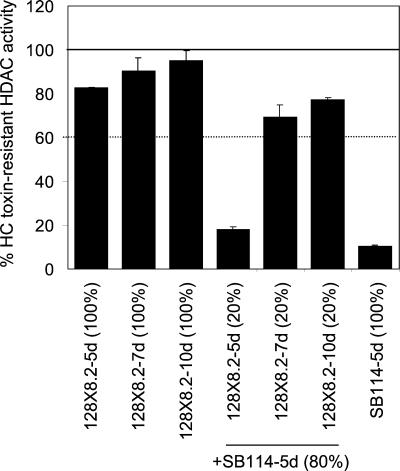
Like intrinsic resistance (Fig. 3), the ability to cross-protect developed with culture age (Fig. 8). Extracts of 5-day cultures of 128X8.2 were resistant to HC-toxin but could not cross-protect SB114 extracts (Fig. 8). The ability to cross-protect developed at 7 days and was still present at 10 days (Fig. 8). This result indicates that the ability to cross-protect can be chronologically separated from resistance.
FIG. 8.
Effect of culture age on ability of resistant C. carbonum extracts to cross-protect sensitive extracts. Extracts of 128X8.2 harvested from mycelial mats of the indicated ages were assayed alone (three left bars) or after mixing with extracts of 5-day-old SB114 in the proportion of one part resistant to four parts sensitive. The error bars indicate the standard deviations of the means of four replicates; some error bars were too small to be visible.
Role of TOXE in cross-protection.
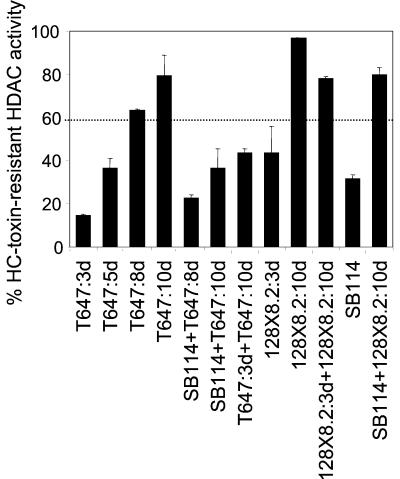
The genetic segregation results (Fig. 2B) suggested that resistance might be linked to TOX2, the HC-toxin biosynthetic gene cluster. HC-toxin per se is not required for resistance or cross-protection because 128X8.2 and 128X9.4, which have functional copies of all of the TOX2 genes except HTS1, are resistant and can cross-protect (Fig. 2A, 3, 6, 7, and 8). TOXE is a transcription factor that is necessary for expression of all of the known TOX2 genes, i.e., HTS1, TOXA, TOXC, TOXD, TOXF, and TOXG (3, 16, 37). Therefore, if resistance and/or the ability to cross-protect are a part of TOX2, these processes might also be regulated by TOXE. To test this, the TOXE null mutant T647 was tested for resistance as a function of age and for the ability to cross-protect. T647 is derived from 164R10 (3), a resistant isolate (Fig. 2B) that as a function of age behaved similarly to other resistant strains, including its parent, SB111 (data not shown). Development of resistance in T647 was delayed. At 3 days, T647 was sensitive, like SB111 (Fig. 3), but unlike SB111 it remained sensitive at 5 days and showed greater than 60% resistance only at 8 days (Fig. 9). Furthermore, even at 10 days it had a greatly reduced or no ability to cross-protect the HDAC activity of SB114 (Fig. 9). This experiment indicates that resistance and cross-protection are under the control of TOXE, at least in part, and therefore are coregulated with HC-toxin biosynthesis itself.
FIG. 9.
HC-toxin resistance and cross-protection by extracts of the C. carbonum TOXE mutant T647 as a function of culture age. In the mixing experiments, the ratio of extract of the first isolate listed (sensitive) to the second isolate (resistant) was four to one. The error bars indicate the standard deviations of the means of four replicates; some error bars were too small to be visible.
