Abstract
Genes transcribed by RNA polymerase II are silenced when introduced near the mat2 or mat3 mating-type loci of the fission yeast Schizosaccharomyces pombe. Silencing is mediated by a number of gene products and cis-acting elements. We report here the finding of novel trans-acting factors identified in a screen for high-copy-number disruptors of silencing. Expression of cDNAs encoding the putative E2 ubiquitin-conjugating enzymes UbcP3, Ubc15 (ubiquitin-conjugating enzyme), or Rhp6 (Rad homolog pombe) from the strong nmt1 promoter derepressed the silent mating-type loci mat2 and mat3 and reporter genes inserted nearby. Deletion of rhp6 slightly derepressed an ade6 reporter gene placed in the mating-type region, whereas disruption of ubcP3 or ubc15 had no obvious effect on silencing. Rhp18 is the S. pombe homolog of Saccharomyces cerevisiae Rad18p, a DNA-binding protein that physically interacts with Rad6p. Rhp18 was not required for the derepression observed when UbcP3, Ubc15, or Rhp6 was overproduced. Overexpressing Rhp6 active-site mutants showed that the ubiquitin-conjugating activity of Rhp6 is essential for disruption of silencing. However, high dosage of UbcP3, Ubc15, or Rhp6 was not suppressed by a mutation in the 26S proteasome, suggesting that loss of silencing is not due to an increased degradation of silencing factors but rather to the posttranslational modification of proteins by ubiquitination. We discuss the implications of these results for the possible modes of action of UbcP3, Ubc15, and Rhp6.
Transcriptional silencing is observed in the mating-type region of Schizosaccharomyces pombe. The mating-type region occupies approximately 30 kb in the right arm of chromosome II and contains three cassettes, mat1, mat2-P, and mat3-M (Fig. 1A) (reviewed in references 2 and 25). The mating-type genes located at the mat1 locus are transcribed and determine the mating type of the cells. The mating type can be switched by duplicative transposition of the genes and promoters contained within the silent locus mat2-P or mat3-M (39). Silencing affects a region of approximately 16 kb that contains mat2 and mat3 and whose limits are controlled by boundary elements called IR-L and IR-R (inverted repeat left and right [59, 84]). This domain is a “cold spot” for recombination as well as for transcription and adopts a particular chromatin structure with features similar to those of heterochromatin (59).
FIG. 1.
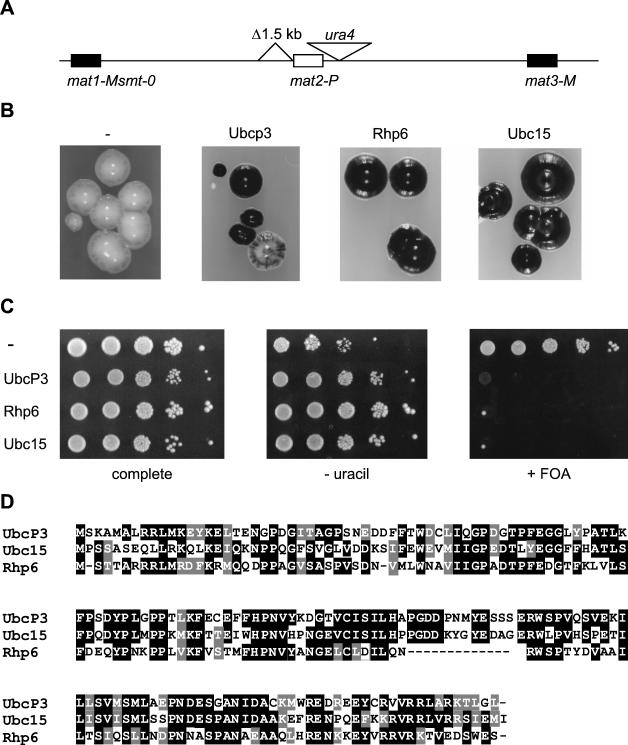
Screen for high-copy-number disruptors of silencing. (A) Mating-type region of the tester strain PG1608. (B) Haploid meiosis phenotypes of PG1608 overexpressing ubcP3, rhp6, ubc15, or no cDNA (−). Colonies grown on MSA plus uracil plus adenine were stained with iodine. (C) Silencing of ura4 in transformed PG1608 cells was estimated by plating 10-fold serial dilutions of cell suspensions on medium lacking uracil (AA-thiamine-leucine-uracil [− uracil]) or containing 5-FOA (FOA-thiamine-leucine [+ FOA]) and on complete medium (AA-thiamine-leucine). (D) Multiple-sequence alignment showing the homology between UbcP3, Ubc15, and Rhp6. Black shading indicates identical residues, while gray shading indicates conserved residues.
Many parallels can be drawn between the position effects exerted on the donor loci of the fission yeast mating-type region and the heterochromatic inactivation of gene expression in higher eukaryotes. Position effects are exerted on the mating-type region by a number of trans-acting factors that are homologous to heterochromatin-associated proteins in higher eukaryotes (16, 36). Several chromodomain proteins affect silencing: the two HP1 homologs Swi6 (switching deficient [44]) and Chp2 (chromodomain protein [83]) and the SUV39H1 homolog Clr4 (cryptic locus regulator [20, 33, 80]). Like SUV39H1, Clr4 contains a SET domain in addition to its chromodomain and displays histone H3 methyltransferase activity in vitro and in vivo (55, 62). Methylation of histone H3 lysine 9 by Clr4 creates a binding site for Swi6 (5, 55). Likewise, methylation of histone H3 lysine 9 by SUV39H1 creates a binding site for mammalian HP1 proteins (43). Clr3 and Clr6, two histone deacetylase homologs, are also required for silencing the mating-type region (20, 26, 80). Histones are underacetylated in the mating-type region and at centromeres (19, 60). Hence, hypoacetylation is another shared feature between S. pombe heterochromatin and heterochromatin in higher eukaryotes. These conserved histone acetylation and methylation patterns stress the fundamental role of histone modifications in the establishment of chromatin structure and transcriptional states (72).
DNA polymerase α was recently shown to play a role in transcriptional silencing in fission yeast (56). Other factors that affect silencing in the mating-type region are Clr1, a zinc-finger protein (G. Thon and A. Klar, unpublished observations); Clr2, which has no described homologs in other systems (7); Rik1 (recombination in K [8]), a protein with homology to UV-damage DNA-binding proteins; and the products of the esp1-3 genes (enhancer of Swi6 phenotype [81]). It is likely that mutations in additional trans-acting silencers will be identified, like csp2 (centromere suppressor of position effect), which causes partial derepression of transcription in the mating-type region (17). One striking feature of the trans-acting factors characterized so far is that single mutations or deletions do not abolish silencing but merely reduce it, suggesting that these factors act in overlapping pathways.
