Abstract
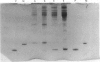
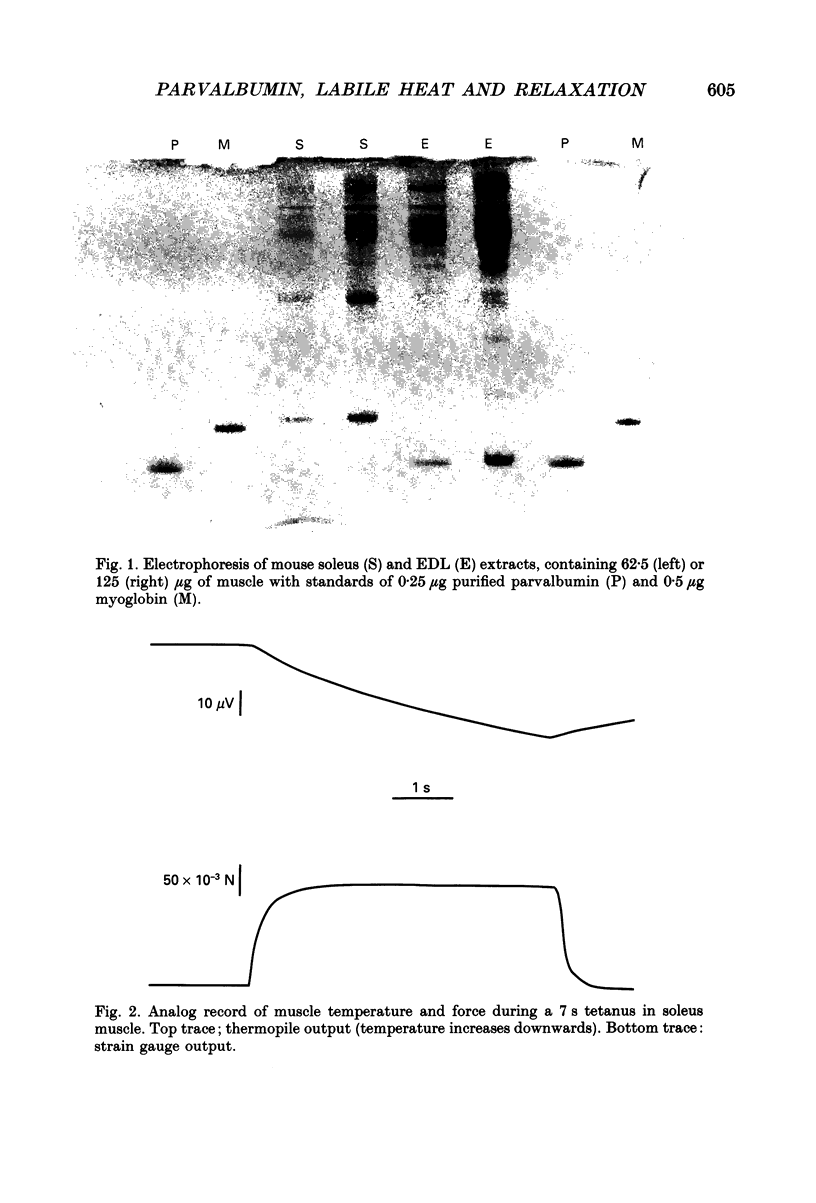
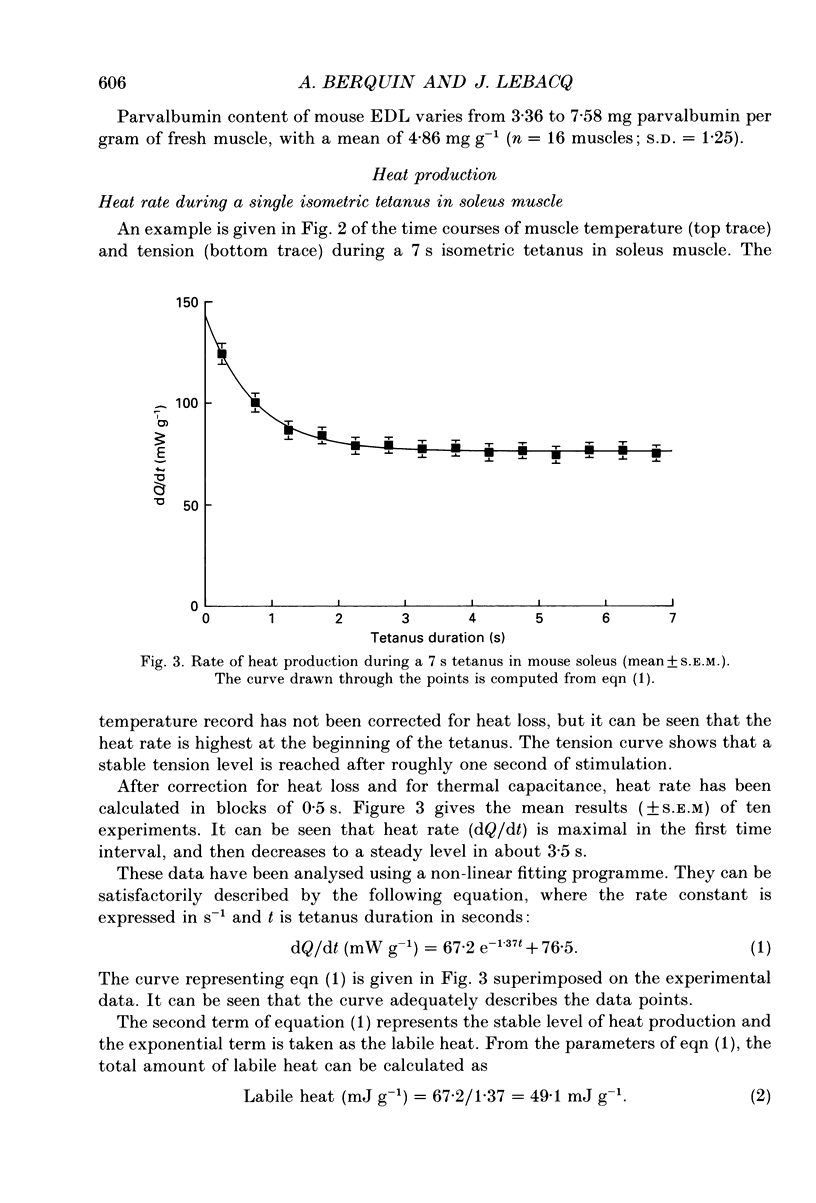
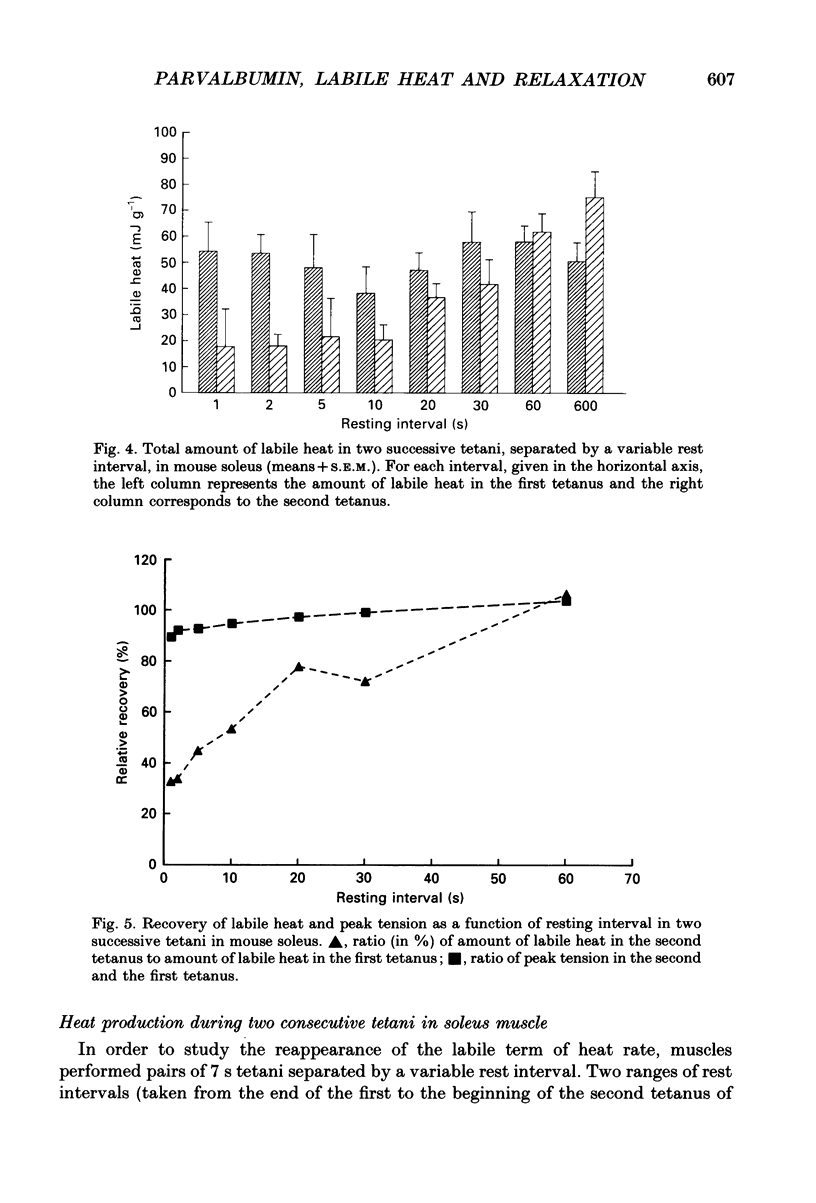
1. Parvalbumin content, heat rate and rate of relaxation were measured in two mouse muscles: the slow-twitch soleus and the fast-twitch extensor digitorum longus (EDL). 2. No trace of parvalbumin was found in the soleus; EDL contained a mean of 4.86 mg of this protein per gram of fresh muscle (S.D. = 1.25). 3. Heat rate during 7 s isometric tetani in isolated soleus muscle at 20 degrees C can be described by the sum of an exponentially decaying term and a constant term. The exponential term is reduced by 67% in a second tetanus performed 1 s after a first one; its repriming is complete after a resting period of about 1 min. The exponential term has therefore the properties of labile heat. 4. Relaxation rate measured during 15 s of isometric interrupted tetani at 20 degrees C is nearly constant in the soleus, but decreases continuously with increasing tetanus duration in the EDL. In the latter, isometric tension also decreases continuously. 