DISCUSSION
The results presented here and earlier suggest that C. carbonum uses multiple mechanisms to protect itself against HC-toxin (11, 38). TOXA, which is predicted to encode a drug transporter and is tightly clustered with HTS1, was originally considered to be a possible mechanism of self-protection. However, there is no evidence that TOXA actually has a protective function (38). In search of an alternative self-protection mechanism, the HDAC activity of C. carbonum was tested and found to be insensitive to HC-toxin compared with the HDAC activity of other organisms, including other filamentous fungi (11). This insensitivity appears to have two components. One is intrinsic—a particular chromatographic peak of resistant HDAC activity is higher in extracts of insensitive isolates than in sensitive isolates (11). The second is extrinsic—resistant extracts contain a factor or factors that render sensitive extracts resistant.
Is the HC-toxin insensitivity of the HDACs of C. carbonum biologically significant, that is, an integral part of the trait of HC-toxin production and hence of pathogenesis? Several features of the phenomenon argue that it is. First, all toxin-producing isolates of C. carbonum examined were resistant, whereas the one known sensitive isolate was Tox2−. Second, in a cross between this sensitive isolate and a resistant isolate, all of the Tox2+ progeny were resistant and all of the Tox2− isolates were sensitive. Third, the ability to cross-protect was regulated by TOXE, the transcription factor that regulates all of the known TOX2 genes. The second and third points indicate a genetic relationship between HC-toxin production and resistance. Fourth, cross-protection was highly specific, operating only between C. carbonum species. Fifth, two other filamentous fungi that are known to produce secondary metabolite inhibitors of HDAC also had resistant HDAC activity, arguing that resistance is a general adaptation to inhibitor production.
In regard to the phylogenetic distribution of resistance, HDAC activities in ascomycetes that are distantly related to C. carbonum, namely A. nidulans and N. crassa, were sensitive, whereas HDAC activities in other Cochliobolus species were either sensitive or resistant. With two exceptions, pathogenic species of Cochliobolus were resistant and saprophytic species were sensitive. Therefore, resistance to HC-toxin is not a fixed or essential attribute of filamentous fungi nor even of Cochliobolus. The association between pathogenicity and resistance could be due to the fact that the pathogenic species of Cochliobolus are more closely related to each other than they are to the saprophytic species (9), and therefore, HDAC resistance is simply a coincidental ancestral trait of the pathogenic group with no adaptive significance for most species. If this is the case, then C. carbonum appears to have been fortuitously preadapted for acquisition of the trait of HC-toxin biosynthesis.
An alternative explanation for the dichotomy between pathogenic and saprophytic species is that the other resistant pathogenic species also produce HDAC inhibitors (that may or may not have a role in disease), and therefore, the other species have also evolved a self-protection mechanism based on HDAC insensitivity. If so, these other HDAC inhibitors would not necessarily be chemically related to HC-toxin, since the resistance phenomenon is not specific for HC-toxin but also functions against trichostatin. This hypothesis is consistent with the fact that two other filamentous fungi known to make HDAC inhibitors also have resistant HDAC activities. One of these, A. brassicicola, is a bona fide plant pathogen. The significance of the fact that two pathogenic Cochliobolus species have HC-toxin-sensitive HDACs is not clear at this point and will require further investigation, including confirmation of the identity and pathogenicity of the two species, analysis of more isolates of each species, and analysis of additional pathogenic and saprophytic species of Cochliobolus (9).
Resistance is not a conserved trait within the genus Cochliobolus nor even within the species C. carbonum. At least one isolate of C. carbonum, SB114, was sensitive. SB114 is an immediate descendant of a naturally occurring Tox2− isolate and not a laboratory-derived mutant. In what respect this isolate differs from the resistant isolates is not yet known, but there were no major qualitative differences in its HDAC HPLC profile nor in its known HDAC genes at the DNA or RNA level. SB114 should be useful for the elucidation of the mechanism or mechanisms of resistance.
When the HDAC activities of fungi were analyzed by anion-exchange chromatography, two peaks of HDAC activity were observed in all isolates and species, a small first one that was almost completely resistant to HC-toxin and a second, larger peak that was almost completely sensitive. Although peak 1 was consistently higher in extracts from resistant strains, it seems unlikely that peak 1 makes a major contribution to the resistance of crude extracts of C. carbonum because, after fractionation, the majority of the HDAC activity in both sensitive and resistant strains was sensitive. However, it is possible that peak 1 accounts for the residual resistant activity seen in all of the sensitive fungi. The size of peak 1 is of the correct relative magnitude compared to the total HDAC activity, and it is present in all fungi, including SB114 and N. crassa. The HDAC gene whose product accounts for peak 1 activity is not yet known. It might be HDC4, the C. carbonum ortholog of yeast Hos3, which is insensitive to trichostatin (12, 15). From our experiments it cannot be excluded that this peak consists of two gene products and that variation in the height of peak 1 between isolates and treatments is due to variable expression of one of them.