Chromosomal deletion analyses have identified a number of cis-acting elements that are required for transcriptional silencing at the donor loci and have confirmed that redundant mechanisms contribute to silencing. Deletion of a 1.5-kb DNA fragment on the centromere proximal side of mat2 (80) or of a 0.2-kb subelement, called REII (3), causes a small silencing defect. The presence of a mat2 silencing element was previously indicated by deletion analysis of a mat2-containing plasmid (18). When a chromosomal deletion of that silencing element is combined with a mutation in rik1, chp2, swi6, clr1, clr2, clr3, or clr4, full derepression of mat2 and its flanking regions occurs (80, 83) (Thon, unpublished). Deletions introduced near the mat3 cassette behave in a manner similar to that of deletion of the mat2 silencer (79). These synergistic effects indicate the existence of two epistasis groups of silencing factors (80). Together with further genetic analyses, they suggest that factors encoded by the esp genes act via the mat2-P and mat3-M silencers, whereas rik1, chp2, swi6, clr1, clr2, clr3, and clr4 act via an element located in the K region between mat2 and mat3. Some cross talk is likely to occur between the two pathways, although the details of the interactions are not known (27, 54, 81). clr6 clr1 or clr6 clr3 double-mutant backgrounds give rise to a strong derepression of transcription in the mating-type region and at centromeric locations (26). However, no cumulative effect is observed when a clr6 mutant allele is combined with a clr2, clr4, or swi6 mutant allele. Taken together, these results indicate that Clr6 functions in a pathway different from that of Clr1 and Clr3 and suggest that Clr6 may act via the silencing elements centromere proximal to mat2 and mat3 (26).
Here we report the results of experiments designed to identify novel factors affecting position effects in trans. A screen for genes that cause disruption of silencing when overexpressed was performed. The screen was expected to reveal genes whose products or enzymatic activities are required for silencing in precise dosage. Such screens were pioneered in Saccharomyces cerevisiae (48), and they were subsequently conducted in S. pombe to identify factors involved in centromere function (29, 34), cytoskeletal dynamics (14), or cell shape (10). The approach seemed well suited to the study of position-effect variegation, a phenomenon particularly sensitive to gene dosage. Our screen led to the identification of UbcP3, Ubc15, and Rhp6, three members of a large family of proteins in fission yeast involved in attaching ubiquitin to proteins. High dosage of UbcP3, Ubc15, or Rhp6 disrupted silencing of the donor loci as well as silencing of heterologous reporter genes inserted nearby. We determined which of the two silencing pathways was affected by overproduction of UbcP3, Ubc15, or Rhp6. We investigated whether the 26S proteasome, Rhp18, a protein that potentially interacts with Rhp6, or the ubiquitin-conjugating activity of Rhp6 was required for the observed effects. We also examined the phenotypes of ubcP3, ubc15, and rhp6 null alleles. We propose that ubcP3, ubc15, and rhp6 participate in the formation of chromatin structures that influence the localization of silencing factors.
MATERIALS AND METHODS
DNA sequences of ubcP3, ubc15, and rhp6.
The sequences described in this study are accessible via cosmid numbers at the Sanger Centre fission yeast genomic database or via accession numbers at SWISS-PROT: SPBP16F5 and Q9HDP3 (ubcP3), SPBC1105, Q9Y818 (ubc15), and SPAC18B11 and P23566 (rhp6).
Strains and media.
The S. pombe strains used in this study are listed in Table 1 with their genotypes and origins. S. pombe cells were propagated and tested using the following media: yeast extract supplemenetd (YES) (81); minimum salts-arginine (MSA) (15) supplemented with 100 mg of adenine, 100 mg of uracil, and 200 mg of l-leucine per liter as indicated; medium containing amino acids and supplements (AA) (66; substituting yeast nitrogen base lacking thiamine for yeast nitrogen base when indicated); fluoroorotic acid (FOA)-thiamine-leucine (81; substituting yeast nitrogen base lacking thiamine for yeast nitrogen base); and PM (6) + 5-FOA (1 g of 5-FOA and 50 mg of uracil per liter). The Escherichia coli strain DH5 (30) was used for cloning plasmids. It was propagated in Luria broth (67). Ampicillin was used at 200 μg/ml. Yeast extract, Casamino Acids, and tryptone were purchased from Difco (Detroit, Mich.); yeast nitrogen base, yeast nitrogen base lacking thiamine, and 5-FOA were purchased from United States Biological; salts were purchased from Merck; and samino acids, nucleotides, and ampicillin were purchased from Sigma.
TABLE 1.
S. pombe strains used in this study
| Strain | Mating-type region | Auxotrophic markers | Others |
|---|---|---|---|
| FY340 | h+N | leu1-32 ura4-DS/E ade6-M210 | TM1(Ncol)::ura4 R.I. |
| HU52 | h90 mat3-M(EcoRV)::ade6 | leu1-32 ura4-D18 ade6-DN/N | |
| PB141 | h90 mat3-M(EcoRV)::ade6 | leu1-32 ura4-D18 ade6-M210 | |
| PG9 | h90 mat3-M(EcoRV)::ura4 | leu1-32 ura4-D18 ade6-M216 | |
| PG506 | mat1-Msmt-0 mat3-M(EcoRV)::ura4 | leu1-32 ura4-D18 ade6-M210 | clr1-5 |
| PG595 | h90 mat3-M(EcoRV)::ura4 | leu1-32 ura4-D18 ade6-M210 | swi6-115 |
| PG1141 | h90 | leu1-32 ura4-D18 ade6-M216 | |
| PG1247 | hΔK1 mat3-M(EcoRV)::ura4 | leu1-32 ura4-D18 ade6-M210 | |
| PG1598 | mat1-Msmt-O mat2-P(XbaI)::ura4 | leu1-32 ura4-D18 ade6-M210 | |
| PG1608 | mat1-Msmt-O Δ(BglII-BssHII)mat2-P(XbaI)::ura4 | leu1-32 ura4-D18 ade6-M210 | |
| PG1786 | mat1-Msmt-O Δ(BglII-BssHII)mat2-P(XbaI)::ura4 | leu1-32 ura4-DS/E ade6-M216 | |
| PG1789 | mat1-Msmt-O mat2-P(XbaI)::ura4 | leu1-32 ura4-DS/E ade6-M216 | |
| PG1899 | h90 mat3-M(EcoRV)::ura4 | leu1-32 ura4-DS/E ade6-M216 | |
| PG2875 | h+N | leu1-32 ura4-DS/E ade6-M210 | ubc2::kanr TM1(Ncol)::ura4 R.I. |
| PG2876 | h90 | leu1-32 ura4-DS/E ade6-M210 | ubc2::kanr TM1(Ncol)::ura4 R.I. |
| PG2883 | mat1-Msmt-0 mat2-P(XbaI)::ura4 | leu1-32 ura4-D18 ade6-M210 | esp3-1 |
| PG2887, PG2888 | h90 mat3-M(EcoRV)::ura4 | leu1-32 ura4-DS/E ade6 | ubc2::kanr |
| PG2889, PG2890 | h90 mat2-P(XbaI)::ura4 | leu1-32 ura4-DS/E ade6 | ubc2::kanr |
| PG2891, PG2892 | h90 Δ(BglII-BssHII)mat2-P(XbaI)::ura4 | leu1-32 ura4-DS/E ade6 | ubc2::kanr |
| PG2895 | h90 mat3-M(EcoRV)::ade6 | leu1-32 ura4-D18 ade6-DN/N | rhp18::ura4 |
| PI131 | h90 mat3-M(EcoRV)::ura4 | leu1-32 ura4-D18 ade6-M216 | mts3-1 |
| SP837 | h90 | leu1-32 ura4-D18 ade6-M210 |
S. pombe cDNA library and screen.
An S. pombe cDNA library was provided by C. J. Norbury (ICRF, Cell Cycle Group, Oxford, United Kingdom) and B. Edgar. In this library, S. pombe cDNAs are placed under the control of the nmt1 promoter in the S. pombe expression vector pREP3X (23, 46, 47). The library was transformed into S. pombe by the lithium acetate method (51). Transformants were plated onto MSA supplemented with adenine and uracil and grown at 33°C. Spo+ colonies were identified by iodine staining and streaked on YES plates. Subsequently, the YES plates were replica plated onto MSA supplemented with adenine and uracil and onto MSA supplemented with adenine.
