Abstract
Growth and development are regulated using cyclic AMP (cAMP)-dependent and -independent pathways in Neurospora crassa. The cr-1 adenylyl cyclase mutant lacks detectable cAMP and exhibits numerous defects, including colonial growth habit, short aerial hyphae, premature conidiation on plates, inappropriate conidiation in submerged culture, and increased thermotolerance. Evidence suggests that the heterotrimeric Gα protein GNA-1 is a direct positive regulator of adenylyl cyclase. Δgna-1 strains are female-sterile, and Δgna-1 strains have reduced apical extension rates on normal and hyperosmotic medium, greater resistance to oxidative and heat stress, and stunted aerial hyphae compared to the wild-type strain. In this study, a Δgna-1 cr-1 double mutant was analyzed to differentiate cAMP-dependent and -independent signaling pathways regulated by GNA-1. Δgna-1 cr-1 mutants have severely restricted colonial growth and do not produce aerial hyphae on plates or in standing liquid cultures. Addition of cAMP to plates or standing liquid cultures rescues cr-1, but not Δgna-1 cr-1, defects, which is consistent with previous results demonstrating that Δgna-1 mutants do not respond to exogenous cAMP. The females of all strains carrying the Δgna-1 mutation are sterile; however, unlike cr-1 and Δgna-1 strains, the Δgna-1 cr-1 mutant does not produce protoperithecia. The Δgna-1 and cr-1 mutations were synergistic with respect to inappropriate conidiation during growth in submerged culture. Thermotolerance followed the order wild type < Δgna-1 < cr-1 = Δgna-1 cr-1, consistent with a cAMP-dependent process. Taken together, the results suggest that in general, GNA-1 and CR-1 regulate N. crassa growth and development using parallel pathways, while thermotolerance is largely dependent on cAMP.
Development in fungal systems frequently occurs in response to specific environmental cues and stressors. In the presence of abundant nutrients, the filamentous fungus Neurospora crassa extends hyphae that elongate and fuse to form the multicellular mycelium (for a review, see reference 51). Desiccation or nutrient deprivation causes the mycelium to differentiate aerial hyphae that give rise to conidiophores and multinucleate asexual spores, macroconidia (referred to here as conidia). Elaboration of conidiophores and production of conidia require an air-water interface; however, submerged cultures can be induced to undergo conidiation by carbon or nitrogen starvation or exposure to high temperatures (7, 18, 43, 55). Nitrogen limitation initiates the sexual cycle by stimulating production of female reproductive structures, or protoperithecia (reviewed in reference 44). Fertilization by a conidium or hypha of the opposite mating type results in formation of the fertilized structure (perithecium), within which sexual spores (ascospores) develop. Mature ascospores are subsequently ejected from the perithecium in the direction of blue light.
Heterotrimeric GTP-binding proteins, consisting of α, β, and γ subunits, transduce various environmental signals to stimulate morphogenesis and cellular responses in fungi (for reviews, see references 5, 19, and 34). Ligands, such as nutrients and pheromones, are recognized by seven transmembrane helical receptors coupled to G proteins. Ligand binding results in a conformational change within the receptor, leading to the exchange of GDP for GTP on the Gα subunit and subsequent dissociation of the GTP-Gα and Gβγ moiety. Depending on the system, both the freed GTP-Gα and Gβγ moiety may regulate downstream effectors, such as adenylyl cyclase and mitogen-activated protein kinase (MAPK) cascades.
The N. crassa cr-1 mutant lacks adenylyl cyclase activity and cyclic AMP (cAMP) (47, 53, 54), and studies support cr-1 as the structural gene for the adenylyl cyclase enzyme (28, 29). When the cr-1 mutant is cultured on a solid medium, it grows colonially, lacks appreciable aerial hyphae, and prematurely produces macroconidia on the surface of the mycelium, yielding a “crisp” appearance. Previous work from our laboratory suggests that the Gα subunit GNA-1 positively stimulates CR-1 activity and participates in pathways regulating female fertility, apical extension, osmotic sensitivity, conidiation, and resistance to heat and oxidative stress (23, 24, 59).
Incongruous development has been observed in signal transduction mutants from N. crassa and the related filamentous fungus Aspergillus nidulans. Deletion of the N. crassa Gα subunit gna-3 gene or the putative glucose sensor rco-3 gene causes inappropriate differentiation of conidiophores in submerged cultures and transcription of the conidiation-specific gene con-10 (27, 38). The cr-1 mutant also conidiates in submerged culture; however, the submerged conidiation phenotype is not as dramatic as that observed in Δgna-3 and rco-3 strains (27, 38, 41). These results suggest that development in N. crassa utilizes both cAMP-dependent and -independent pathways. In A. nidulans, overexpression of the RGS (named for regulator of G-protein signaling) protein gene flbA, expression of the dominant negative Gα allele fadAG203R, or deletion of the Gβ gene sfaD leads to submerged culture conidiation (32, 33, 46, 60). Deletion of the cAMP-dependent protein kinase A catalytic subunit gene pkaA does not cause inappropriate conidiation in submerged cultures, suggesting that the conidiation defects of FlbA, FadA, and SfaD are cAMP independent (50).
Results from studies of N. crassa and Saccharomyces cerevisiae support an inverse correlation between cAMP levels and resistance to high temperatures. In N. crassa, cr-1 and gna-1 mutants possess increased thermotolerance (8, 59). Conversely, strains with GTPase-deficient alleles of gna-1 are more sensitive to lethal temperatures than the wild type (59). Low cAMP levels induced by loss-of-function mutations in ras2 or by deletion of the Gα subunit GPA2 lead to increased heat stress resistance in S. cerevisiae (6, 22). In contrast, the elevated cAMP levels of strains carrying the constitutively activated ras2Val19 mutation are correlated with hypersensitivity to lethal temperatures (11, 26).
In this study, we investigate cAMP-dependent and -independent pathways regulated by adenylyl cyclase and the stimulatory Gα protein GNA-1 in N. crassa. Wild-type, Δgna-1, cr-1, and Δgna-1 cr-1 strains are analyzed for phenotypes and expression of G-protein subunits and adenylyl cyclase. Thermotolerance, submerged culture conidiation, and transcript levels of a conidiation-regulated gene are also investigated. Our results suggest that thermotolerance is largely dependent on cAMP and that GNA-1 and CR-1 act synergistically to regulate development in N. crassa.
MATERIALS AND METHODS
Strains, media, and culture conditions.
