Abstract
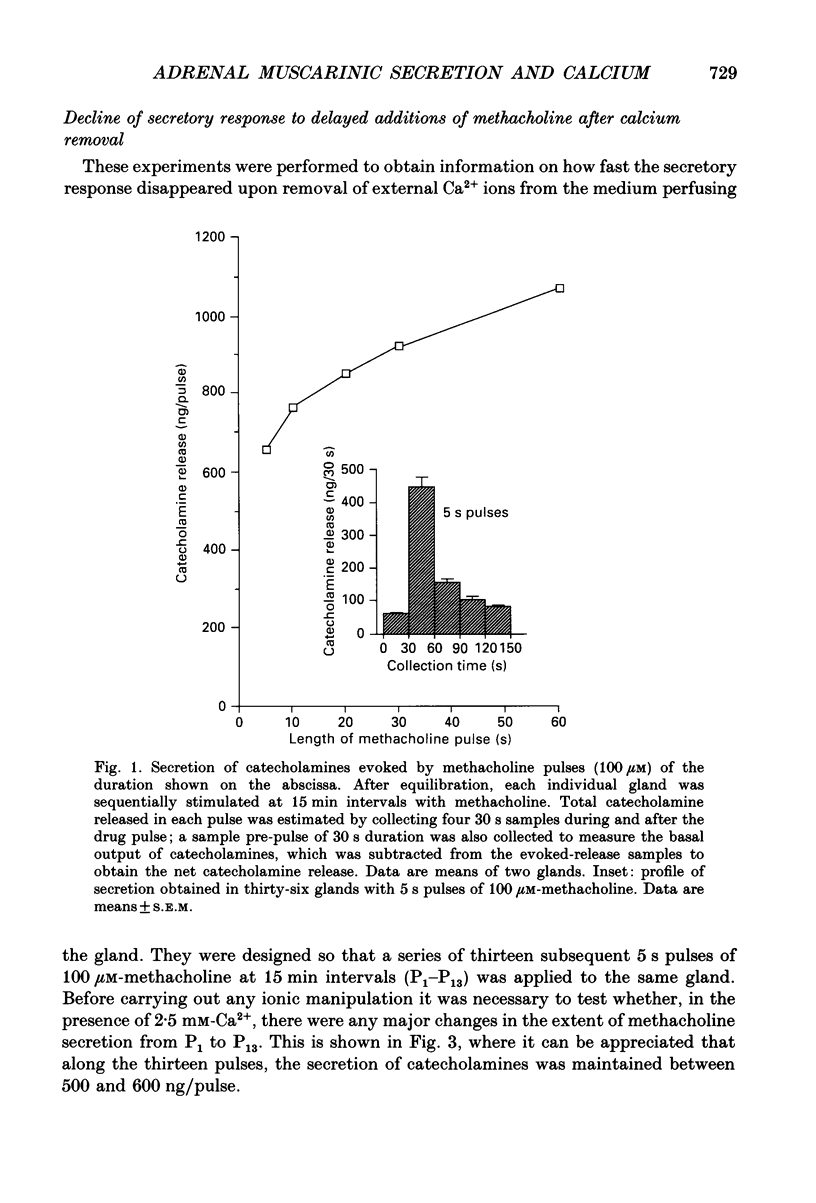
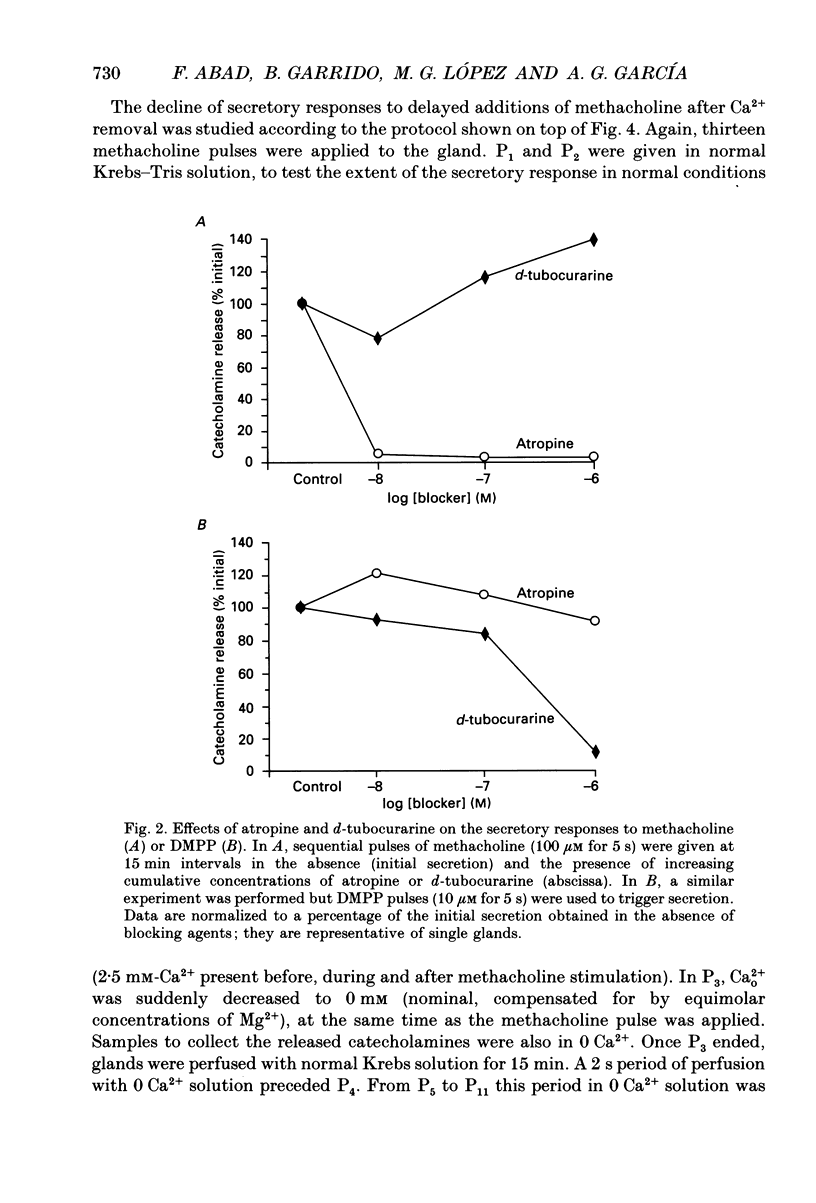
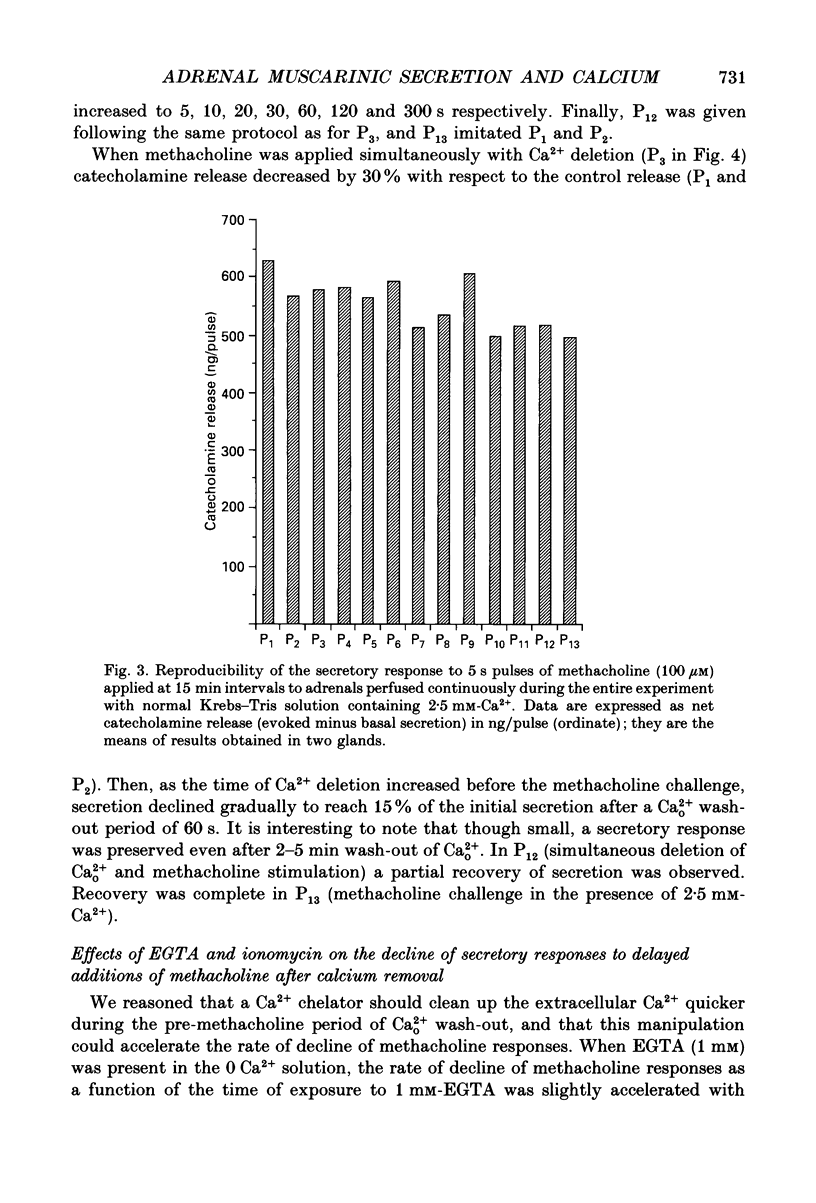
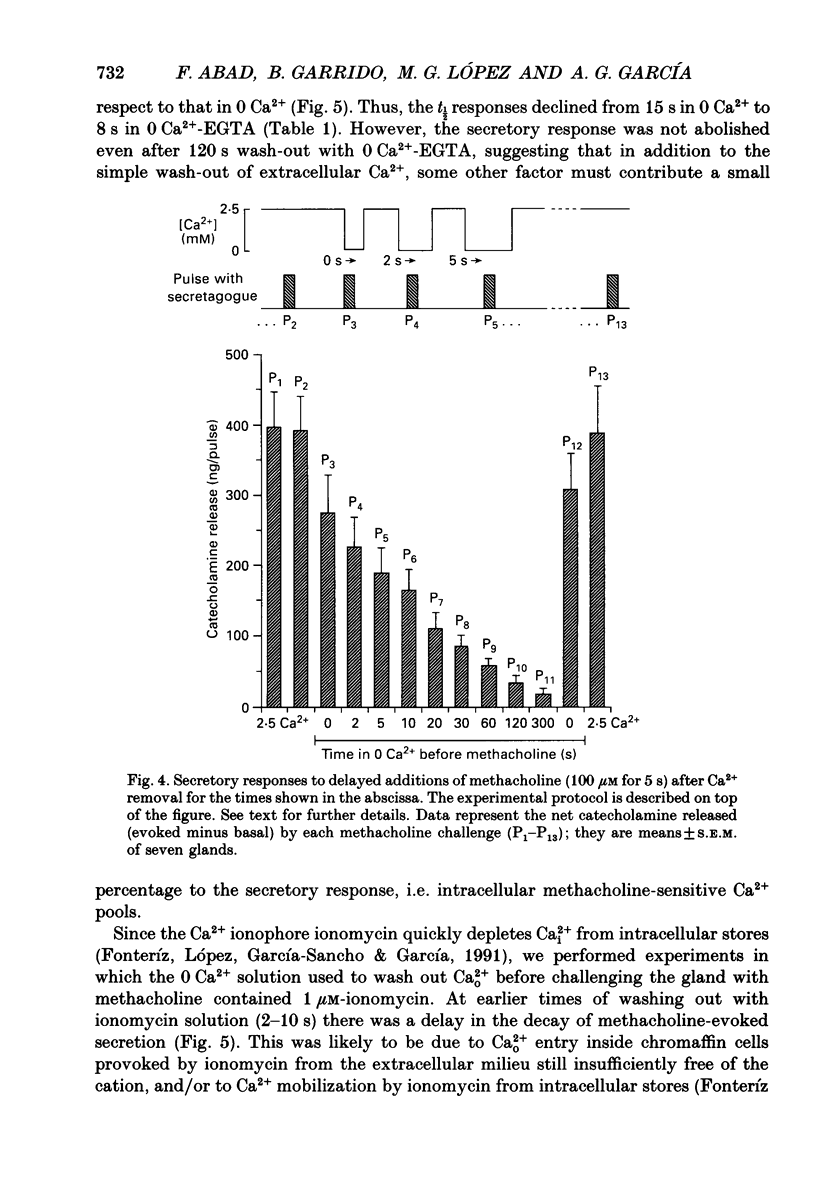
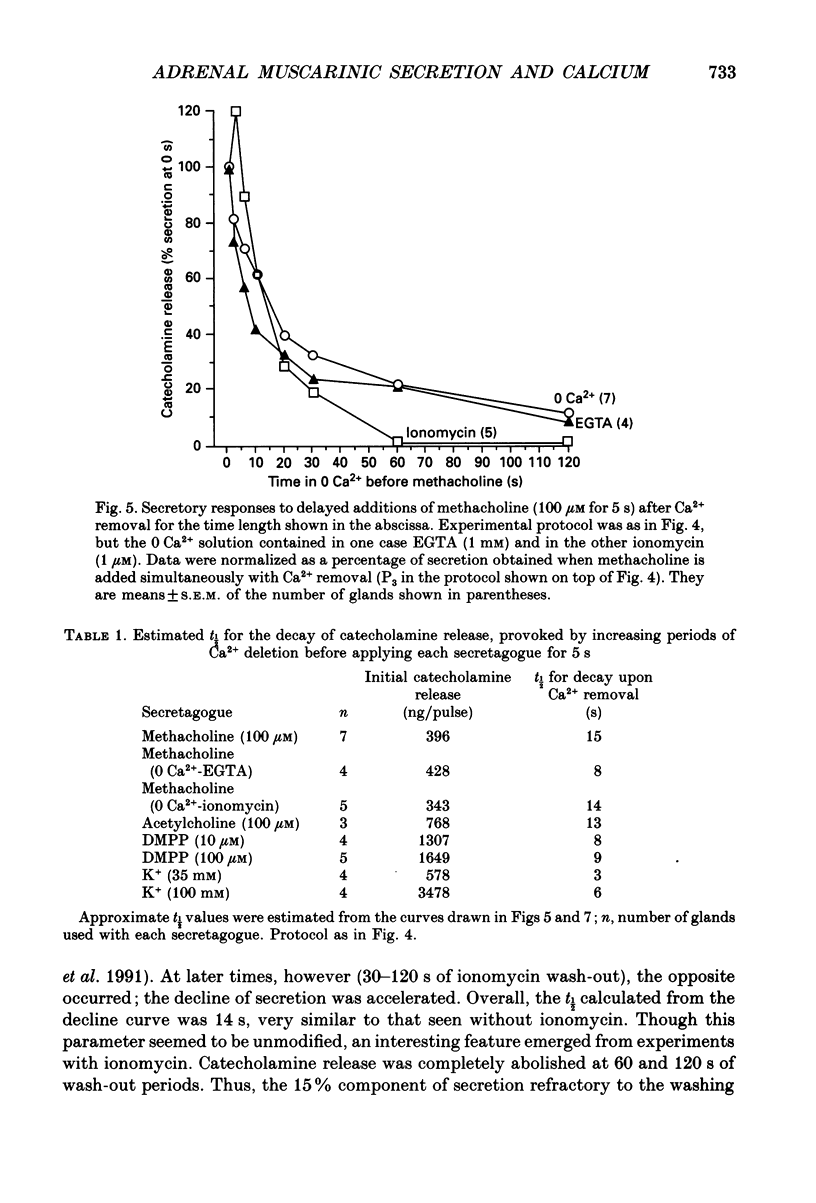
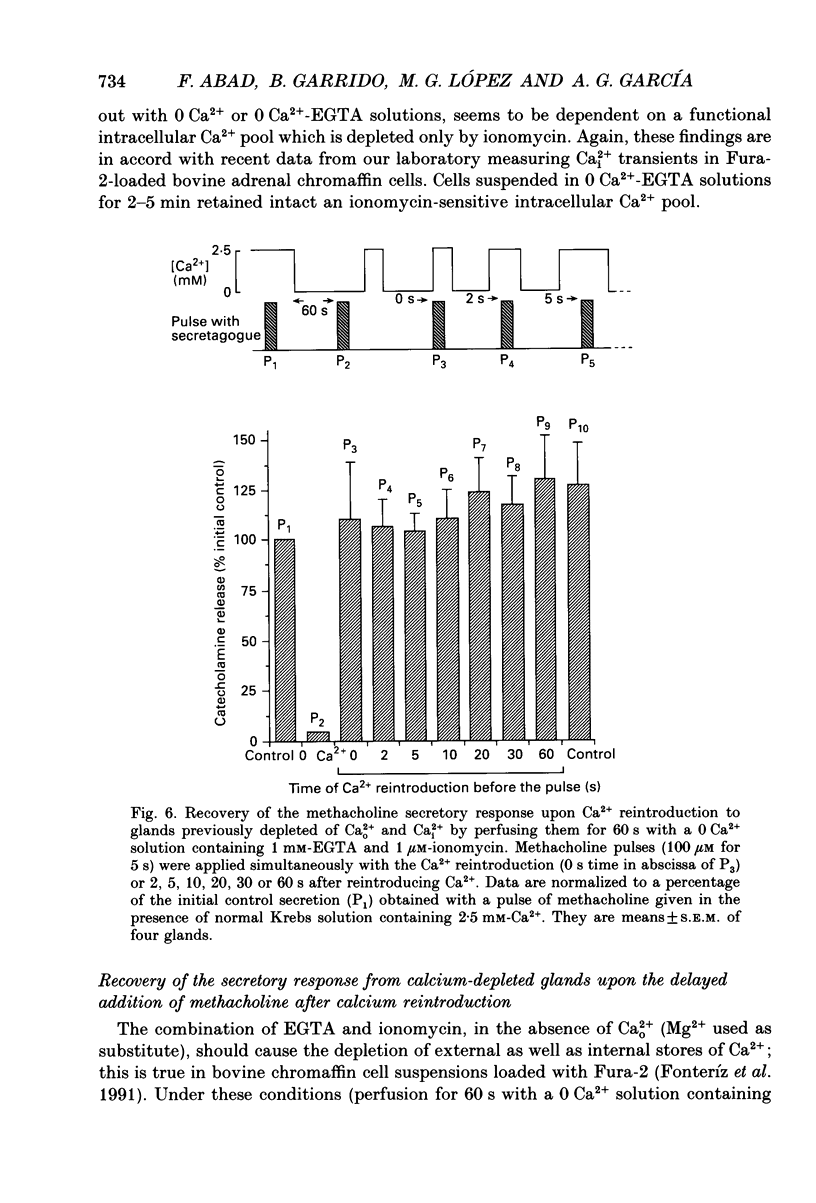
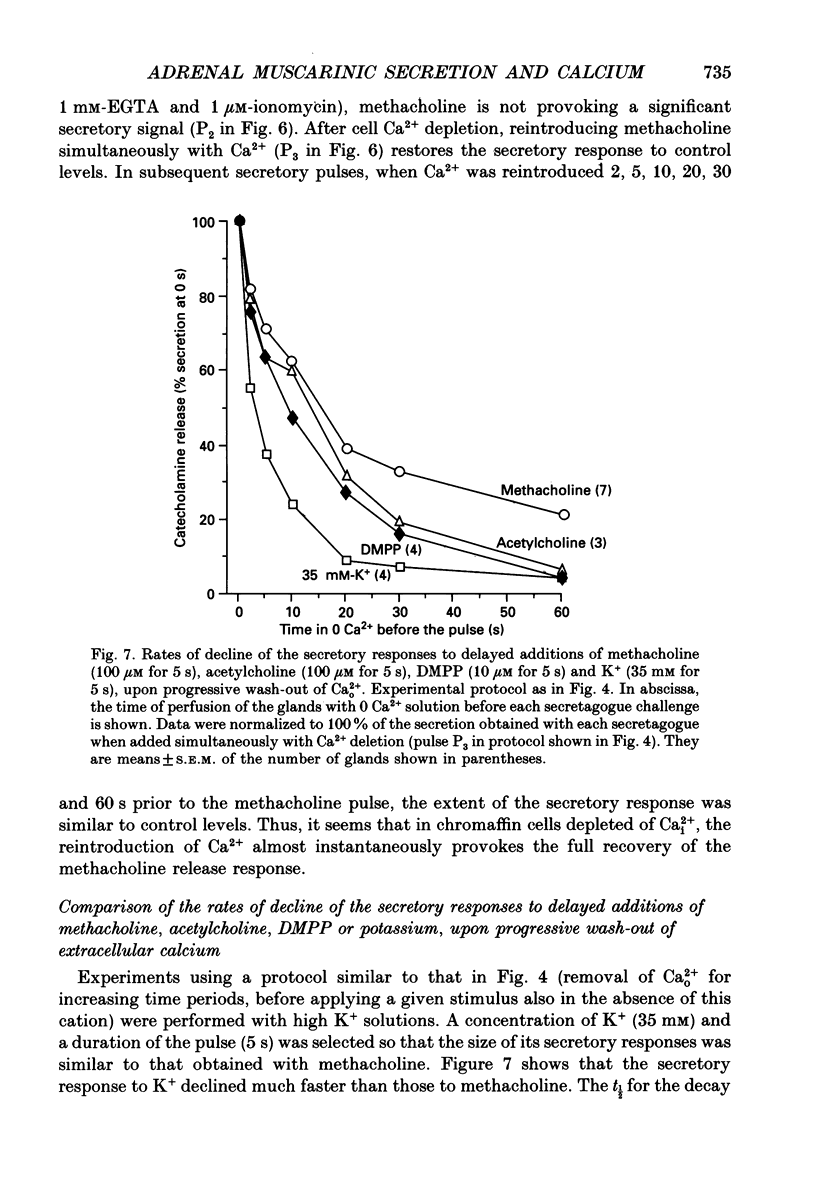
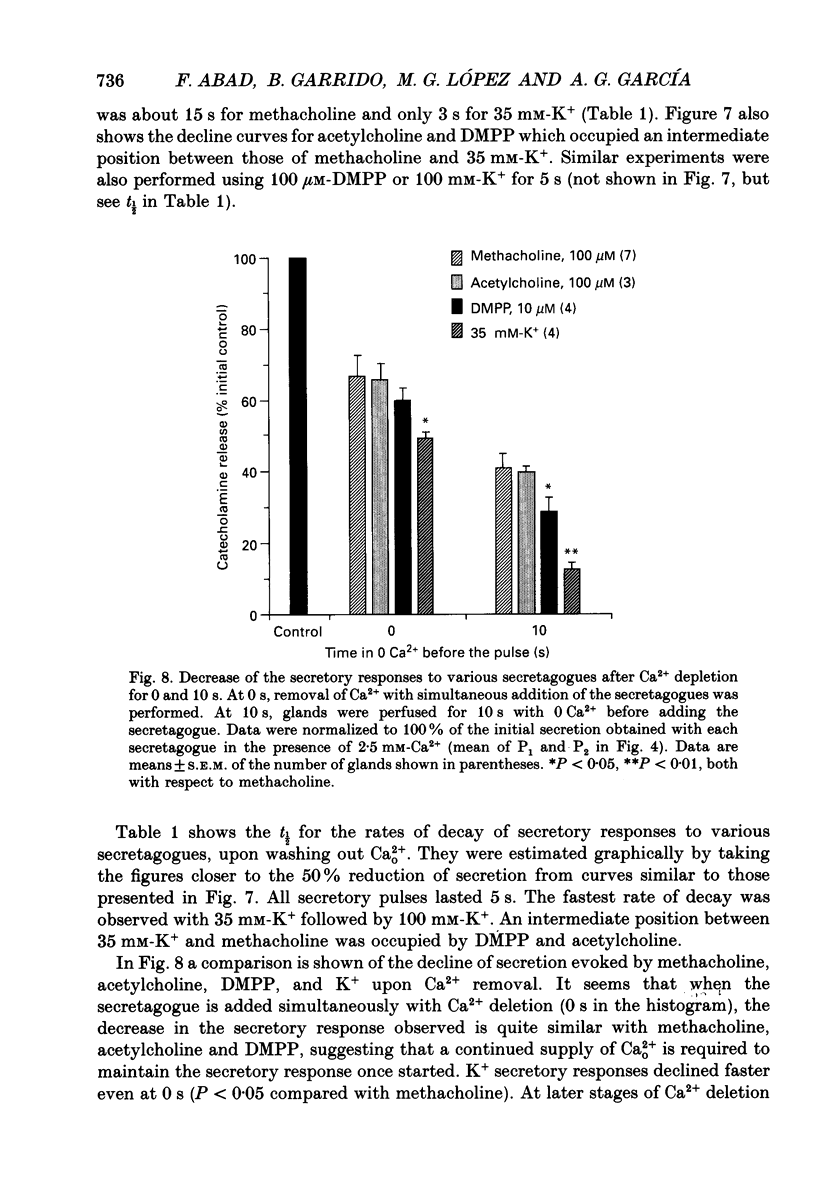
1. In view of conflicting reports on the source of Ca2+ needed to trigger the secretory response to muscarinic stimulation of chromaffin cells, we have reinvestigated this problem in the cat adrenal gland perfused with oxygenated Krebs solution at 37 degrees C. Above a basal rate of secretion of 60 ng/30 s of total catecholamines, 5 s pulses of 100 microM-methacholine evoked 10-fold increases of secretion. This response was entirely mediated by muscarinic receptors, since it was blocked by submicromolar concentrations of atropine but not by d-tubocurarine. 2. Delayed application of methacholine pulses after Ca2+ removal from the Krebs solution led to a progressive decline of the secretory response with a t1/2 of 15 s. Secretion was blocked by 85% after a 60 s period of Ca2+ deprivation; extension of the external Ca2+ (Ca2+o) wash-out period up to 5 min did not further reduce the secretory response. 