Silencing assay.
Spot tests were performed to evaluate the expression levels of reporter genes placed at silenced loci in the mating-type region. S. pombe cultures were grown to saturation at 33°C. Series of 10-fold dilutions were spotted onto plates of YES, AA lacking appropriate supplements, supplemented MSA, or 5-FOA as indicated. Plates were incubated at 33°C for 4 to 6 days until full growth was achieved except for ubc2::kanr strains, which were incubated at 28°C.
Plasmid recovery and identification.
Plasmids were recovered from S. pombe in E. coli DH5 using the miniprep DNA extraction procedure previously described by Moreno et al. (51). Sequence data were obtained by ABI prism big dye terminator cycle sequencing (PE Applied Biosystems, Perkin-Elmer) using primers specific for the nmt promoter (5′-CTGACTTATAGTCGCTTTG) to sequence the 5′ end of the cDNA inserts and for the nmt terminator (5′-GCAGTACTGGCAAGGGAG) to sequence the 3′ end of the cDNAs. The cDNA encoding UbcP3 was isolated from the ubcP3-rps4-1 hybrid clone obtained from the cDNA library by PCR using the forward primer 5′-ACGCGTCGACAATGAGTAAAGCTATGCCGTTG, reverse primer 5′-CGGGATCCTTATAATCCCAAGGTTTTACG, and native Pfu polymerase (Stratagene). rps4-1 was amplified by PCR using the forward primer 5′-CGGGATCCGTCGACATGGTTCGAGGTCCTAAAAAGC, the reverse primer 5′-CGGGATCCTTAGGCAAGTCCCTTGAG, and native Pfu polymerase (Stratagene). Both PCR products were digested with SalI and BamHI and cloned into pREP3X digested with SalI and BamHI using T4 DNA Ligase (Roche). The resulting clones were checked by sequencing, and silencing phenotypes were assayed in S. pombe.
Construction of ubcP3 and ubc15 disruption strains.
Disruptions were carried out in an SP837/PG1141 diploid strain. The disruption constructs were prepared in pBluescript SK (Stratagene). ubc15 upstream and downstream flanking sequences, of 800 and 1,000 bp, respectively, were amplified from genomic DNA by PCR using the upstream forward primer 5′-GGGGTACCTATTTTAGTAACCACTTGTATC and reverse primer 5′-GCCGCTCGAGGATCCACTTCCGATATTTTAATAATTAC, the downstream forward primer 5′-CGGGATCCAGATAATGTTCAACCTAGGTC and reverse primer 5′-ATAAGAATGCGGCCGCATGTTTACTGCAGAACGCTG, and the DNA polymerase Pfu (Stratagene). The upstream KpnI-XhoI fragment, the downstream BamHI-NotI fragment, an 1,800-bp XhoI-BamHI fragment containing ura4, and pBluescript SK digested with KpnI and NotI were combined in a four-way ligation using T4 DNA ligase (Roche) to create the plasmid pIN101. A ubcP3 disruption construct was prepared in the following manner: a 700-bp fragment containing upstream ubcP3 coding genomic sequence was amplified using the forward primer 5′-ACGCGTCGACAATGAGTAAAGCTATGCCGTTG and the reverse primer 5′-GCCCAAGCTTCCGTCCCATCTTTGTAAAC, and a 500-bp fragment containing downstream ubcP3 coding genomic DNA was amplified by the forward primer 5′-GCCCAAGCTTCCATCCTACATGCACCTG and reverse primer 5′-CGGGATCCTTATAATCCCAAGGTTTTACG. The 700-bp SalI-HindIII fragment, the 500-bp HindIII-BamHI fragment, and pBluescript digested with SalI and BamHI were combined in a three-way ligation to create the plasmid pIN102. Subsequently an 1,800-bp HindIII ura4 fragment was inserted in pIN102 digested with HindIII and dephosphorylated with shrimp alkaline phosphatase (United States Biological) to create the ubcP3 disruption construct pIN103. In addition to disrupting ubcP3, the ura4 fragment deletes the sequence TTTGCAT containing the putative ubcP3 active-site cysteine. The resulting ubc15::ura4 plasmid (pIN101) was digested with KpnI and NotI, and the ubcP3::ura4 plasmid (pIN103) was digested with SalI and BamHI. They were transformed into the diploid S. pombe strain SP837/PG1141 by the lithium acetate procedure (51), and the cells were plated on AA-uracil. Transformants with the correct integrations were identified by PCR and Southern blot analysis.
Northern blots.
RNA preparations and Northern blotting were performed as described previously (83). The membranes were hybridized either to a ura4 riboprobe (83) or to an ade6 probe made by random priming on a 2.8-kb XmnI-SpeI DNA fragment containing the ade6 open reading frame (78). Hybridization intensities were quantified using a Storm 840 PhosphorImager and ImageQuant software (Molecular Dynamics).
RESULTS
A screen for S. pombe cDNAs that in elevated dosage disrupt transcriptional silencing in the mating-type region.
The preponderance of evidence indicates that transcriptional silencing is mediated by a large multicomponent structure. Overexpression of one component may perturb the stoichiometric relationship between subunits and cause defects in silencing because of improper assembly or maintenance of the repressive state. To identify such gene products with potential roles in the formation of repressive chromatin, we screened an S. pombe cDNA library (B. Edgar and C. Norbury) for cDNAs that in elevated dosage cause derepression of genes residing in the silent part of the mating-type region. The cDNAs were expressed from the strong, inducible nmt1 promoter (no message on thiamine [46]). Since silencing defects can be masked by a redundancy of the silencing mechanisms, the cDNA library was introduced into a partially derepressed strain, PG1608. In PG1608 cells, silencing is reduced by the deletion of a cis-acting element adjacent to mat2 (Fig. 1A). Further derepression of the region such as that caused by mutations in trans-acting factors of the Swi6 epistasis group gives rise to easily observable phenotypes. Because they contain the unswitchable mat1-Msmt-0 allele, PG1608 cells express the M mating type stably. Expression of the P mating-type genes residing in the normally silent mat2 cassette in conjunction with the M mating-type genes from mat1-M causes cells to undergo haploid meiosis. Haploid meiosis can be detected by exposure to iodine vapors, upon which spore-containing colonies stain darkly, whereas colonies containing no spores are not stained. Stable mat1-M cells in which silencing is not compromised appear totally Spo− in this assay. PG1608 cells are stained very lightly.