N. crassa strain 74-OR23-1A (mating type A) (Fungal Genetics Stock Center [FGSC]) (University of Kansas Medical Center, Kansas City, Kans.) was used as the wild type in all experiments except those involving fertilization of mating type A strains; in such cases, wild-type strain Sta73a (mating type a) was used as the male. A Δgna-1 cr-1 mating type A double mutant (strain 1.2) was constructed through a sexual cross between the parental cr-1 mating type A strain (FGSC 4008; allele B123) and Δgna-1::hph+ mating type a strain (3B10a) (24). Ascospores were plated on medium containing sorbose (9) containing 200 μg of hygromycin B per ml. Southern blot analysis was performed (23) to verify that hygromycin-resistant progeny contained the Δgna-1::hph+ gene replacement (data not shown). Since the cr-1 gene and the mating type gene are linked, hygromycin B-resistant progeny were tested for mating type to determine the cr-1 allele present (9, 41). Escherichia coli strain DH5α was used to maintain plasmids (20).
N. crassa strains were cultured on Vogel's minimal medium containing 1.5% sucrose (VM) (56) to facilitate production of vegetative hyphae in liquid cultures or production of vegetative hyphae, aerial hyphae, and conidia on plates. Synthetic crossing medium (SCM) (57) was used to induce production of female reproductive structures during the sexual cycle. Conidia were used to inoculate shaken or standing liquid cultures and agar plates. Media were supplemented with peptone (2% wt/vol) or cAMP (2.0 mM) where indicated.
Overexpression of N. crassa adenylyl cyclase (CR-1) and antiserum generation.
Cosmid pSV50-4:7C (4:7C) containing the structural gene for N. crassa adenylyl cyclase (nac/cr-1) has previously been identified (28). This cosmid was used to generate a construct to allow overexpression of a 56-kDa protein corresponding to the 5′ end of the cr-1 open reading frame (ORF). PCR mutagenesis was used to create restriction sites for cloning into the pET22 vector (Novagen, Madison, Wis.) (allows protein overexpression in E. coli) and to remove the first two introns from the 5′ end of the ORF in the 4:7C genomic clone. Templates for PCR were generated by cloning (i) a 9.14-kb NdeI fragment from 4:7C into the NdeI site of pGEM5Zf (pDIV6), (ii) the PstI-BamHI fragment from pDIV6 into pBluescript II KS+ digested with the same enzymes (pDIV6.5), and (iii) a 4-kb EcoRI fragment from 4:7C that contains the 5′ end of the cr-1 ORF and ∼1-kb untranslated sequence into pBluescript II KS+ to yield pDIV8.
The first cr-1 intron was deleted through creation of a silent mutation at the intron splice junction that also encodes an NheI restriction site. Two PCR products flanking the intron were generated using pDIV8 as a template and ligated together to create a fragment extending from the ATG translational initiation codon to the end of the second exon of cr-1. The primers used for the two PCRs are (i) NAC1 (creates an NdeI site at the translational start site of nac; plus strand) (G GTG CAT ATG ACG AGA AAT GAC), (ii) NAC2 (encodes an NheI site at the 3′ end of exon 1; minus strand) (TCA GCT AGC AAA TTG GGT GCC GTC), (iii) NAC3 (creates an NheI site at the 5′ end of exon 2; plus strand) (ATC GCT AGC AGG TCA TCG AGA G), and (iv) NAC4 (recognizes an EcoRI site in exon 2; minus strand) (GG GAA TTC AAT GAC AAC TGC). The NAC1/NAC2 and NAC3/NAC4 PCRs were performed using Pfu polymerase and 625 ng (each) of the two indicated primers according to the manufacturer's recommendations (Stratagene, La Jolla, Calif.). The NAC1/NAC2 and NAC3/NAC4 PCR products were then digested with the appropriate enzymes and then ligated into pSP72 (Promega, Madison, Wis.) digested with NdeI and EcoRI to create pDIV13.
The second intron in cr-1 was removed using inverse PCR with pDIV6.5 as a template. The primers were NAC5 (recognizes the 5′ end of intron 2 junction; minus strand) (GTC GGC TTC TTG GTA CAG AAA CGG) and NAC6 (anneals at 3′ end of intron 2 junction; plus strand) (GAC ATC GCT CGC TAT GGC GAA GCA CCC). The NAC5/NAC6 reactions were performed using Pfu polymerase and 125 ng (each) of the two indicated primers according to the manufacturer's recommendations (Stratagene), except for the presence of 5% dimethyl sulfoxide. The NAC5/NAC6 3.7-kb linear PCR product containing vector and insert was ligated and transformed into E. coli DH5α cells. Approximately 56% (27 of 48) of the resulting clones contained a 700-bp PstI-BamHI fragment, indicating deletion of the second intron. Sequence analysis revealed that all clones contained additional small deletions at the intron splice site. Clone pDIV12 contained a n−3 mutation that resulted in an in-frame deletion of one of two successive aspartate residues (at position 441 or 442 in the amino acid-coding sequence). The absence of one amino acid is not expected to dramatically affect the ability of the protein to function as an effective antigen in rabbits; therefore, this clone was used to make the final overexpression construct (see below).
The 700-bp PstI-BamHI fragment from pDIV12 and the 673-bp NdeI-PstI fragment from pDIV13 were ligated into pET22b (Qiagen, Inc., Valencia, Calif.) digested with NdeI and BamHI to create the final overexpression construct, pDIV18. E. coli strain HMS174(plysS) (52) was transformed with pDIV18, and the strain was cultured to facilitate overexpression of the CR-1 protein fragment as recommended by the manufacturer (Qiagen). The 56-kDa protein was subsequently purified by virtue of its carboxy-terminal six-His epitope tag using Ni-nitrilotriacetic acid agarose according to the manufacturer's recommendations (Qiagen). The fractions enriched for the CR-1 protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide), and the CR-1 protein band was excised and used to raise a specific antiserum in rabbits (Cocalico Biologicals, Reamstown, Pa.).
Western and Northern blot analyses.
The particulate fractions used for the adenylyl cyclase assays described above were subjected to Western blot analysis using GNA-1 (23), GNA-2 (3), GNB-1 (24), or CR-1 antisera at final dilutions of 1:2,500, 1:5,000, 1:5,000, and 1:10,000, respectively. The proteins were detected by using the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, N.J.) with a horseradish peroxidase-conjugated goat anti-rabbit antibody (Bio-Rad, Hercules, Calif.) as the secondary antibody.
For isolation of total RNA, 8-day-old conidia were inoculated into 50-ml portions of liquid VM and cultured in constant darkness for 16 h at 30°C with shaking at 200 rpm. Mycelia were collected by vacuum filtration and ground to a fine powder in liquid nitrogen, and total RNA was isolated as previously described (48). Northern blot analysis was performed as described previously (4), using a 200-bp BamHI-EcoRI fragment of pBW100 containing the con-10 cDNA (45) or the cox-5 gene insert from pSRCOX-5 (48) as probes.
Adenylyl cyclase assays.