5. Therefore, parvalbumin can account neither for the labile heat production in mouse soleus nor for the slowing of relaxation associated with muscle fatigue observed after a few seconds of tetanus in EDL. The role of parvalbumin in striated muscles is thus reassessed, and other possible causes of labile heat production and slowing of relaxation are discussed.
Full text
PDF



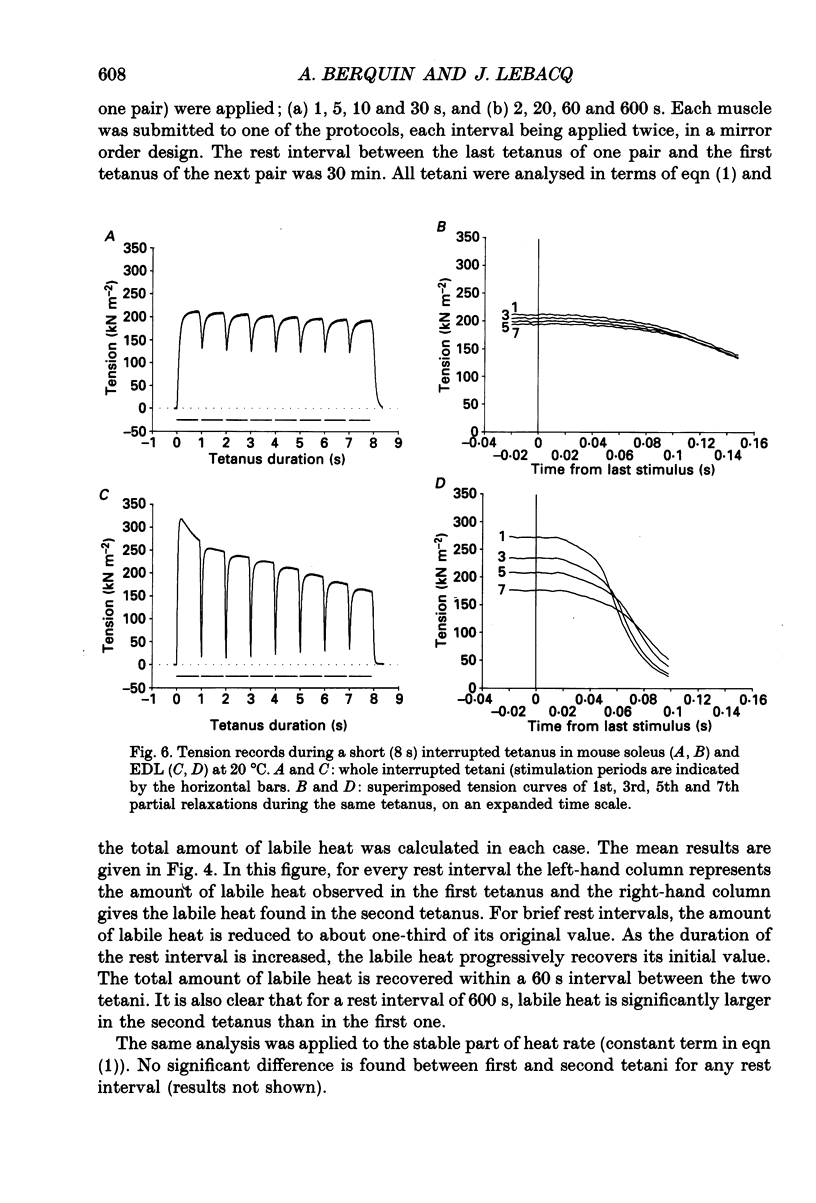
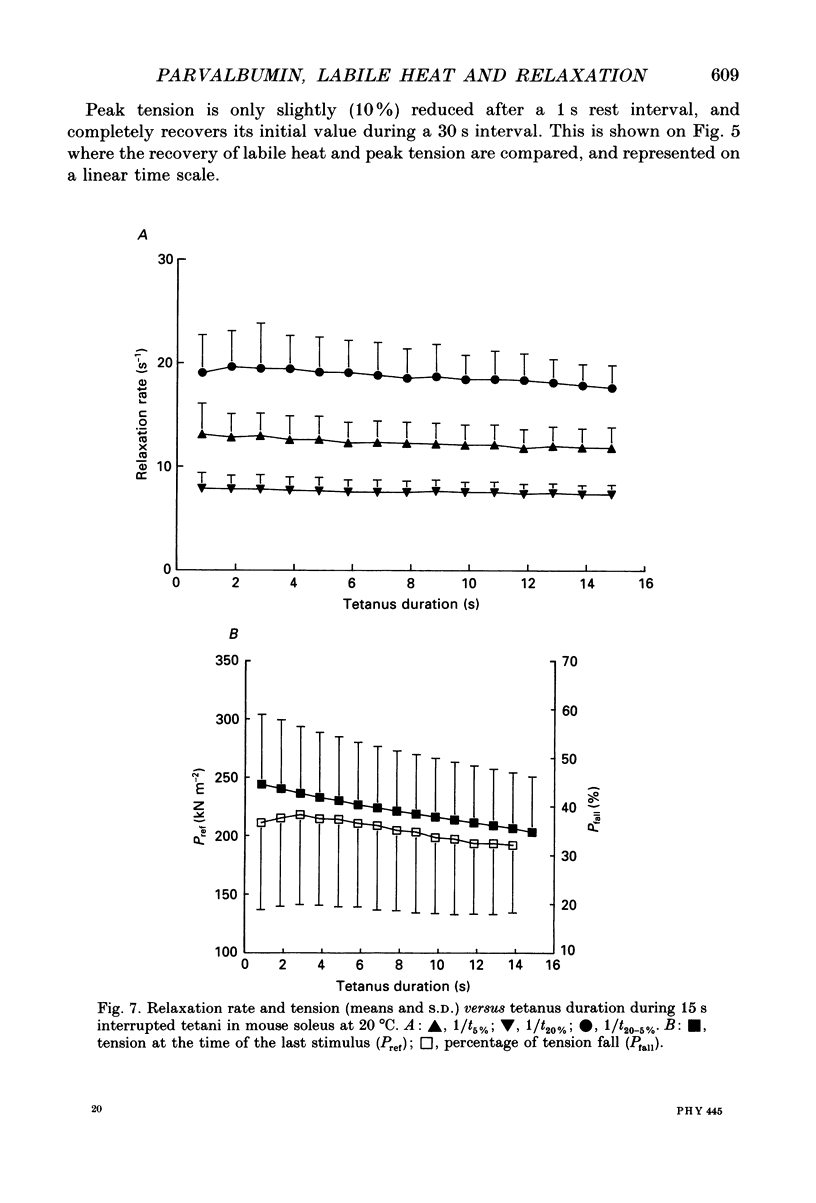
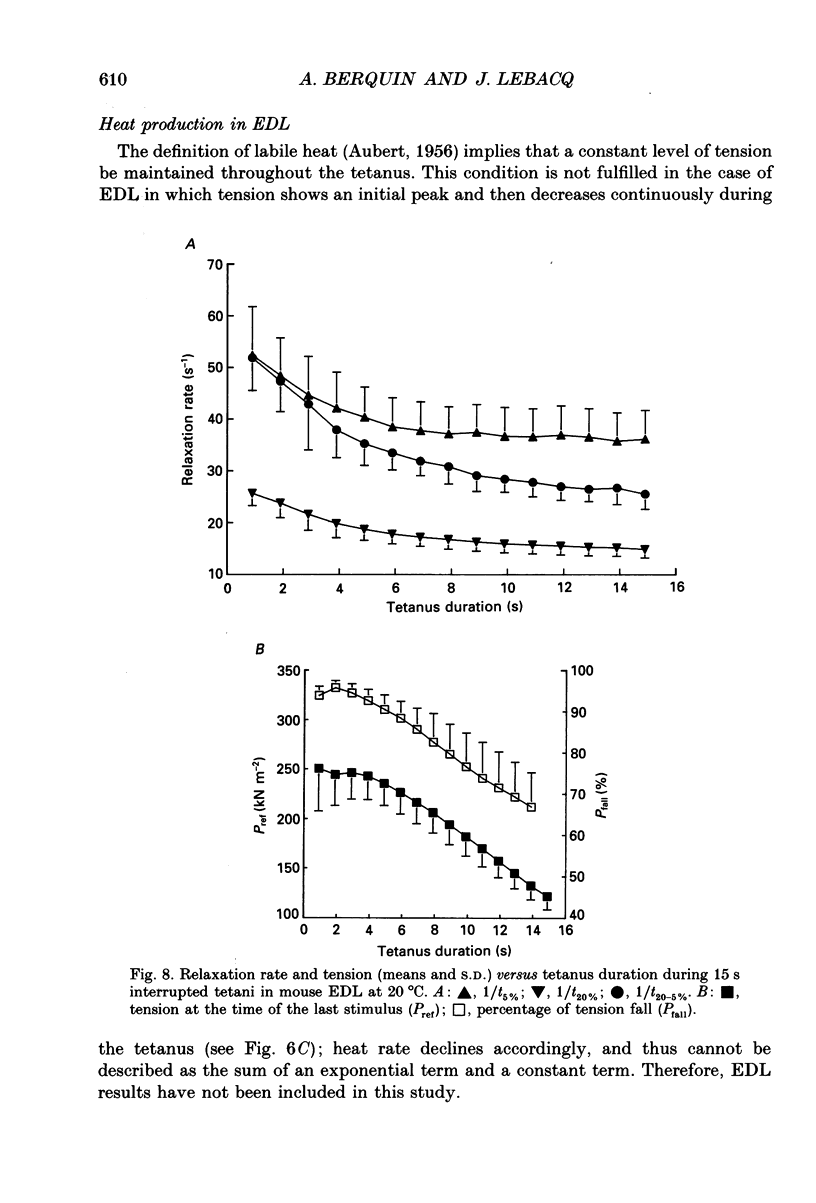






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C. The heat production associated with the maintenance of a prolonged contraction and the extra heat produced during large shortening. J Physiol. 1951 Feb;112(3-4):438–445. doi: 10.1113/jphysiol.1951.sp004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B. Effect of tetanus duration on the free calcium during the relaxation of frog skeletal muscle fibres. J Physiol. 1986 Jul;376:203–218. doi: 10.1113/jphysiol.1986.sp016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982 Jan;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Correlated reduction of velocity of shortening and the rate of energy utilization in mouse fast-twitch muscle during a continuous tetanus. J Gen Physiol. 