The observation that the majority of the HDAC activity even in resistant fungi appeared sensitive after chromatographic fractionation suggested that resistant isolates have one or more extrinsic factors that in some manner protect their normally sensitive HDAC activities against HC-toxin and other inhibitors. The mixing experiments indicated that resistant extracts could protect sensitive extracts, which supports this hypothesis. The cross-protection activity was heat labile and species specific, failing to protect any sensitive HDACs except those of C. carbonum itself. Conversely, extracts of other resistant species, including C. heterostrophus could not protect C. carbonum HDAC activity. Thus, cross-protection is not caused by chemical detoxification of HC-toxin. The other resistant fungi must either have a different mechanism of self-protection or respond only to their own extrinsic self-protection factors.
The following model to explain these observations is based on the extrinsic factor being the primary mechanism of self-protection. Resistant C. carbonum isolates accumulate in an age-dependent manner an extrinsic protection factor (or factors). At day 5 the fungus has accumulated a sufficient amount of this factor to protect its own HDACs but not enough to cross-protect, but at day 7 an excess of protective factor has accumulated to allow cross-protection. (Alternatively, the cross-protecting factor acts by stimulating the intrinsically resistant HDAC activity of peak 1.) It seems more likely that the protective effect of the putative extrinsic factor is mediated by interaction with one or more of the sensitive HDACs themselves rather than with HC-toxin. This is because cross-protection was species specific and because cross-protection also worked against HDAC inhibitors that are chemically unrelated to HC-toxin. Because full cross-protection required a ratio of resistant extract to sensitive extract of at least 3:7, it seems more likely that the cross-protection factor acts stoichiometrically with the sensitive HDAC rather than catalytically; that is, the protective factor is a protein that binds to the HDAC of peak 2 in a 1:1 ratio rather than enzymatically altering it. Binding that was sufficiently weak to be disrupted by the anion-exchange HPLC conditions would explain the observation that the majority of the HDAC activity, even from resistant isolates, appeared to be sensitive after chromatography.
Although resistance and cross-protection did not depend on the synthesis of HC-toxin, both phenomena had at least some dependence on ToxE, the transcription factor that regulates the TOX2 genes. In TOXE mutants, development of resistance was delayed from days 5 to 8, and the ability to cross-protect was not present even at day 10. This indicates that TOXE is not required for the development of resistance but is required for the development of the ability to cross-protect, which in turn suggests that different processes underlie the two phenomena. Both intrinsic and extrinsic resistance may be important in protecting C. carbonum against HC-toxin. Self-protection against the nonspecific toxin cercosporin in the fungus C. kikuchii also has multiple mechanisms (19).
Acknowledgments
This work was supported by grants from the Division of Energy Biosciences, U.S. Department of Energy, and the Competitive Research Grants Program, U.S. Department of Agriculture, to J.D.W., by grant no. P13209 from the Austrian Science Foundation to G.B., and by an APART fellowship from the Austrian Academy of Sciences to G.B.
We thank Gillian Turgeon (Cornell University and Torrey Mesa Research Institute, La Jolla, Calif.), George Barron (University of Guelph), Willem Broekaert (Katholieke Universiteit Leuven), and the Fungal Genetics Stock Center for fungal isolates. We thank Gillian Turgeon and Olen Yoder for access to the Torrey Mesa Research Institute C. heterostrophus genome database.