Eight-day-old conidia were inoculated at a final concentration of 2.5 × 106 cells/ml in 500 ml of VM and cultured at 30°C for 16 h in darkness with shaking at 200 rpm. Mycelia were collected by filtration and ground to a fine powder in liquid nitrogen. Samples were suspended in 10-ml portions of extraction buffer [25 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) (pH 6.9), 1 mM MgCl2, 0.25 mM EDTA, 1 mM phenylmethylsulfonyl fluoride] and stirred gently at 4°C for 20 min. Extracts were centrifuged at 1,000 × g for 15 min at 4°C. The resulting supernatant was centrifuged at 180,000 × g at 4°C. The pellet (particulate fraction) was resuspended in extraction buffer, and protein concentration was determined using the Bradford microassay kit (Bio-Rad) with bovine serum albumin as a standard. Samples were assayed for Mn2+-ATP- and Mg2+ -ATP-dependent adenylyl cyclase activity as described previously (24).
Phenotypic analysis and thermotolerance measurements.
Colony growth and morphology were assessed by inoculating VM plates with conidia followed by incubation at 30°C in the dark for approximately 36 h. Standing liquid cultures were inoculated and grown as previously described (27) in the presence or absence of 2.0 mM cAMP. For analysis of conidiation of submerged cultures, liquid VM cultures were inoculated with conidia at a density of 1 × 106 or 3 × 106 cells/ml, incubated for 16 h with shaking at 200 rpm in the dark, and then photographed. For analysis of female fertility, strains were cultured on SCM plates for 10 days in constant light at room temperature to induce production of protoperithecia. Perithecia were photographed as described previously (23) 6 days after fertilization with conidia of the opposite mating type. Resistance of 3-h-old germlings to a 52°C lethal heat treatment with and without prior exposure to 45°C (induced versus uninduced thermotolerance, respectively) was measured as previously described (59). Percent survival was obtained by dividing the number of colonies from plates containing heated germlings (induced or uninduced) by the number of colonies on plates from germlings held at 30°C throughout the experiment (controls).
RESULTS
Δgna-1 cr-1 strains contain wild-type levels of GNA-2 and GNB-1 but lack full-length CR-1 protein and adenylyl cyclase activity.
A regulatory component is required for adenylyl cyclase to utilize Mg2+-ATP as a substrate for both basal and GTP-stimulated activities; in contrast, Mn2+-ATP adenylyl cyclase activity is independent of auxiliary proteins and thus serves as a measurement of the amount of active enzyme (15). N. crassa cr-1 strains do not contain Mg2+- or Mn2+-ATP adenylyl cyclase activity or intracellular cAMP (47). We have previously shown that while Mn2+-ATP adenylyl cyclase activity is only slightly reduced in Δgna-1 mutants, basal and GTP-stimulated Mg2+-ATP activities are severely affected in these strains (24). These results, in combination with the observation that an anti-GNA-1 antibody inhibits Mg2+-ATP-dependent adenylyl cyclase activity in preparations of the wild-type strain, suggested that GNA-1 is the regulatory component in N. crassa adenylyl cyclase assays (24).
Certain Δgna-1 defects, such as female sterility, are not shared by cr-1 strains, suggesting that the affected functions are regulated using cAMP-independent pathways. Furthermore, exogenous cAMP does not rescue Δgna-1 phenotypes (23), despite the apparent requirement for GNA-1 in regulation of adenylyl cyclase activity. To differentiate between cAMP-dependent and -independent functions of gna-1, a Δgna-1 cr-1 mutant was constructed through a sexual cross. An observation of identical defects in cr-1 and Δgna-1 cr-1 strains would be evidence for cAMP-dependent functions for gna-1. In contrast, an observation of different phenotypes for these two strain backgrounds would offer support for cAMP independence.
The Δgna-1 mutation (strain 3b10a [mating type a]) is marked by an E. coli hph gene insertion, and the cr-1 mutation (FGSC 4008 [mating type A]) is followed by linkage to the mating type (41). Therefore, homokaryotic hygromycin-resistant (hygR) ascospore progeny should carry the gna-1 gene replacement (data not shown) and should not contain the GNA-1 protein (Fig. 1A). HygR progeny that were phenotypically identical to the Δgna-1 parent were tested for mating type, and all were mating type a, consistent with the presence of the cr-1+ allele. Of 10 hygR phenotypically crisp progeny, 2 were mating type a and 8 were mating type A. Southern blot analysis demonstrated that all 10 strains carried the Δgna-1::hph+ deletion mutation (data not shown). Thus, the mating type a hygR crisp progeny represented strains that were cr-1 mating type recombinants.
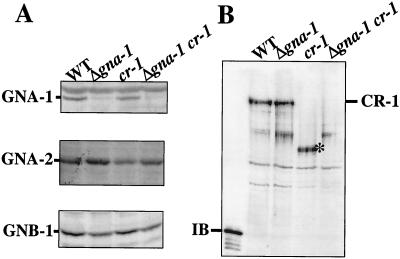
FIG. 1.
Levels of Gα, Gβ, and adenylyl cyclase proteins. (A) Levels of GNA-1, GNA-2, and GNB-1. Samples containing 30 μg of total membrane protein from strains with the indicated genotypes were subjected to Western blot analysis using specific antisera. WT, wild type. (B) Levels of CR-1. Samples containing 30 μg of total membrane protein were analyzed by Western blot analysis using a CR-1 antiserum. The position of full-length CR-1 in the wild-type (WT) and Δgna-1 mutant preparations is indicated to the right of the blot. The migration position of the amino-terminal 56-kDa CR-1 protein fragment (IB) used as an antigen is indicated. The asterisk indicates the 150-kDa species found in the cr-1 strain preparation.
Western blot analysis showed that cr-1 and wild-type strains contain wild-type levels of GNA-1, GNA-2, and GNB-1, as previously observed (Fig. 1A) (27). The Δgna-1 cr-1 mutants lack GNA-1 but contain wild-type levels of the Gα and Gβ subunits GNA-2 and GNB-1, respectively (Fig. 1A). A 56-kDa protein corresponding to the extreme amino terminus of CR-1 was overexpressed in E. coli and used to generate a polyclonal antibody in rabbits. Western blot analysis with the preimmune serum showed no hybridizing proteins (data not shown). Analysis with the immune serum indicated reaction with a 220-kDa species present at similar levels in Δgna-1 and wild-type strains (Fig. 1B). The 220-kDa protein was not observed in cr-1 preparations (Fig. 1B), but a prominent ∼150-kDa species was present. Since the CR-1 antibody is directed against the amino terminus, this 150-kDa protein may be the result of a nonsense mutation at the carboxy terminus, producing a truncated protein. Mutation of gna-1 in the cr-1 background leads to loss of the ∼150-kDa species, implying that GNA-1 influences the level of the mutant protein; however, we do not know the mechanism underlying this effect. The observation that levels of the 220-kDa protein are similar in wild-type and Δgna-1 strains is consistent with the modest effect of the Δgna-1 mutation on Mn2+-ATP activity noted in our earlier study (24).