1983 Nov;82(5):703–720. doi: 10.1085/jgp.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Edman K. A. Effects of fatigue and reduced intracellular pH on segment dynamics in 'isometric' relaxation of frog muscle fibres. J Physiol. 1989 Jun;413:159–174. doi: 10.1113/jphysiol.1989.sp017647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Howarth J. V., Rall J. A., Wilson M. G., Woledge R. C. Absolute values of myothermic measurements on single muscle fibres from frog. J Muscle Res Cell Motil. 1986 Aug;7(4):327–332. doi: 10.1007/BF01753653. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. A comparison of the energy balance in two successive isometric tetani of frog muscle. J Physiol. 1977 Sep;270(2):455–471. doi: 10.1113/jphysiol.1977.sp011962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Chemical change and energy production during contraction of frog muscle: how are their time courses related? J Physiol. 1979 Mar;288:353–366. [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Effect of muscle length on energy balance in frog skeletal muscle. J Physiol. 1981 Jul;316:453–468. doi: 10.1113/jphysiol.1981.sp013800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Energy changes and muscular contraction. Physiol Rev. 1978 Jul;58(3):690–761. doi: 10.1152/physrev.1978.58.3.690. [DOI] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. J Physiol. 1980 Feb;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mattiazzi A. R. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motil. 1981 Sep;2(3):321–334. doi: 10.1007/BF00713270. [DOI] [PubMed] [Google Scholar]
- Gibbs C. L., Gibson W. R. Energy production of rat soleus muscle. Am J Physiol. 1972 Oct;223(4):864–871. doi: 10.1152/ajplegacy.1972.223.4.864. [DOI] [PubMed] [Google Scholar]
- Gilbert C., Kretzschmar K. M., Wilkie D. R., Woledge R. C. Chemical change and energy output during muscular contraction. J Physiol. 1971 Oct;218(1):163–193. doi: 10.1113/jphysiol.1971.sp009609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis J. M. Relaxation of vertebrate skeletal muscle. A synthesis of the biochemical and physiological approaches. Biochim Biophys Acta. 1985 Jun 3;811(2):97–145. doi: 10.1016/0304-4173(85)90016-3. [DOI] [PubMed] [Google Scholar]