REFERENCES
- 1.Ahn, J.-H., Y.-Q. Cheng, and J. D. Walton. 2002. An extended physical map of the TOX2 locus of Cochliobolus carbonum required for biosynthesis of HC-toxin. Fungal Genet. Biol. 35:31-38. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, J.-H., and J. D. Walton. 1996. Chromosomal organization of TOX2, a complex locus required for host-selective toxin biosynthesis in Cochliobolus carbonum. Plant Cell 8:887-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn, J.-H., and J. D. Walton. 1998. Regulation of cyclic peptide biosynthesis and pathogenicity in Cochliobolus carbonum by TOXEp, a novel protein with a bZIP basic DNA-binding motif and four ankyrin repeats. Mol. Gen. Genet. 260:462-469. [DOI] [PubMed] [Google Scholar]
- 4.Alexander, N. J., S. P. McCormick, and T. M. Hohn. 1999. TRI12, a trichothecene efflux pump from Fusarium sporotrichoides: gene isolation and expression in yeast. Mol. Gen. Genet. 261:977-984. [DOI] [PubMed] [Google Scholar]
- 5.Almouzni, G., S. Khochbin, S. Dimitrov, and A. P. Wolffe. 1994. Histone acetylation influences both gene expression and development of Xenopus laevis. Dev. Biol. 165:654-669. [DOI] [PubMed] [Google Scholar]
- 6.Apel-Birkhold, P. C., and J. D. Walton. 1996. Cloning, disruption, and expression of two endo-β1,4-xylanase genes, XYL2 and XYL3, from Cochliobolus carbonum. Appl. Environ. Microbiol. 62:4129-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baidyaroy, D., G. Brosch, J.-H. Ahn, S. Graessle, S. Wegener, N. J. Tonukari, O. Caballero, P. Loidl, and J. D. Walton. 2001. A gene related to yeast HOS2 histone deacetylase affects extracellular depolymerase expression and virulence in a plant pathogenic fungus. Plant Cell 13:1609-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender, C. L., F. Alarcón-Chaidez, and D. C. Gross. 1999. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthases. Microbiol. Mol. Biol. Rev. 63:266-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berbee, M. L., M. Pirseyedi, and S. Hubbard. 1999. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 91:964-977. [Google Scholar]
- 10.Borges-Walmsley, M. I., and A. R. Walmsley. 2001. The structure and function of drug pumps. Trends Microbiol. 9:71-79. [DOI] [PubMed] [Google Scholar]
- 11.Brosch, G., M. Dangl, S. Graessle, A. Loidl, P. Trojer, E.-M. Brandtner, K. Mair, J. D. Walton, D. Baidyaroy, and P. Loidl. 2001. An inhibitor-resistant histone deacetylase in the plant pathogenic fungus Cochliobolus carbonum. Biochemistry 40:12855-12863. [DOI] [PubMed] [Google Scholar]
- 12.Brosch, G., R. Ransom, T. Lechner, J. D. Walton, and P. Loidl. 1995. Inhibition of maize histone deacetylases by HC toxin, the host-selective toxin of Cochliobolus carbonum. Plant Cell 7:1941-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bursnall, L. A., and H. T. Tribe. 1974. Fungal parasitism in cysts of Heterodera. Trans. Br. Mycol. Soc. 62:595-601. [Google Scholar]
- 14.Callahan, T. M., M. S. Rose, M. J. Meade, M. Ehrenshaft, and R. G. Upchurch. 1999. CFP, the putative cercosporin transporter of Cercospora kikuchii, is required for wild type cercosporin production, resistance, and virulence on soybean. Mol. Plant-Microbe Interact. 12:901-910. [DOI] [PubMed] [Google Scholar]
- 15.Carmen, A. A., P. R. Griffin, J. R. Calaycay, S. E. Rundlett, Y. Suka, and M. Grunstein. 1999. Yeast HOS3 forms a novel trichostatin A-insensitive homodimer with intrinsic histone deacetylase activity. Proc. Natl. Acad. Sci. USA 96:12356-12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng, Y.-Q., and J. D. Walton. 2000. A eukaryotic alanine racemase involved in cyclic peptide biosynthesis. J. Biol. Chem. 275:4906-5004. [DOI] [PubMed] [Google Scholar]
- 17.Cundliffe, E. 1989. How antibiotic-producing organisms avoid suicide. Annu. Rev. Microbiol. 43:207-233. [DOI] [PubMed] [Google Scholar]