As expected, no Mn2+-ATP or Mg2+-ATP-dependent adenylyl cyclase activity could be detected in cr-1 or Δgna-1 cr-1 strains (data not shown). Δgna-1 mutant preparations contain substantial levels of Mn2+-ATP-dependent activity, consistent with previous results (87% of wild-type activity [24]). Similar to what has been noted previously (24), Δgna-1 mutants have basal and GTP-stimulated Mg2+-ATP-dependent activities that are only 18 and 9.5% of wild-type activity, respectively.
cr-1 and gna-1 contribute to female fertility.
Previous work from our laboratory demonstrated that Δgna-1 mutants are male-fertile but female-sterile. Δgna-1 strains have aberrant protoperithecia that do not develop into normal perithecia after fertilization and that only rarely produce ascospores (Fig. 2) (23). In contrast, cr-1 mutants are able to function as males or females during a sexual cross but exhibit delayed perithecial and ascospore development compared to the wild type (Fig. 2) (41). These observations supported the hypothesis that cAMP is not essential for female fertility in N. crassa.
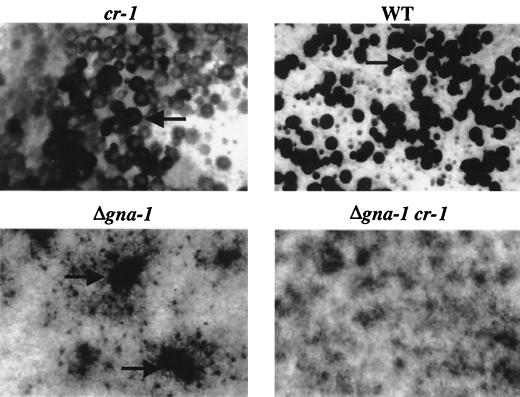
FIG. 2.
Female fertility. Strains with the indicated genotypes were cultured on SCM for 7 days prior to fertilization using wild-type (WT) strain 74-OR23-1A or Sta73a conidia. Perithecia were photographed 6 days after fertilization. Perithecia (in cr-1 and wild-type strains) and aberrant perithecia (in a Δgna-1 strain) are indicated by arrows.
Functions for GNA-1 and cAMP in regulating fertility were further examined by analyzing the sexual cycle in strains lacking both gna-1 and cr-1. Similar to Δgna-1 mutants, the males of Δgna-1 cr-1 strains are fertile but the females are sterile (data not shown and Fig. 2). However, unlike Δgna-1 strains, Δgna-1 cr-1 double mutants do not form observable protoperithecia or perithecia (data not shown and Fig. 2). Therefore, the cr-1 mutation blocks protoperithecial formation in the Δgna-1 background, suggesting that cAMP may play a role in protoperithecial development. This contrasts with the known effects of cr-1 on perithecial development and may reflect a requirement for cAMP during protoperithecial formation that is exacerbated by mutation of gna-1 and which impedes later perithecial development.
Loss of gna-1 blocks the response of cr-1 mutants to exogenous cAMP.
As mentioned above, cr-1 mutants have a greatly reduced apical extension rate and produce much shorter aerial hyphae compared to those of the wild type. The apical extension rate of Δgna-1 strains is approximately 50% of the wild-type rate, and Δgna-1 mutants produce shorter aerial hyphae (23). Supplementation of cr-1 plate and standing liquid cultures with cAMP restores the wild-type morphology and growth rate to cr-1 mutants but has no effect on Δgna-1 mutants (23, 27; unpublished observations in reference 53).
The inability to rescue the apical extension rate and aerial hypha height defects of Δgna-1 strains by adding cAMP could stem from the fact that these processes are controlled using distinct cAMP-dependent (cr-1) and cAMP-independent (gna-1) mechanisms in N. crassa. Alternatively, our previous observations could reflect a more general requirement for gna-1 in the response to exogenous cAMP. To distinguish between these possibilities, we explored the effect of cAMP supplementation on the Δgna-1 cr-1 double mutant. Loss of gna-1 in a cr-1 background causes a greater restriction of apical growth than that observed in a cr-1 background alone (57% decrease) (Fig. 3 and data not shown). Similar to previous observations with Δgna-1 mutants, exogenous cAMP has no effect on plate cultures of Δgna-1 cr-1 strains (data not shown). In standing liquid cultures, cr-1 strains produce short aerial hyphae, while hyphae are completely absent in Δgna-1 cr-1 mutants (Fig. 3B). Addition of cAMP restored wild-type aerial hypha height to cr-1 mutants but had no effect on Δgna-1 cr-1 (Fig. 3B). Thus, not only are Δgna-1 strains refractory to exogenous cAMP, but the presence of the Δgna-1 mutation also blocks the normally robust response of cr-1 mutants. A similar effect is observed upon loss of gna-1 in the Δgna-3 genetic background (A.M. Kays and K. A. Borkovich, unpublished observations). These observations are consistent with a more general requirement for gna-1 in the response of N. crassa to exogenous cAMP.
FIG. 3.
Vegetative phenotypes in the presence of an air-water interface. (A) Growth of colonies on solid medium. Strains with the indicated genotypes were grown on VM plates at room temperature under constant light and photographed when the Δgna-1 colony had reached the edge of the plate (approximately 36 h). Wild-type colonies are approximately two times larger than Δgna-1 colonies under these conditions (data not shown). (B) Standing liquid cultures. Strains were inoculated into 2-ml portions of liquid VM and incubated in a stationary position first for 3 days at 30°C in the dark and then for 4 days at room temperature in constant light. The presence (+) or absence (−) of 2.0 mM cAMP is indicated below the test tubes.
Relationship between cr-1 and gna-1 in regulating thermotolerance.
The heat shock response is a biological phenomenon that occurs in all cells as a mechanism to survive the stress of nonphysiological temperatures. In response to heat stress, cells quickly synthesize heat shock proteins (Hsps) that act to stabilize endogenous proteins, prevent denaturation, and protect cells from subsequent lethal heat exposure. The mechanism of Hsp induction is referred to as induced thermotolerance (for reviews, see references 39 and 49). A sudden increase in temperatures is lethal when cells do not have the opportunity to adapt and is referred to as a lethal or uninduced heat shock. Exposure to temperatures over 50°C is lethal to N. crassa; however, previous exposure of cells to temperatures of 40 to 47°C allows the induction of Hsps (25, 42).
Studies have shown that mutation of cr-1 or gna-1 results in heightened thermotolerance in N. crassa (8, 59). Analysis of thermotolerance in Δgna-1, cr-1, and Δgna-1 cr-1 strains was performed to determine the relationship between Δgna-1 and cr-1 in regulating this phenomenon. If GNA-1 exerts its effect through control of cAMP levels and cAMP alone controls thermotolerance, then the response to high temperatures in the cr-1 and Δgna-1 cr-1 strains should be the same. Alternatively, if GNA-1 also controls a cAMP-independent pathway that negatively regulates the heat shock response, loss of gna-1 in the cr-1 background should cause increased thermotolerance.