Approximately 105 transformants expressing cDNAs from the aforementioned library were screened for an increase in the intensity of iodine staining. Thirty-seven transformants with increased sporulation were isolated and subjected to a second screen. In PG1608 cells, the S. pombe ura4 gene is inserted at an XbaI site approximately 400 bp centromere distal to mat2 (Fig. 1A). The ura4 gene placed at that location is stringently repressed in a wild-type background and partially derepressed in PG1608 (80) (Fig. 1C). The 37 transformants displaying increased haploid meiosis were examined for their ability to grow in the absence of uracil. Twelve isolates showed a plasmid-dependent sporulation phenotype and enhanced ability to grow in the absence of uracil. Plasmids were recovered from these isolates and sequenced. They could be divided into three classes: class 1 with eight clones containing rhp6 (Rad homolog pombe 6 [63]), which encodes the S. pombe Rad6 homolog; class 2 with three clones containing ubc15, which encodes a hitherto-uncharacterized putative E2 ubiquitin-conjugating enzyme; and class 3, consisting of one clone containing ubcP3, which encodes a protein previously identified on the basis of its ability to bind ubiquitin (61) (Fig. 1D). In addition to ubcP3, the last clone also contained a cDNA (rps4-1) encoding the ribosomal protein S4. The two cDNAs were cloned individually in pREP3X and overexpressed separately in PG1608. All effects originally observed using the hybrid clone were found to be due to overexpression of ubcP3. Figure 1B shows the sporulation phenotypes of cells overexpressing ubcP3, ubc15, or rhp6, and Fig. 1C shows their ability to grow in the absence of uracil and in the presence of 5-FOA, a toxigenic substrate of the Ura4 enzyme.
Effect of high UbcP3, Ubc15, or Rhp6 dosage on the expression of the S. pombe ade6 and ura4 genes placed near mat2 or mat3.
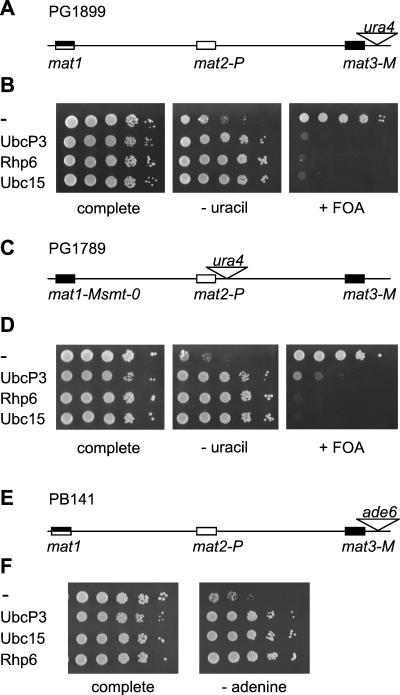
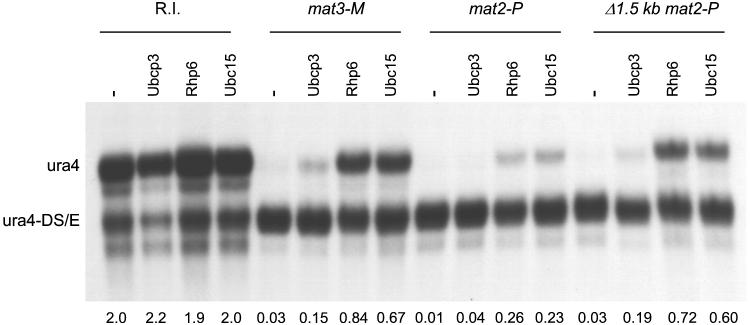
To determine whether the silencing defects caused by overexpressing ubcP3, ubc15, or rhp6 were specific to genes residing at or near the mat2 locus or to cells with the 1.5-kb deletion flanking mat2-P, these cDNAs were overexpressed in a number of different backgrounds. The S. pombe ura4 gene is repressed in a wild-type background when placed at the mat2 centromere-distal XbaI site (PG1789 [80]) or when placed at an EcoRV site located approximately 150 bp away from mat3-M (PG1899) (Fig. 2A and B [82]). We transformed PG1789 and PG1899 with the pREP3X plasmid lacking an insert or containing ubcP3, ubc15, or rhp6. Expression of the cDNAs was induced by depletion of thiamine, and the silencing phenotypes were assayed by spot tests and Northern blot analysis. In the Northern blot analysis, the amount of ura4 transcript originating from the mating-type region was compared with the amount of transcript originating from a truncated ura4 allele, ura4-DS/E (1). As shown in Fig. 2D and 3 (lanes labeled mat2-P and Δ1.5 kb mat2-P), expression of ubcP3, ubc15, or rhp6 increased expression of ura4 placed at the XbaI site near mat2-P. Similarly, expression of ura4 placed at the EcoRV site near mat3 increased upon induction of ubcP3, ubc15, or rhp6 expression (Fig. 2B and Fig. 3, lanes labeled mat3-M). Expression of the full-length ura4 gene randomly integrated in the genome (R.I. [1]) was not similarly affected by overexpression of the ubiquitin-conjugating enzymes (Fig. 3, lanes labeled R.I.), indicating that the effect observed near mat2 or mat3 is on silencing per se. Furthermore, the S. pombe ade6 gene placed at the EcoRV site was also derepressed when ubcP3, ubc15, or rhp6 was overexpressed (Fig. 2E and F). In summary, derepression of silencing by high dosage of UbcP3, Ubc15, or Rhp6 affects the genes contained within mat2-P, ura4 inserted near mat2-P, and ura4 and ade6 inserted near mat3-M. Derepression can occur when all cis-acting elements are present, albeit to a lesser degree than when one of the cis-acting elements is deleted (Fig. 3, compare mat2-P and Δ1.5 kb mat2-P lanes; see also below). Finally, derepression is observed in both switchable (PG1899 and PB141) and nonswitchable (PG1608 and PG1789) backgrounds.
FIG. 2.
High dosage of UbcP3, Rhp6, or Ubc15 generally affects silencing in the mating-type region. (A) Mating-type region of the switchable (h90) strain PG1899. (B) Silencing in PG1899 was assayed by plating serial dilutions of cells transformed with plasmids containing no cDNA (−), ubcP3, ubc15, or rhp6 on the same media as in Fig. 1C. (C) Mating-type region of the nonswitchable strain PG1789. (D) Silencing was assayed in PG1789 by spotting serial dilutions of cells on the same media as in Fig. 1C. (E) Mating-type region of PB141. (F) Silencing of ade6 placed near mat3-M was assayed by plating dilution series of transformed PB141 cells on medium lacking adenine (PM plus uracil) (− adenine) and complete medium (PM plus uracil plus adenine).
FIG. 3.