- 18.Darkin-Rattray, S. J., A. M. Gurnett, R. W. Myers, P. M. Dulski, T. M. Crumley, J. J. Allocco, C. Cannova, P. T. Meinke, S. L. Colletti, M. A. Bednarek, S. B. Singh, M. A. Goetz, A. W. Dombrowski, J. D. Polishook, and D. M. Schmatz. 1996. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 93:13143-13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daub, M. E., and M. Ehrenshaft. 2000. The photoactivated Cercospora toxin cercosporin: contributions to plant disease and fundamental biology. Annu. Rev. Phytopathol. 38:461-490. [DOI] [PubMed] [Google Scholar]
- 20.Desjardins, A. E., R. H. Proctor, G. Bai, S. P. McCormick, G. Shaner, G. Buechley, and T. M. Hohn. 1996. Reduced virulence of trichothecene-nonproducing mutants of Gibberella zeae in wheat field tests. Mol. Plant-Microbe Interact. 9:775-781. [Google Scholar]
- 21.Ghannoum, M. A., and L. B. Rice. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12:501-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graessle, S., M. Dangl, H. Haas, K. Mair, P. Trojer, E.-M. Brandtner, J. D. Walton, P. Loidl, and G. Brosch. 2000. Characterization of two putative histone deacetylase genes from Aspergillus nidulans. Biochim. Biophys. Acta 1492:120-126. [DOI] [PubMed] [Google Scholar]
- 23.Graessle, S., G. Brosch, and P. Loidl. 2001. Histone acetylation: plants and fungi as model systems for the investigation of histone deacetylases. Cell Mol. Life Sci. 58:704-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itazaki, H., K. Nagashima, K. Sugita, H. Yoshida, Y. Kawamura, Y. Yasuda, K. Matsumoto, K. Ishii, N. Uotani, H. Nakai, et al. 1990. Isolation and structural elucidation of new cyclotetrapeptides, trapoxins A and B, having detransformation activities as antitumor agents. J. Antibiot. 43:1524-1532. [DOI] [PubMed] [Google Scholar]
- 25.Jack, D. L., N. M. Yang, and M. H. Saier, Jr. 2001. The drug/metabolite transporter superfamily. Eur. J. Biochem. 268:3620-3639. [DOI] [PubMed] [Google Scholar]
- 26.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293:1074-1080. [DOI] [PubMed] [Google Scholar]
- 27.Johal, G., and S. P. Briggs. 1992. Reductase activity encoded by the HM1 disease resistance gene in maize. Science 258:985-987. [DOI] [PubMed] [Google Scholar]
- 28.Jung, M. 2001. Inhibitors of histone deacetylase as new anticancer agents. Curr. Med. Chem. 8:1505-1511. [DOI] [PubMed] [Google Scholar]
- 29.Karlovsky, P. 1999. Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins 7:1-23. [DOI] [PubMed] [Google Scholar]
- 30.Kijima, M., M. Yoshida, K. Suita, S. Horinouchi, and T. Beppu. 1993. Trapoxin, an antitumor cyclic tetrapeptide, is an irreversible inhibitor of mammalian histone deacetylase. J. Biol. Chem. 268:22429-22435. [PubMed] [Google Scholar]
- 31.Kimura, M., I. Kaneko, M. Komiyama, A. Takatsuki, H. Koshino, K. Yoneyama, and I. Yamaguchi. 1998. Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. Cloning and characterization of Tri101. J. Biol. Chem. 273:1654-1661. [DOI] [PubMed] [Google Scholar]
- 32.Köhler, T., J.-C. Pechère, and P. Plésiat. 1999. Bacterial antibiotic efflux systems of medical importance. Cell Mol. Life Sci. 56:771-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon, H. J., T. Owa, C. A. Hassig, J. Shimada, and S. L. Schreiber. 1998. Depudecin induces morphological reversion of transformed fibroblasts via the inhibition of histone deacetylase. Proc. Natl. Acad. Sci. USA 95:3356-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meeley, R. B., G. S. Johal, S. P. Briggs, and J. D. Walton. 1992. A biochemical phenotype for a disease resistance gene in maize. Plant Cell 4:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosqueda, G., G. Van den Broeck, E. Saucedo, A. M. Bailey, A. Alvarez-Morales, and L. Herrera-Estrella. 1990. Isolation and characterization of the gene from Pseudomonas syringae pv. phaseolicola encoding the phaseolotoxin-insensitive ornithine carbamoyltransferase. Mol. Gen. Genet. 222:461-466. [DOI] [PubMed] [Google Scholar]