In agreement with previous work from our laboratory, Δgna-1 mutants possess approximately sixfold-greater uninduced thermotolerance than that of the wild type and 4.5-fold-greater induced thermotolerance than that of the wild type (Fig. 4). In contrast to a previous report (8), we found that thermotolerance in cr-1 strains is inducible; strains containing the cr-1 mutation have a greater than twofold-higher survival if preexposed to a nonlethal heat treatment (Fig. 4). cr-1 and Δgna-1 cr-1 strains were extremely thermotolerant relative to wild-type and Δgna-1 strains, and thermotolerance is inducible in both cr-1 and Δgna-1 cr-1 mutants. Importantly, the thermotolerance of cr-1 and Δgna-1 cr-1 strains is similar within experimental error (Fig. 4), implying that GNA-1 regulates thermotolerance through modulation of cAMP levels. The data are consistent with previous reports that the observed increase and decrease in thermotolerance of strains carrying either null or constitutively activated gna-1 alleles result from their lower or higher levels of cAMP, respectively (59).
FIG. 4.
Thermotolerance assays. The viability of 3-h-old germlings was determined after exposure to a lethal temperature (52°C) with (induced thermotolerance) and without (uninduced thermotolerance) prior exposure to a sublethal heat shock temperature (45°C). The error bars show standard errors of the means.
Δgna-1 mutants conidiate in submerged cultures at higher cell densities.
Macroconidiation in N. crassa requires an air-water interface; thus, conidiation typically occurs on solid surfaces. However, wild-type N. crassa strains can be induced to conidiate in submerged cultures when starved for carbon or nitrogen. Conidiation of submerged cultures has also been observed for cr-1, rco-1, and gna-3 mutants (27, 38, 41). On the basis of these observations, we postulated that the Δgna-1 cr-1 mutant would be derepressed for conidiation in submerged cultures.
The wild-type control strain grew to confluence and did not conidiate in submerged culture under any of the conditions tested (Fig. 5A). The Δgna-1 mutant conidiated in submerged culture only when cells were inoculated at a high cell density (3 × 106 cell/ml [Fig. 5A]). A threefold decrease (106 cell/ml) in cell density resulted in small, tight mycelial aggregates, with no observable conidiophores (Fig. 5A). cr-1 and Δgna-1 cr-1 strains produced large numbers of conidiophores at both cell densities, with more conidiophores visible in the Δgna-1 cr-1 mutant (Fig. 5A). Therefore, submerged culture conidiation in the Δgna-1 mutant is cell density dependent, and the Δgna-1 mutation intensifies the conidiation defect of cr-1 strains.
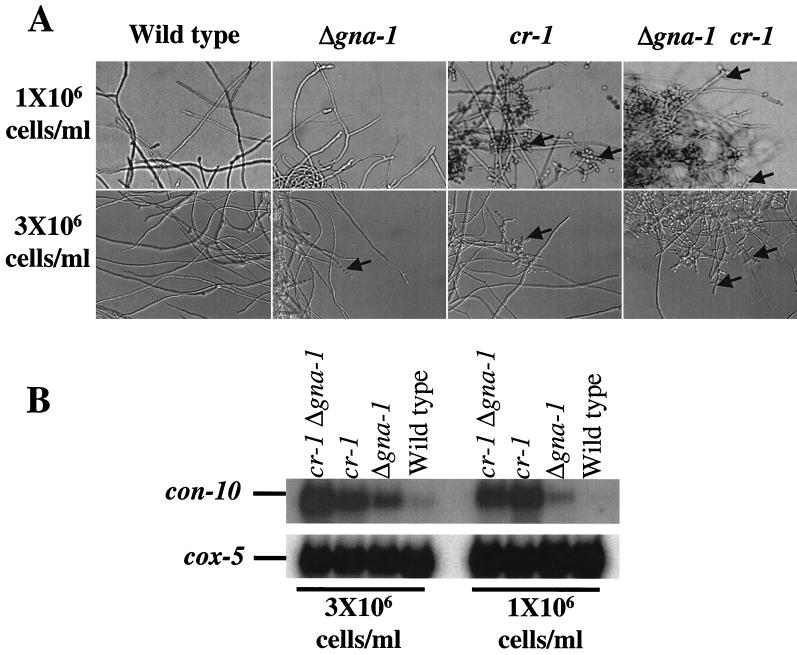
FIG. 5.
Conidiophore development and conidiation-specific gene expression in submerged cultures. (A) Conidiophore development. Conidia were inoculated into liquid VM at either 1 × 106 or 3 × 106 cells/ml, incubated with shaking in the dark at 30°C for 16 h, and then photographed. Arrows indicate conidiophores. (B) Correlation between conidiophore development and conidiation-specific gene expression. Total RNA (20 μg) isolated from strains with the indicated genotypes was analyzed by Northern blot analysis using a 32P-labeled con-10 cDNA as a conidiation-specific probe; the cox-5 gene was used as a probe to control for RNA loading and transfer.
It has been previously demonstrated that cr-1 mutants express the conidiation-specific gene con-10 in submerged culture (27). To determine if the conidiation observed in Δgna-1 and Δgna-1 cr-1 strains correlated with expression of con-10, RNA was isolated and analyzed for the presence of the con-10 transcript using Northern blot analysis. Although no conidiophores were observed, con-10 was weakly transcribed at the higher cell density for the wild-type strain, suggesting that conidiation may just be initiating. For the Δgna-1 strain, con-10 was expressed at both cell densities, with greater transcription occurring at the higher inoculation density (Fig. 5B). cr-1 and Δgna-1 cr-1 strains transcribe the con-10 message at both cell densities (Fig. 5B), with the highest level produced by the Δgna-1 cr-1 mutant at the high cell density. The relative amounts of con-10 in cr-1 and Δgna-1 cr-1 cultures inoculated at the lower concentration were more variable; however, the amounts of transcript produced by Δgna-1 cr-1 strains were generally the same as or greater than that made by cr-1 mutants (data not shown and Fig. 5B).
These results indicate that loss of cr-1 and gna-1 is synergistic with respect to derepression of con-10 expression, particularly in cultures inoculated at a high cell density, as the Δgna-1 cr-1 strain contains more con-10 mRNA than the cr-1 strain. Furthermore, because adenylyl cyclase activity is absent from both cr-1 and Δgna-1 cr-1 strains, the observation that the Δgna-1 cr-1 strain contains the greatest amount of con-10 transcript indicates that con-10 gene expression is also regulated by a pathway involving GNA-1, independent of cAMP.