High dosage of UbcP3, Rhp6, or Ubc15 increases the amount of ura4 transcript originating from the mating-type region. Strains containing the ura4-DS/E allele (1) and a random integration of ura4 (FY340 [R.I.] [1]), the mat3-M (EcoRV)::ura4 allele depicted in Fig. 2A (PG1899 [mat3-M]), the mat2-P (XbaI)::ura4 allele depicted in Fig. 2C (PG1789 [mat2-P]), or the Δ(BglII-BssHII) mat2-P (XbaI)::ura4 allele depicted in Fig. 1A (PG1786 [Δ1.5 kb mat2-P]) were transformed with the indicated cDNAs and used to prepare a Northern blot. “ura4” indicates the full-length ura4 mRNA transcribed from the mating-type region, while “ura4-DS/E” indicates the truncated mRNA transcribed from the ura4-DS/E allele. The ratio of ura4 to ura4-DS/E transcript is indicated below each lane.
Combination of cis- and trans-acting mutations: epistasis analysis.
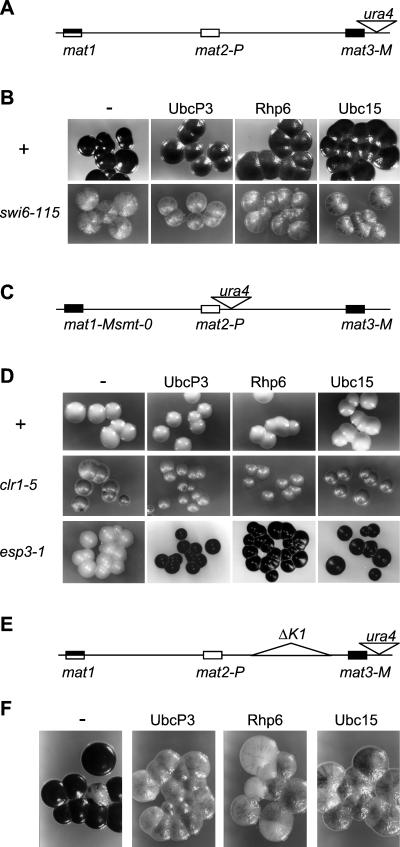
As previously mentioned, silencing factors that repress transcription in the mating-type region can be assigned to one of two epistasis groups. swi6, clr1, clr2, clr3, and clr4 belong to one group that acts in part via the K region; clr6, esp1, esp2, and esp3 belong to a second group that possibly exerts its effect via the mat2/mat3 elements (26, 81). To determine whether ubcP3, ubc15, or rhp6 could be assigned to one of the two pathways, an epistasis analysis was performed. The fact that the cDNA clones were obtained in a partially derepressed strain caused by deletion of the element in front of mat2 suggested that the silencing defects caused by overexpressing ubcP3, ubc15, or rhp6 might be exerted via the swi6, clr1, clr2, clr3, and clr4 pathway. If this was true, combining overexpression of ubcP3, ubc15, or rhp6 with a mutation in swi6, clr1, clr2, clr3, or clr4 should not give rise to cumulative derepression. Indeed, overexpression of ubcP3, ubc15, or rhp6 in swi6-115 (Fig. 4A and B), clr1-5 (Fig. 4C and D), clr4-681, clr3-735, or clr2-760 (data not shown) cells does not change their level of haploid meiosis. Furthermore, in a manner similar to the derepression caused by mutations in the Swi6 epistasis group (22, 27, 81), high dosage of ubcP3, ubc15, or rhp6 induced high levels of haploid meiosis in unswitchable esp3-1 cells (Fig. 4C and D) and derepressed transcription in a ΔK1 strain (Fig. 4 E and F). These phenotypes are consistent with ubcP3, ubc15, and rhp6 affecting silencing via the swi6, clr1, clr2, clr3, and clr4 pathway.
FIG. 4.
High dosage of UbcP3, Ubc15, or Rhp6 combined with mutations in cis- and trans-acting silencers. (A) Mating-type region of the switchable strains PG9 (h90) and PG595 (h90, swi6-115). (B) Sporulation of PG9 (swi6+) (+) and PG595 (swi6-115) cells transformed with plasmids containing no cDNA (−), ubcP3, ubc15, or rhp6 was assayed by staining colonies grown on sporulation medium (MSA plus adenine plus uracil) with iodine. (C) Mating-type region of the nonswitchable strains PG1598 (mat1-Msmt-0), PG506 (mat1-Msmt-0, clr1-5), and PG2883 (mat1-Msmt-0, esp1-3). (D) Sporulation of PG1598 (+), PG506 (clr1-5), and PG2883 (esp1-3) transformed with the indicated plasmids was assayed as done for panel B. (E) PG1247 contains an ∼8-kb deletion between mat2-P and mat3-M. (F) Sporulation in PG1247 cells transformed with plasmids containing no cDNA (−), ubcP3, ubc15, or rhp6 was assayed by iodine staining.
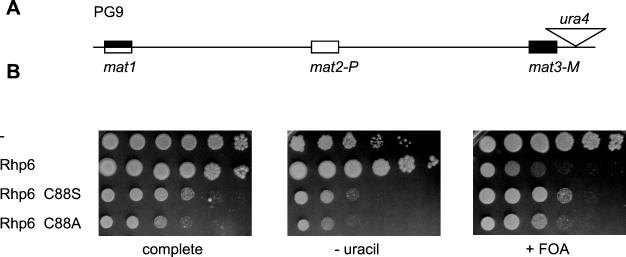
The antisilencing activity of Rhp6 requires an intact active site.
The silencing defects caused by high dosage of UbcP3, Ubc15, or Rhp6 could be a result of these ubiquitin-conjugating enzymes titrating away silencing complexes from their cognate site of action by direct association. If this was the case, a catalytically inactive E2 ubiquitin-conjugating enzyme would most likely exert an effect on silencing similar to that of a catalytically active enzyme. To test this possibility, we created active-site mutants of rhp6. In the process of covalently attaching ubiquitin to lysines in target proteins, ubiquitin-conjugating enzymes form a transient thioester bond with ubiquitin at an active-site cysteine (22). We mutated the predicted active-site cysteine of Rhp6, cysteine 88, by site-directed mutagenesis. Cysteine 88 is the only cysteine present in Rhp6. By analogy with S. cerevisiae Rad6p active-site mutants (75, 76), Rhp6(C88A) with its active-site cysteine changed to alanine should be unable to form a thioester conjugate with ubiquitin, whereas Rhp6(C88S), with a change of cysteine to serine, should be able to bind ubiquitin but should be unable to transfer it to target proteins. Overexpressed rhp6 carrying either of the two point mutations, rhp6(C88S) or rhp6(C88A), caused cell elongation (not shown) and slow growth (Fig. 5B, complete) but failed to derepress transcription (Fig. 5B, − uracil and + 5-FOA). Hence, the ubiquitin-conjugating activity of Rhp6 is required for its effects on silencing when overproduced. These effects are therefore not caused by a titration of silencing complexes via direct binding to Rhp6.
FIG. 5.
The Rhp6 active site is required for the antisilencing effect of Rhp6. (A) Mating-type region of PG9. (B) The effects on silencing of overexpressing rhp6, rhp6-C88S, rhp6-C88A, or no cDNA were compared by testing the ability of transformed PG9 strains to grow on complete medium (MSA plus adenine plus uracil), medium lacking uracil (MSA plus adenine) (− uracil), and medium containing 5-FOA (PM plus adenine plus uracil plus FOA) (+ FOA).
Effect of disrupting ubcP3, ubc15, or rhp6 on silencing in the mating-type region.