3. When EGTA (1 mM) was present in the 0 Ca2+ solution, the rate of decline of methacholine responses, as a function of the time of exposure to 1 mM-EGTA, was similar to that obtained with 0 Ca2+. Again, about 15-20% of the secretory response was resistant even to prolonged periods of washing out with the 0 Ca(2+)-EGTA solution. 4. The Ca2+ ionophore ionomycin (1 microM) first decreased and then accelerated the rate of decline of methacholine responses upon Ca2+o wash-out. Particularly relevant is the complete blockade of secretion when the Ca2+o wash-out is performed in the presence of this ionophore. This suggests the existence of a small intracellular functional Ca2+ store sensitive to ionomycin. 5. After abolition of the secretory response through 60 s periods of wash-out with a 0 Ca(2+)-EGTA-ionomycin solution, followed by delayed 5 s methacholine pulses after Ca2+o reintroduction, the glands instantly recovered their normal muscarinic-mediated secretory response. This suggests that upon muscarinic stimulation, Ca2+ required by the secretory machinery to trigger such response immediately comes from extracellular sources. How Ca2+o gains the cell interior so fast upon muscarinic stimulation is unknown; we have previously suggested that the muscarinic receptor in the cat chromaffin cell could be coupled to an ionophore channel which might be chemically activated by muscarinic agonists. 6. Secretory responses to 5 s pulses with 35 or 100 mM-K+ declined faster (t1/2 of 3 and 6 s, respectively) upon Ca2+o wash-out than those of methacholine.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almazan G., Aunis D., García A. G., Montiel C., Nicolás G. P., Sánchez-García P. Effects of collagenase on the release of [3H]-noradrenaline from bovine cultured adrenal chromaffin cells. Br J Pharmacol. 1984 Apr;81(4):599–610. doi: 10.1111/j.1476-5381.1984.tb16124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta J. J., Borges R., García A. G., Hidalgo M. J. Secretory and radioligand binding studies on muscarinic receptors in bovine and feline chromaffin cells. J Physiol. 1989 Nov;418:411–426. doi: 10.1113/jphysiol.1989.sp017849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges R., Ballesta J. J., García A. G. M2 muscarinoceptor-associated ionophore at the cat adrenal medulla. Biochem Biophys Res Commun. 