DISCUSSION
Previous work has demonstrated that GNA-1 is necessary for normal stress responses and female fertility and that GNA-3 is a global negative regulator of conidiation (23, 27, 59). Biochemical studies indicate dual regulation of adenylyl cyclase by GNA-1 and GNA-3 in N. crassa: GNA-1 positively modulates adenylyl cyclase activity, and GNA-3 maintains adenylyl cyclase enzyme levels (24, 27). Here we have further defined the role of GNA-1 in cAMP-dependent and -independent pathways by constructing and analyzing a Δgna-1 cr-1 double mutant. In contrast to the cr-1 strain, Δgna-1 cr-1 grows even more slowly and is refractory to exogenous cAMP. Deletion of gna-1 in the cr-1 background leads to female sterility. Thermotolerance experiments show that Δgna-1 cr-1 and cr-1 strains are similarly resistant to high temperatures. Conidiation in Δgna-1 submerged cultures is dependent on cell density, and the Δgna-1 cr-1 strain conidiates more abundantly than the cr-1 strain.
The inability of Δgna-1 cr-1 strains to respond to exogenous cAMP was consistent with our earlier observation that Δgna-1 mutants are insensitive to cAMP supplementation (23). As mentioned above, the same phenomenon is seen in Δgna-1 Δgna-3 strains (Kays and Borkovich, unpublished). Previous work has shown that both wild-type and Δgna-1 strains secrete similar amounts of cAMP into the medium during growth in submerged cultures (24). This indicates that cAMP may function as an extracellular signal in N. crassa and that the mechanism that allows movement of cAMP in and out of the cell is intact in Δgna-1 mutants. Therefore, GNA-1 may function in a pathway that senses extracellular cAMP levels to inhibit conidiation of submerged cultures or other developmental processes. Alternatively, GNA-1 may be required for the expression or stability of a component in the cAMP sensing or signaling pathway in N. crassa.
A requirement for a Gα protein in the response to exogenous cAMP has not been previously reported in N. crassa or other related fungi. In fact, there are several instances of fungal adenylyl cyclase and Gα mutants that can respond to exogenous cAMP. As mentioned above, the wild-type morphology is restored to standing liquid cultures of cr-1 and Δgna-3 strains by cAMP supplementation (27, 53). Treatment with cAMP suppresses the developmental defects of the Magnaporthe grisea Δmac1 and Ustilago maydis Δuac1 adenylyl cyclase mutants (1, 16). Phenotypic defects resulting from mutation of the gna-3 homologue in the saprophyte Podospora anserina and the pathogens U. maydis, Ustilago hordei, and Cryptococcus neoformans are all rescued by exogenous cAMP (2, 31, 35, 37). Addition of cAMP to a mutant of the gna-1 homologue, cpg-1, in the plant pathogen Cryphonectria parasitica suppresses its virulence defect (13).
Sexual development in N. crassa is initiated by formation of protoperithecia in response to nitrogen limitation. Deletion of gna-1 causes an early stage defect in the sexual cycle, resulting in the formation of aberrant protoperithecia (23, 59). In contrast, although cr-1 strains lack intracellular cAMP, they are still able to complete the sexual cycle and are only delayed in postfertilization development, including perithecial and ascospore production (41). Female sterility in the cr-1 strain background is observed only upon mutation of gna-1, supporting a cAMP-independent role for GNA-1 in regulating female fertility. Furthermore, cAMP supplementation has no effect on the sexual cycle defects of either Δgna-1 or Δgna-3 mutants (27; F. D. Ivey and K. A. Borkovich, unpublished observations). In contrast to N. crassa, the sexual cycle of M. grisea and U. maydis is dependent on cAMP, and the Δmac1 and Δuac1 adenylyl cyclase mutants mate only when exogenous cAMP is supplied (1, 16).
Thermotolerance in N. crassa and S. cerevisiae is inversely correlated with cAMP levels (12). Strains with mutations in genes encoding either adenylyl cyclase or its positive regulators are more resistant to heat stress (6, 8, 22, 59), and heat shock transcription factors are active during periods of low protein kinase A activity in S. cerevisiae (17). The thermotolerance of Δgna-1 cr-1 and cr-1 strains is identical, consistent with this process being largely dependent on cAMP in N. crassa. However, the observation that thermotolerance can be induced in strains carrying the cr-1 mutation suggests that there may also be a cAMP-independent mechanism for the induction of heat shock stress regulators. Studies in S. cerevisiae support cAMP-independent pathways in regulating the heat shock response. Disturbing the integrity of the cell wall by chemical agents or by mutation of genes important for cell wall synthesis leads to phosphorylation and activation of the MAPK Slt2p and changes in cell wall composition (10). Such disruptions to the cell wall also result in an increased resistance to high temperatures, supporting the observation that additional kinases may also function during the heat shock response (10, 14).
Conidiation of submerged cultures occurs in Δgna-1 mutants in a cell density-dependent manner. Conidiation of submerged cultures is much more extensive in cr-1 cultures than in Δgna-1 mutant cultures. Deletion of gna-1 and cr-1 is synergistic, with respect to expression of the conidiation-specific gene con-10 at high cell densities. The observations that Δgna-1 strains produce conidiophores only at higher cell densities and that Δgna-1 cr-1 strains exhibit more conidiation than cr-1 mutants suggest that GNA-1 functions independently of cAMP as a negative regulator of conidiation of submerged cultures. The GNA-1 homologue FadA in A. nidulans also serves as a negative regulator of conidiation of submerged cultures (21, 60). cAMP independence is supported by the observation that A. nidulans pkaA catalytic subunit mutants do not conidiate in submerged culture (50).
The results from comparison of Δgna-1, cr-1, and Δgna-1 cr-1 strains support the hypothesis that hyphal development and conidiation involve cAMP-dependent and MAPK pathways. A gene encoding a MAPK kinase kinase (MAPKKK), nrc-1, has been identified in N. crassa (30). NRC-1 is most similar to the MAPKKK proteins Ste11p and Byr-2 from S. cerevisiae and Schizosaccharomyces pombe, respectively (30). Mutation of nrc-1 results in formation of short aerial hyphae, constitutive conidiation on solid medium and in submerged culture, thin basal hyphae, semicolonial growth morphology, defective conidial separation, and female sterility (30); some of these phenotypes are also found in cr-1 and Δgna-1 strains. The presence of shared defects for nrc-1 and gna-1 mutants suggests that GNA-1 may act upstream of NRC-1 to suppress submerged conidiation. Another possibility is that a conidiation-specific, cAMP-dependent pathway influences the NRC-1 MAPK cascade. Cross talk between cAMP and MAPK pathways during regulation of fungal development has been observed in M. grisea; the MAPK PMK-1, the GNA-1 homologue MAGB, and cAMP all regulate the invasive growth habit of this organism (36, 40).