We constructed two diploid strains, one containing a ubcP3 allele disrupted with ura4 and one containing a ubc15 allele entirely replaced with ura4. Examining the progeny of these diploids showed that neither ubcP3 nor ubc15 is essential for cell viability. To assay for silencing phenotypes, ubcP3::ura4 or ubc15::ura4 was crossed into strains with ade6 integrated near mat3-M with or without deletion of the mat3-M silencer. Disruption of ubcP3 or ubc15 did not cause obvious defects in silencing of ade6 at mat3-M (data not shown). In addition, the ubcP3::ura4 ubc15::ura4 double mutant failed to reveal a silencing defect (data not shown). Furthermore, h90 ubcP3::ura4 and h90 ubc15::ura4 cells had mating and sporulation phenotypes indistinguishable from those of wild-type h90 cells (data not shown).
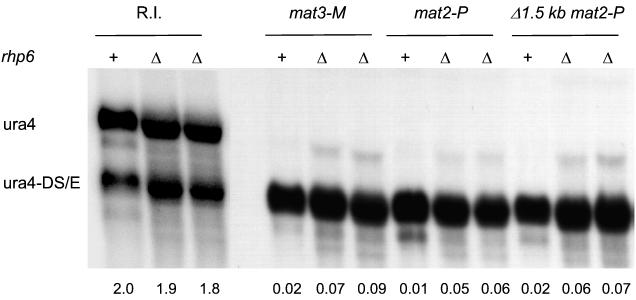
We used the ubc2::kanr allele described in reference 40 to test whether Rhp6 depletion had an effect on silencing. Northern blot analyses revealed a small increase in the amount of ura4 transcript originating from sites within the mating-type region in ubc2::kanr strains (Fig. 6), indicating that Rhp6 participates in the repression of marker genes placed near the silent cassettes in wild-type strains.
FIG. 6.
Deletion of rhp6 slightly derepresses silencing in the mating-type region. Strains containing a random integration of ura4 (R.I.), the mat3-M (EcoRV)::ura4 allele depicted in Fig. 2A (mat3-M), the mat2-P (XbaI)::ura4 allele depicted in Fig. 2C (mat2-P), or the Δ(BglII-BssHII) mat2-P (XbaI)::ura4 allele depicted in Fig. 1A (Δ1.5 kb mat2-P) and a wild-type (+) or null (Δ) allele of rhp6 were used to estimate the amount of ura4 transcript originating from the mating-type region relative to the ura4-DS/E control. ura4-to-ura4-DS/E ratios are reported below the blot. The strains were as follow, from left to right: R.I. +, FY340; R.I. Δ, PG2875; R.I. Δ, PG2876; mat3-M +, PG1899; mat3-M Δ, PG2887; mat3-M Δ, PG2888; mat2-P +, PG1789; mat2-P Δ, PG2889; mat2-P Δ, PG2890; Δ1.5 kb mat2-P +, PG1786; Δ1.5 kb mat2-P Δ, PG2891; and Δ1.5 kb mat2-P Δ, PG2892.
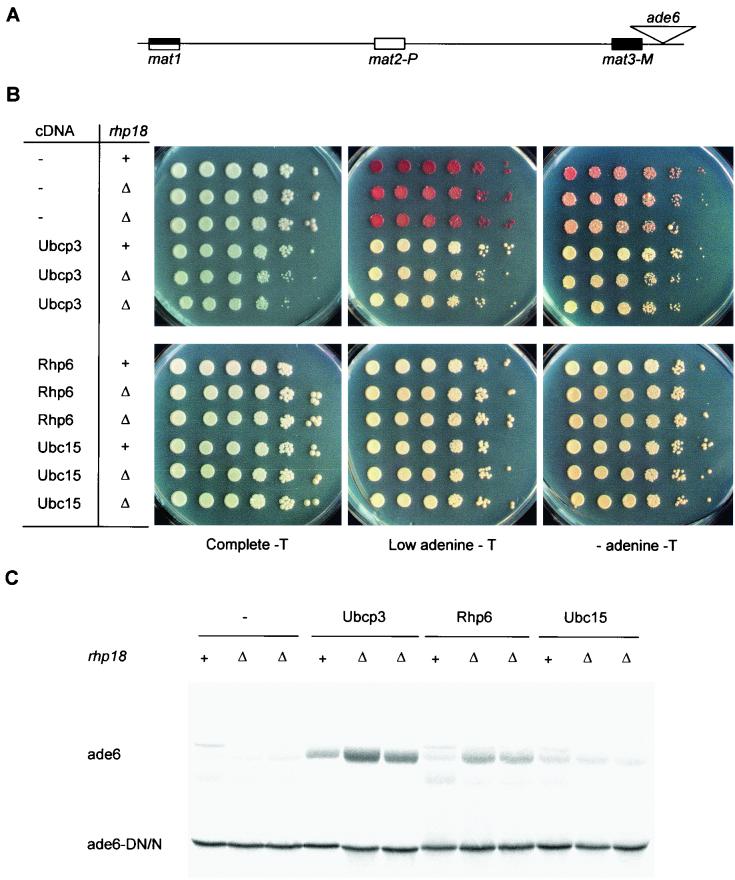
The derepression caused by high dosage of UbcP3, Ubc15, or Rhp6 does not require rhp18.
The S. cerevisiae RAD18 gene encodes a protein with single-stranded-DNA-binding activity that interacts with the ubiquitin-conjugating enzyme Rad6p and functions in postreplication repair (4). Similarly, stable hRad18p-HHR6A and hRad18p-HHR6B protein complexes are formed when the human Rad6p homologs HHR6A and HHR6B are coexpressed with human hRAD18 in yeast cells (88). Rhp18, the S. pombe Rad18 homolog, could possibly be involved in the silencing defects observed in our study by providing an interface between ubiquitin-conjugating enzymes and DNA. In order to test this possibility, we overexpressed ubcP3, ubc15, or rhp6 in rhp18::ura4 cells containing ade6 near the mat3-M cassette (86) (Fig. 7A). Expression of ade6 was first assayed by plating cells on medium lacking adenine and on medium containing a low concentration of adenine on which cells lacking Ade6 accumulate a red pigment (Fig. 7B). Expression of ade6 was further analyzed by Northern blotting, using a truncated ade6 transcript originating from the ade6-DN/N allele (19) as internal control (Fig. 7C). Derepression of ade6 occurred in rhp18::ura4 cells, indicating that the silencing defects caused by overproduction of UbcP3, Ubc15, or Rhp6 do not depend on Rhp18.
FIG. 7.
Disruption of silencing caused by overexpression of ubcP3, ubc15, or rhp6 does not require rhp18. (A) Mating-type region of Hu52 and PG2895. (B) Silencing of ade6 near mat3-M was tested by plating dilution series of rhp18+ (Hu52) or rhp18Δ::ura4 (PG2895) cells transformed with plasmids containing no cDNA (−), ubcP3, ubc15, or rhp6 on medium lacking adenine (AA-thiamine-leucine-adenine) (− adenine - T), on medium poor in adenine (AA-thiamine-leucine + 12 mg of adenine per liter) (Low adenine - T), and on complete medium (AA-acid-thiamine-leucine). (C) A Northern blot prepared with RNA from the strains displayed in panel B was hybridized to a probe for the ade6 open reading frame. “ade6” indicates the full-length ade6 transcript originating from the mating-type region, while “ade6-DN/N” indicates the truncated ade6 transcript originating from the ade6-DN/N allele.