- Gillis J. M., Thomason D., Lefèvre J., Kretsinger R. H. Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil. 1982 Dec;3(4):377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Gower D., Kretzschmar K. M. Heat production and chemical change during isometric contraction of rat soleus muscle. J Physiol. 1976 Jul;258(3):659–671. doi: 10.1113/jphysiol.1976.sp011439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann C. W., Berchtold M. W., Rowlerson A. M. Correlation of parvalbumin concentration with relaxation speed in mammalian muscles. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7243–7247. doi: 10.1073/pnas.79.23.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E. Phosphate release and force generation in skeletal muscle fibers. Science. 1985 Jun 14;228(4705):1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Homsher E., Kean C. J., Wallner A., Garibian-Sarian V. The time-course of energy balance in an isometric tetanus. J Gen Physiol. 1979 May;73(5):553–567. doi: 10.1085/jgp.73.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Lacktis J., Yamada T., Zohman G. Repriming and reversal of the isometric unexplained enthalpy in frog skeletal muscle. J Physiol. 1987 Dec;393:157–170. doi: 10.1113/jphysiol.1987.sp016817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T. T., Johnson J. D., Rall J. A. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J Physiol. 1991 Sep;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Reichmann H., Pette D. Rapid reduction in parvalbumin concentration during chronic stimulation of rabbit fast twitch muscle. FEBS Lett. 1983 Feb 21;152(2):180–182. doi: 10.1016/0014-5793(83)80374-3. [DOI] [PubMed] [Google Scholar]
- Peckham M., Woledge R. C. Labile heat and changes in rate of relaxation of frog muscles. J Physiol. 1986 May;374:123–135. doi: 10.1113/jphysiol.1986.sp016070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Woledge R. C. Thermodynamic analysis of calcium binding to frog parvalbumin. J Muscle Res Cell Motil. 1985 Dec;6(6):757–768. doi: 10.1007/BF00712240. [DOI] [PubMed] [Google Scholar]
- Wendt I. R., Gibbs C. L. Energy production of rat extensor digitorum longus muscle. Am J Physiol. 1973 May;224(5):1081–1086. doi: 10.1152/ajplegacy.1973.224.5.1081. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. Slowing of relaxation during fatigue in single mouse muscle fibres. J Physiol. 1991 Mar;434:323–336. doi: 10.1113/jphysiol.1991.sp018472. [DOI] [PMC free article] [PubMed] [Google Scholar]