1987 Apr 29;144(2):965–972. doi: 10.1016/s0006-291x(87)80058-x. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., Burgoyne R. D. Effect of activation of muscarinic receptors on intracellular free calcium and secretion in bovine adrenal chromaffin cells. Biochim Biophys Acta. 1985 Jul 30;846(1):167–173. doi: 10.1016/0167-4889(85)90122-3. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. Spatial localization of the stimulus-induced rise in cytosolic Ca2+ in bovine adrenal chromaffin cells. Distinct nicotinic and muscarinic patterns. FEBS Lett. 1989 Apr 24;247(2):429–434. doi: 10.1016/0014-5793(89)81385-7. [DOI] [PubMed] [Google Scholar]
- Cheek T. R. Spatial aspects of calcium signalling. J Cell Sci. 1989 Jun;93(Pt 2):211–216. doi: 10.1242/jcs.93.2.211. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Preferential release of adrenaline from the adrenal medulla by muscarine and pilocarpine. Nature. 1965 Dec 11;208(5015):1102–1103. doi: 10.1038/2081102a0. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Minz B., Tsudzimura H. The mechanism of the nervous discharge of adrenaline. J Physiol. 1934 Jun 9;81(3):286–304. doi: 10.1113/jphysiol.1934.sp003136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. K., Holz R. W., Agranoff B. W. Muscarinic receptors in chromaffin cell cultures mediate enhanced phospholipid labeling but not catecholamine secretion. J Neurochem. 1981 Aug;37(2):491–497. doi: 10.1111/j.1471-4159.1981.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Fonteríz R. I., López M. G., García-Sancho J., García A. G. Alamethicin channel permeation by Ca2+, Mn2+ and Ni2+ in bovine chromaffin cells. FEBS Lett. 1991 May 20;283(1):89–92. doi: 10.1016/0014-5793(91)80560-p. [DOI] [PubMed] [Google Scholar]
- Gandía L., Casado L. F., López M. G., García A. G. Separation of two pathways for calcium entry into chromaffin cells. Br J Pharmacol. 1991 May;103(1):1073–1078. doi: 10.1111/j.1476-5381.1991.tb12302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. G., Hernandez M., Horga J. F., Sanchez-Garcia P. On the release of catecholamines and dopamine-beta-hydroxylase evoked by ouabain in the perfused cat adrenal gland. Br J Pharmacol. 