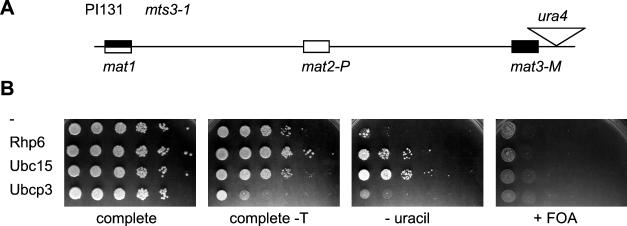
The derepression caused by high dosage of Ubc15 or Rhp6 can occur in a 26S proteasome mutant.
Polyubiquitination can target proteins for degradation via the 26S proteasome (22). Degradation recycles misfolded proteins and participates in cell cycle control (22, 41, 53, 85). Elevated doses of UbcP3, Ubc15, or Rhp6 might therefore cause the observed silencing defects by increasing turnover of silencing factors via the 26S proteasome. If this was the case, defects in the 26S proteasome might prevent derepression from occurring or at least attenuate it. In order to test this hypothesis, we assayed whether mts3-1, a thermosensitive mutation in the 19S regulatory cap of the 26S proteasome (68), suppressed the silencing defects caused by high dosage of UbcP3, Ubc15, or Rhp6. mts3-1 cells displayed a partial growth defect at the semipermissive temperature of 30°C, and they grew very poorly on FOA-containing plates, preventing use of this medium to assess expression of ura4. Overexpression of UbcP3 also proved toxic to mts3-1 cells, precluding any test of silencing (Fig. 8). Overexpression of Ubc15 or Rhp6 could be assayed, and Fig. 8 shows that it caused derepression of transcription even when the 26S proteasome was compromised, leading to increased growth in the absence of uracil. This indicates that disruption of silencing by Ubc15 or Rhp6 overproduction is not due to the degradation of silencing factors.
FIG. 8.
The silencing defects caused by overexpression of ubcP3 or rhp6 do not require a functional 26S proteasome. (A) Mating-type region of PI131. (B) PI131 cells overexpressing the indicated proteins were plated on medium lacking uracil (MSA plus adenine) (− uracil) or containing 5-FOA (PM plus adenine plus uranine plus FOA) (+ FOA). The effect of overexpressing ubcP3, ubc15, or rhp6 on the growth of PI131 was tested by plating on noninducing (AA-leucine [complete]) and inducing (MSA plus adenine plus uracil [complete-T]) media. Plate contents were incubated at 30°C.
DISCUSSION
In this study, we present the identification of a group of proteins that influence transcriptional silencing in fission yeast and an initial characterization of their mode of action. These proteins belong to the family of ubiquitin-conjugating enzymes. One of them, Rhp6, was previously implicated in transcriptional repression (70), as was its S. cerevisiae homolog Rad6p (11, 32). Both Rhp6 and Rad6p have been extensively characterized because of their role in DNA damage repair. Two other ubiquitin-conjugating enzymes identified by our screen, UbcP3 and Ubc15, were not obtained in previous mutant searches. Their effects on silencing are similar to those of Rhp6, which raises the possibility that the three ubiquitin-conjugating enzymes act on related substrates.
Ubiquitin-conjugating enzymes and silencing in S. pombe.
Overexpression of UbcP3, Ubc15, or Rhp6 impaired silencing of genes residing within or near the mat2 or mat3 mating-type cassettes, whereas disruption of ubcP3 or ubc15 did not significantly affect silencing. Deletion of rhp6 had pleiotropic phenotypes, including a partial loss of silencing of marker genes introduced in the mating-type region. The lack of phenotype of the ubcP3 or ubc15 disruptions and the relatively small effect of the rhp6 deletion suggest that ubiquitin-conjugating enzymes might affect silencing redundantly. Fourteen putative E2 ubiquitin-conjugating enzymes have been identified in fission yeast (Sanger Centre fission yeast genomic database). Our data do not rule out the possibility that ubiquitin-conjugating enzymes other than UbcP3, Ubc15, and Rhp6 affect transcriptional silencing. Ubc4, for example, a ubiquitin-conjugating enzyme that causes chromosome loss when overexpressed (34), could influence position effects, a possibility remaining to be investigated. Remarkably, UbcP3, Ubc15, Rhp6 and Ubc4 are more closely related to each other than to any other putative ubiquitin-conjugating enzyme in S. pombe, UbcP3 and Ubc15 being most similar with 50% identical amino acids (Fig. 1D).
A point mutation in the 5′ splice junction at the second intron of rhp6/sng1 was previously reported to cause a 10-fold reduction in mature rhp6 mRNA level (70). That mutation derepresses the mat2-P and mat3-M genes but not heterologous reporter genes inserted in the silent regions flanking the donor cassettes (70). In addition, Singh and coworkers (70) observed derepression only in cells that were able to switch their mating type, which suggests a role for rhp6 in reassembly of chromatin after the DNA replication that accompanies mating-type switching. We found that rhp6 overexpression caused derepression not only of the mating-type genes contained within the silent cassettes but also of heterologous genes introduced near the cassettes and that the derepression occurred both in switchable and nonswitchable cells. These results are not incompatible with those of Singh et al. (70). Ubiquitination of a substrate by Rhp6 might be required for the spreading of a silencing factor from regions that flank the cassettes into the freshly used donor cassettes. Reduced concentrations of Rhp6, such as those caused by the sng1 mutation, would not allow spreading of the silencing factor, whereas overproduction of Rhp6 might cause ubiquitination of an excess of substrate and targeting of the silencing factor to novel sites. This would lead to depletion of the factor not only within the donor cassettes but more generally in the entire region that is normally silenced. Our observation that deletion of rhp6 reduces silencing of a ura4 marker placed near mat3-M is more difficult to reconcile with the conclusions of Singh et al. (70). That derepression might not have occurred or might have escaped detection in cells containing a residual amount of Rhp6.
Ubiquitin-processing enzymes and silencing.
S. cerevisiae RAD6/UBC2 is required for silencing at telomeres and the HM loci (32). RAD6 is also required for transcriptional silencing of Ty1 elements in the RDN1 locus (11). These effects of Rad6p can be explained in terms of alterations of chromatin structure. Gross structural alterations of chromatin are also consistent with the role of RAD6 and rhp6+ in sporulation (24, 50, 63) and with the role of the mouse Rad6p homolog HHR6B in the histone-to-protamine transition during spermatogenesis (65, 42). Overexpression of rhp6 mutated at cysteine 88 did not disrupt silencing (this study), and active-site mutants of RAD6 (32) are silencing deficient, suggesting that in both cases the ubiquitination event itself is necessary for silencing. Other ubiquitin-processing enzymes play a role in transcriptional silencing. E(var)3-64E, a putative ubiquitin-specific protease, affects position-effect variegation in Drosophila melanogaster (31). In S. cerevisiae, the ubiquitin hydrolases Ubp3p and Dot4p, respectively, counteract (49) and enhance (37, 69) silencing. Both Ubp3p and Dot4p interact with the silencing factor Sir4p, and these deubiquitinating enzymes may regulate silencing by controlling either the activity or the assembly of SIR protein complexes, possibly helping restrict transcriptional repression to normally silenced loci (37, 49). In the present study we show that, in addition to Rhp6, two hitherto-uncharacterized putative E2 ubiquitin-conjugating enzymes, UbcP3 and Ubc15, influence silencing in the mating-type region of S. pombe. Collectively, these studies demonstrate a role for ubiquitination in silencing, possibly by the regulation of chromatin structure.