- 36.Panaccione, D. G., J. S. Scott-Craig, J.-A. Pocard, and J. D. Walton. 1992. A cyclic peptide synthetase gene required for pathogenicity of the fungus Cochliobolus carbonum on maize. Proc. Natl. Acad. Sci. USA 89:6590-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedley, K. F., and J. D. Walton. 2001. Regulation of cyclic peptide biosynthesis in a plant pathogenic fungus by a novel transcription factor. Proc. Natl. Acad. Sci. USA 98:14174-14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitkin, J. W., D. G. Panaccione, and J. D. Walton. 1996. A putative cyclic peptide efflux pump encoded by the TOXA gene of the plant-pathogenic fungus Cochliobolus carbonum. Microbiology 142:1557-1565. [DOI] [PubMed] [Google Scholar]
- 39.Rowley, K. B., R. Xu, and S. S. Patil. 2000. Molecular analysis of thermoregulation of phaseolotoxin-resistant ornithine carbamoyltransferase (argK) from Pseudomonas syringae pv. phaseolocola. Mol. Plant-Microbe Interact. 13:1071-1080. [DOI] [PubMed] [Google Scholar]
- 40.Rundlett, S. E., A. A. Carmen, R. Kobayashi, S. Bavykin, B. M. Turner, and M. Grunstein. 1996. HDA1 and RPD3 are members of distinct yeast histone deacetylase complexes that regulate silencing and transcription. Proc. Natl. Acad. Sci. USA 93:14503-14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott-Craig, J. S., D. G. Panaccione, J.-A. Pocard, and J. D. Walton. 1992. The cyclic peptide synthetase catalyzing HC-toxin production in the filamentous fungus Cochliobolus carbonum is encoded by a 15.7-kb open reading frame. J. Biol. Chem. 67:26044-26049. [PubMed] [Google Scholar]
- 42.Spratt, B. G. 1994. Resistance to antibiotics mediated by target alterations. Science 264:388-393. [DOI] [PubMed] [Google Scholar]
- 43.Taunton, J., C. A. Hassig, S. L. Schreiber. 1996. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272:408-411. [DOI] [PubMed] [Google Scholar]
- 44.Tonukari, N. J., J. S. Scott-Craig, and J. D. Walton. 2000. The Cochliobolus carbonum SNF1 gene is required for cell-wall-degrading enzyme expression and virulence on maize. Plant Cell 12:237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walton, J. D. 1987. Two enzymes involved in biosynthesis of the host-selective phytotoxin HC-toxin. Proc. Natl. Acad. Sci. USA 84:8444-8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walton, J. D. 1996. Host-selective toxins: agents of compatibility. Plant Cell 8:1723-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walton, J. D., E. D. Earle, and B. W. Gibson. 1982. Purification and structure of the host-specific toxin from Helminthosporium carbonum race 1. Biochem. Biophys. Res. Commun. 107:785-794. [DOI] [PubMed] [Google Scholar]
- 48.Walton, J. D., E. D. Earle, H. Stahelin, A. Grieder, A. Hirota, and A. Suzuki. 1985. Reciprocal biological activities of the cyclic tetrapeptides chlamydocin and HC-toxin. Experientia 41:348-350. [DOI] [PubMed] [Google Scholar]
- 49.Walton, J. D., R. F. Ransom, and J. W. Pitkin. 1997. Northern corn leaf spot: chemistry, enzymology, and molecular genetics of a host-selective phytotoxin, p. 94-123. In G. Stacey and N. T. Keen (ed.), Plant-microbe interactions, vol. 3. Chapman & Hall, New York, N.Y.
- 50.Yoshida, H., and K. Sugita. 1992. A novel tetracyclic peptide, trapoxin, induces phenotypic change from transformed to normal in sis-oncogene-transformed NIH3T3 cells. Jpn. J. Cancer Res. 83:324-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, L., and R. G. Birch. 1997. The gene for albicidin detoxification from Pantoea dispersa encodes an esterase and attenuates pathogenicity of Xanthomonas albilineans to sugarcane. Proc. Natl. Acad. Sci. USA 94:9984-9989. [DOI] [PMC free article] [PubMed] [Google Scholar]