1980 Mar;68(3):571–583. doi: 10.1111/j.1476-5381.1980.tb14573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García A. G., Sala F., Reig J. A., Viniegra S., Frías J., Fontériz R., Gandía L. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature. 1984 May 3;309(5963):69–71. doi: 10.1038/309069a0. [DOI] [PubMed] [Google Scholar]
- Harish O. E., Kao L. S., Raffaniello R., Wakade A. R., Schneider A. S. Calcium dependence of muscarinic receptor-mediated catecholamine secretion from the perfused rat adrenal medulla. J Neurochem. 1987 Jun;48(6):1730–1735. doi: 10.1111/j.1471-4159.1987.tb05730.x. [DOI] [PubMed] [Google Scholar]
- Kao L. S., Schneider A. S. Calcium mobilization and catecholamine secretion in adrenal chromaffin cells. A Quin-2 fluorescence study. J Biol Chem. 1986 Apr 15;261(11):4881–4888. [PubMed] [Google Scholar]
- Kao L. S., Schneider A. S. Muscarinic receptors on bovine chromaffin cells mediate a rise in cytosolic calcium that is independent of extracellular calcium. J Biol Chem. 1985 Feb 25;260(4):2019–2022. [PubMed] [Google Scholar]
- Kim K. T., Westhead E. W. Cellular responses to Ca2+ from extracellular and intracellular sources are different as shown by simultaneous measurements of cytosolic Ca2+ and secretion from bovine chromaffin cells. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9881–9885. doi: 10.1073/pnas.86.24.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Schiavone M. T. Effect of muscarine on release of catecholamines from the perfused adrenal gland of the cat. Br J Pharmacol. 1982 Nov;77(3):455–460. doi: 10.1111/j.1476-5381.1982.tb09318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Observations on the muscarinic activation of catecholamine secretion in the chicken adrenal. Neuroscience. 1986 Sep;19(1):357–366. doi: 10.1016/0306-4522(86)90027-8. [DOI] [PubMed] [Google Scholar]
- Ladona M. G., Aunis D., Gandía L., García A. G. Dihydropyridine modulation of the chromaffin cell secretory response. J Neurochem. 1987 Feb;48(2):483–490. doi: 10.1111/j.1471-4159.1987.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Lee F. L., Trendelenburg U. Muscarinic transmission of preganglionic impulses to the adrenal medulla of the cat. J Pharmacol Exp Ther. 1967 Oct;158(1):73–79. [PubMed] [Google Scholar]