The results presented here provide further evidence that GNA-1 controls multiple signal transduction pathways in N. crassa. The previous demonstration of differential complementation of Δgna-1 phenotypes by mammalian Gα protein genes (58), as well as the results from functional analysis of N. crassa gna-3 and P. anserina modD mutants, showed that these three Gα subunits function in more than one pathway (27, 37). GNA-1 regulates cAMP-dependent functions, such as thermotolerance, by positively modulating adenylyl cyclase activity. Female fertility and submerged conidiation are regulated by GNA-1 through cAMP-independent pathways. Of the three N. crassa Gα subunits, GNA-1 has the greatest role in resistance to cellular stresses, including hyperosmotic conditions and elevated temperatures. Identification of signaling components that act upstream and downstream of GNA-1 will elucidate the molecular mechanisms that enable filamentous fungi to sense and respond to environmental stresses and will further reveal the extent of conservation between signaling pathways in yeast and filamentous fungal species.
Acknowledgments
We thank Jennifer Bieszke, Svetlana Krystofova and Qi Yang for many helpful discussions and Thomas Vida and Dale Hereld for assistance with microscopy.
This work was supported in part by Public Health Service grant GM-48626 from the National Institutes of Health (to K.A.B.).
REFERENCES
- 1.Adachi, K., and J. E. Hamer. 1998. Divergent cAMP signaling pathways regulate growth and pathogenesis in the rice blast fungus Magnaporthe grisea. Plant Cell 10:1361-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alspaugh, J. A., J. R. Perfect, and J. Heitman. 1997. Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 11:3206-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baasiri, R. A., X. Lu, P. S. Rowley, G. E. Turner, and K. A. Borkovich. 1997. Overlapping functions for two G protein α subunits in Neurospora crassa. Genetics 147:137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bieszke, J. A., E. L. Braun, L. E. Bean, S. Kang, D. O. Natvig, and K. A. Borkovich. 1999. The nop-1 gene of Neurospora crassa encodes a seven transmembrane helix retinal-binding protein homologous to archaeal rhodopsins. Proc. Natl. Acad. Sci. USA 96:8034-8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borkovich, K. A. 1996. Signal transduction pathways and heterotrimeric G proteins, p. 211-233. In R. Brambl and G. A. Marzluf (ed.), The mycota. II. Biochemistry and molecular biology, vol. III. Springer-Verlag KG, Berlin, Germany.
- 6.Colombo, S., P. Ma, L. Cauwenberg, J. Winderickx, M. Crauwels, A. Teunissen, D. Nauwelaers, J. H. Winde, M. Gorwa, D. Colavizza, and J. M. Thevelein. 1998. Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J. 17:3326-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortat, M., and G. Turian. 1974. Conidiation of Neurospora crassa in submerged culture without mycelial phase. Arch. Mikrobiol. 95:305-309. [DOI] [PubMed] [Google Scholar]
- 8.Cruz, A. K., H. F. Terenzi, J. A. Jorge, and H. F. Terenzi. 1988. Cyclic AMP-dependent, constitutive thermotolerance in the adenylate cyclase-deficient cr-1 (crisp) mutant of Neurospora crassa. Curr. Genet. 13:451-454. [DOI] [PubMed] [Google Scholar]
- 9.Davis, R. H., and F. J. deSerres. 1970. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 71A:79-143. [Google Scholar]
- 10.de Nobel, H., C. Ruiz, H. Martin, W. Morris, S. Brul, M. Molina, and F. M. Klis. 2000. Cell wall perturbation in yeast results in dual phosphorylation of the Slt2/Mpk1 MAP kinase and in an Slt2-mediated increase in FKS2-lacZ expression, glucanase resistance and thermotolerance. Microbiology 146:2121-2132. [DOI] [PubMed] [Google Scholar]
- 11.Engelberg, D., E. Zandi, C. S. Parker, and M. Karin. 1994. The yeast and mammalian Ras pathways control transcription of heat shock genes independently of heat shock transcription factor. Mol. Cell. Biol. 14:4929-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estruch, F. 2000. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol. Rev. 24:469-486. [DOI] [PubMed] [Google Scholar]
- 13.Gao, S., and D. L. Nuss. 1996. Distinct roles for two G protein α subunits in fungal virulence, morphology, and reproduction revealed by targeted gene disruption. Proc. Natl. Acad. Sci. USA 93:14122-14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garreau, H., R. N. Hasan, G. Renault, F. Estruch, E. Boy-Marcotte, and M. Jacquet. 2000. Hyperphosphorylation of Msn2p and Msn4p in response to heat shock and the diauxic shift is inhibited by cAMP in Saccharomyces cerevisiae. Microbiology 146:2113-2120. [DOI] [PubMed] [Google Scholar]
- 15.Gilman, A. G. 1987. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 56:615-649. [DOI] [PubMed] [Google Scholar]
- 16.Gold, S., G. Duncan, K. Barrett, and J. Kronstad. 1994. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 8:2805-2816. [DOI] [PubMed] [Google Scholar]
- 17.Gorner, W., E. Durchschlag, M. T. Martinez-Pastor, F. Estruch, G. Ammerer, B. Hamilton, H. Ruis, and C. Schuller. 1998. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12:586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guignard, R., F. Grange, and G. Turian. 1974. Microcycle conidiation induced by partial nitrogen deprivation in Neurospora crassa. Can. J. Microbiol. 30:1210-1215. [Google Scholar]
- 19.Hamm, H. E. 1998. The many faces of G protein signaling. J. Biol. Chem. 273:669-672. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557. [DOI] [PubMed] [Google Scholar]
- 21.Hicks, J. K., J. H. Yu, N. P. Keller, and T. H. Adams. 1997. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA G alpha protein-dependent signaling pathway. EMBO J. 16:4916-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iida, H. 1988. Multistress resistance of Saccharomyces cerevisiae is generated by insertion of retrotransposon Ty into the 5′ coding region of the adenylate cyclase gene. Mol. Cell. Biol. 8:5555-5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivey, F. D., P. N. Hodge, G. E. Turner, and K. A. Borkovich. 1996. The Gαi homologue gna-1 controls multiple differentiation pathways in Neurospora crassa. Mol. Biol. Cell 7:1283-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivey, F. D., Q. Yang, and K. A. Borkovich. 1999. Positive regulation of adenylyl cyclase activity by a Gαi homologue in Neurospora crassa. Fungal Genet. Biol. 26:48-61. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor, M. 1983. A study of the heat-shock response in Neurospora crassa. Int. J. Biochem. 15:639-649. [DOI] [PubMed] [Google Scholar]
- 26.Kataoka, T., D. Broek, and M. Wigler. 1985. DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell 43:493-505. [DOI] [PubMed] [Google Scholar]