- Livett B. G., Boksa P. Receptors and receptor modulation in cultured chromaffin cells. Can J Physiol Pharmacol. 1984 Apr;62(4):467–476. doi: 10.1139/y84-076. [DOI] [PubMed] [Google Scholar]
- Nakazato Y., Ohga A., Oleshansky M., Tomita U., Yamada Y. Voltage-independent catecholamine release mediated by the activation of muscarinic receptors in guinea-pig adrenal glands. Br J Pharmacol. 1988 Jan;93(1):101–109. doi: 10.1111/j.1476-5381.1988.tb11410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan A. J., Burgoyne R. D. A comparison of bradykinin, angiotensin II and muscarinic stimulation of cultured bovine adrenal chromaffin cells. Biosci Rep. 1989 Apr;9(2):243–252. doi: 10.1007/BF01116001. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisner A. M., Douglas W. W. The need for calcium in adrenomedullary secretion evoked by biogenic amines, polypeptides, and muscarinic agents. Proc Soc Exp Biol Med. 1966 Oct;123(1):62–64. doi: 10.3181/00379727-123-31402. [DOI] [PubMed] [Google Scholar]
- Shellenberger M. K., Gordon J. H. A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem. 1971 Feb;39(2):356–372. doi: 10.1016/0003-2697(71)90426-x. [DOI] [PubMed] [Google Scholar]