- 27.Kays, A. M., P. S. Rowley, R. A. Baasiri, and K. A. Borkovich. 2000. Regulation of conidiation and adenylyl cyclase levels by the Gα protein GNA-3 in Neurospora crassa. Mol. Cell. Biol. 20:7693-7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kore-eda, S., T. Murayama, and I. Uno. 1991. Isolation and characterization of the adenylate cyclase structural gene of Neurospora crassa. Jpn. J. Genet. 66:317-334. [DOI] [PubMed] [Google Scholar]
- 29.Kore-eda, S., T. Murayama, and I. Uno. 1991. Suppression of the cr-1 mutation in Neurospora crassa. Jpn. J. Genet. 66:77-83. [DOI] [PubMed] [Google Scholar]
- 30.Kothe, G. O., and S. J. Free. 1998. The isolation and characterization of nrc-1 and nrc-2, two genes encoding protein kinases that control growth and development in Neurospora crassa. Genetics 149:117-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruger, J., G. Loubradou, E. Regenfelder, A. Hartmann, and R. Kahmann. 1998. Crosstalk between cAMP and pheromone signalling pathways in Ustilago maydis. Mol. Gen. Genet. 260:193-198. [DOI] [PubMed] [Google Scholar]
- 32.Lee, B. N., and T. H. Adams. 1995. fluG and flbA function interdependently to initiate conidiophore development in Aspergillus nidulans through brlAβ activation. EMBO J. 15:299-309. [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, B. N., and T. H. Adams. 1994. Overexpression of flbA, an early regulator of Aspergillus asexual sporulation, leads to activation of brlA and premature initiation of development. Mol. Microbiol. 14:323-334. [DOI] [PubMed] [Google Scholar]
- 34.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lichter, A., and D. Mills. 1997. Fil1, a G-protein α-subunit that acts upstream of cAMP and is essential for dimorphic switching in haploid cells of Ustilago hordei. Mol. Gen. Genet. 256:426-435. [DOI] [PubMed] [Google Scholar]
- 36.Liu, S., and R. A. Dean. 1997. G protein α subunit genes control growth, development, and pathogenicity of Magnaporthe grisea. Mol. Plant-Microbe Interact. 10:1075-1086. [DOI] [PubMed] [Google Scholar]
- 37.Loubradou, G., J. Begueret, and B. Turcq. 1999. MOD-D, a Gα subunit of the fungus Podospora anserina, is involved in both regulation of development and vegetative incompatibility. Genetics 152:519-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madi, L., S. A. McBride, L. A. Bailey, and D. J. Ebbole. 1997. rco-3, a gene involved in glucose transport and conidiation in Neurospora crassa. Genetics 146:499-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mager, W. H., and A. J. J. De Kruijff. 1995. Stress-induced transcriptional activation. Microbiol. Rev. 59:506-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell, T. K., and R. A. Dean. 1995. The cAMP-dependent protein kinase catalytic subunit is required for appressorium formation and pathogenesis by the rice blast pathogen Magnaporthe grisea. Plant Cell 7:1869-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perkins, D. D., A. Radford, D. Newmeyer, and M. Bjorkman. 1982. Chromosomal loci of Neurospora crassa. Microbiol. Rev. 46:426-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plesofsky-Vig, N., and R. Brambl. 1985. Heat shock response of Neurospora crassa: protein synthesis and induced thermotolerance. J. Bacteriol. 162:1083-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plesofsky-Vig, N., D. Light, and R. Brambl. 1983. Paedogenetic conidiation in Neurospora crassa. Exp. Mycol. 7:283-286. [Google Scholar]
- 44.Raju, N. B. 1992. Genetic control of the sexual cycle in Neurospora. Mycol. Res. 96:241-262. [Google Scholar]
- 45.Roberts, A. N., V. Berlin, K. M. Hager, and C. Yanofsky. 1988. Molecular analysis of a Neurospora crassa gene expressed during conidiation. Mol. Cell. Biol. 8:2411-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen, S., J. H. Yu, and T. H. Adams. 1999. The Aspergillus nidulans sfaD gene encodes a G protein beta subunit that is required for normal growth and repression of sporulation. EMBO J. 18:5592-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg, G. B., and M. L. Pall. 1983. Reconstitution of adenylate cyclase in Neurospora from two components of the enzyme. Arch. Biochem. Biophys. 221:254-260. [DOI] [PubMed] [Google Scholar]
- 48.Sachs, M. S., and C. Yanofsky. 1991. Developmental expression of genes involved in conidiation and amino acid biosynthesis in Neurospora crassa. Dev. Biol. 148:117-128. [DOI] [PubMed] [Google Scholar]
- 49.Schoffl, F., R. Prandl, and A. Reindl. 1998. Regulation of the heat-shock response. Plant Physiol. 117:1135-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimizu, K., and N. P. Keller. 2001. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157:591-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Springer, M. L. 1993. Genetic control of fungal differentiation: the three sporulation pathways in Neurospora crassa. Bioessays 15:365-374. [DOI] [PubMed] [Google Scholar]
- 52.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 53.Terenzi, H. F., M. M. Flawia, M. T. Tellez-Inon, and H. N. Torres. 1976. Control of Neurospora crassa morphology by cyclic adenosine 3′,5′-monophosphate and dibutyryl cyclic adenosine 3′,5′-monophosphate. J. Bacteriol. 126:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terenzi, H. F., M. M. Flawia, and H. N. Torres. 1974. A Neurospora crassa morphological mutant showing reduced adenylate cyclase activity. Biochem. Biophys. Res. Commun. 58:990-996. [DOI] [PubMed] [Google Scholar]
- 55.That, T. C., and G. Turian. 1978. Ultrastructural study of microcyclic macroconidiation in Neurospora crassa. Arch. Microbiol. 116:279-288. [DOI] [PubMed] [Google Scholar]
- 56.Vogel, H. J. 1964. Distribution of lysine pathways among fungi: evolutionary implications. Am. Nat. 98:435-446. [Google Scholar]
- 57.Westergaard, M., and H. K. Mitchell. 1947. Neurospora. V. A synthetic medium favoring sexual reproduction. Am. J. Bot. 34:573-577. [Google Scholar]
- 58.Yang, Q., J. A. Bieszke, and K. A. Borkovich. 2000. Differential complementation of a Neurospora crassa Gαi mutation using mammalian Gα protein genes. Mol. Gen. Genet. 263:712-721. [DOI] [PubMed] [Google Scholar]
- 59.Yang, Q., and K. A. Borkovich. 1999. Mutational activation of a Gαi causes uncontrolled proliferation of aerial hyphae and increased sensitivity to heat and oxidative stress in Neurospora crassa. Genetics 151:107-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu, J. H., J. Wieser, and T. H. Adams. 1996. The Aspergillus FlbA RGS domain protein antagonizes G protein signaling to block proliferation and allow development. EMBO J. 15:5184-5190. [PMC free article] [PubMed] [Google Scholar]