Histones as potential substrates.
In higher eukaryotes, histone H2A, histone H2B, and histone H3 can be ubiquitinated and a number of studies suggest that histone ubiquitination contributes to the organization of chromatin (reviewed in references 9, 12, 71, and 87). Ubiquitination of histone H2A and histone H2B has been proposed to regulate chromosome condensation, nucleosomes being deubiquitinated at metaphase and rapidly reubiquitinated at anaphase (45, 52). Ubiquitination of histone H2B, and to a lesser extent of histone H2A, is in some cases associated with transcriptional activity (13, 57). Histone H3 ubiquitination occurs during the maturation of rat spermatids concomitantly with extensive chromatin remodeling (12). The purified S. cerevisiae Rad6p protein can ubiquitinate histone H2A, H2B, and H3 in vitro (28, 74). Rad6p ubiquitinates histone H2B in vivo in S. cerevisiae, and mutation of the conserved ubiquitination site in H2B confers mitotic and meiotic defects (64). However, a silencing-proficient mutant (rad6-149) is unable to ubiquitinate histone H2B to detectable levels, indicating that histone H2B ubiquitination does not directly mediate silencing in S. cerevisiae (64 and references therein). Comparison of S. cerevisiae histone H2A and histone H2A from higher eukaryotes, which can be ubiquitinated at lysine 119 (58, 77), suggested which lysine might be ubiquitinated in yeast. Mutating this lysine, however, produced cells that were indistinguishable from the wild type under a variety of conditions, indicating that ubiquitination of histone H2A does not play a major role in S. cerevisiae cell growth, sporulation, or resistance to heat, stress, or UV radiation (77). This conclusion was confirmed by the examination of a quadruple mutant of histone H2A, in which lysine 119 as well as three neighboring lysines were changed to arginine (64). However, neither study tested whether mutating lysines of histone H2A or histone H2B influenced transcriptional silencing. Future studies will reveal whether and which nucleosomal proteins are ubiquitinated in S. pombe and whether ubiquitination of histones influences silencing.
Repair.
Transcriptional silencing, DNA repair, and recombination are related by their dependence on chromatin structure. Deletions of chromatin assembly factors (CAC1, CAC2, or CAC3) cause sensitivity to UV and reduce telomeric silencing in S. cerevisiae (21, 38, 73). The S. cerevisiae Rad6p interacts with Rad18p, which is a key factor in the RAD6 DNA repair pathway. Rad18p may target Rad6p to damaged DNA by its ability to bind to single-stranded DNA (4). RAD18 is not required for silencing of telomeric markers in S. cerevisiae (32), but its deletion further reduces telomeric silencing in cells lacking CAC1 (24). Here we show that disruption of silencing by overexpressing ubcP3, ubc15, or rhp6 does not require rhp18. Further experiments are required to determine whether Rhp18 is required for UbcP3, Ubc15, or Rhp6 effects on silencing in a wild-type background, a requirement that would be overruled when the UbcP3, Ubc15, or Rhp6 dosage is increased. High dosage of UbcP3, Ubc15, or Rhp6 or disruption of ubcP3 or ubc15 had no effect on UV sensitivity (data not shown), suggesting that the effects exerted on silencing are not directly related to the UV DNA damage response.
Protein degradation.
A large number of proteins are modified covalently by the enzymatic attachment of ubiquitin to side chains of lysines, a modification that often leads to proteolytic degradation (53, 85). Covalently bound polyubiquitin chains are recognized by one or more subunits of the 26S proteasome as the first step in the process that leads to the degradation of the ubiquitinated protein and recycling of the 76-amino-acid ubiquitin molecules (22, 85). Mutations in mts2 and pad1 encoding 19S regulatory cap subunits of the 26S proteasome were reported to cause increased repression of centromeric ura4 (35), indicating that silencing factors might normally be degraded by the proteasome. We found that derepression caused by high dosage of Ubc15 or Rhp6 can occur in a strain carrying a mutation in the 19S regulatory cap of the 26S proteasome (mts3-1 [68]). This suggests that disruption of silencing by Ubc15 or Rhp6 is not caused by increased degradation of silencing factors via the 26S proteasome. However, we cannot rule out that some silencing factors were degraded at the semipermissive temperature because of residual activity of the proteasome.
Models for the role of ubiquitin-conjugating enzymes in silencing.
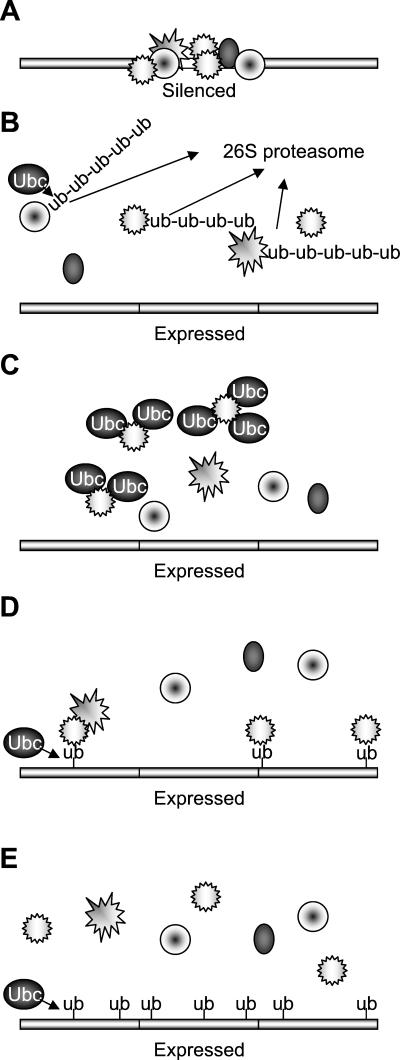
Figure 9 presents four mechanisms by which a high dosage of ubiquitin-conjugating enzymes could reduce transcriptional silencing. The silenced chromatin state is represented in Fig. 9A. In the first model (Fig. 9B), the turnover of histones or chromatin-associated silencing factors is increased when E2-ubiquitinating enzymes are in abundance. Our finding that derepression of silencing occurs in a proteasome mutant argues against this model. In the second model (Fig. 9C), the derepression results from the ubiquitin-conjugating enzymes directly titrating silencing complexes away from their site of action by protein-protein associations, as is, for example, the case in the titration of Sir4p by overexpressed Dot4p in S. cerevisiae (37). This model predicts that catalytically inactive ubiquitin-conjugating enzymes would be able to derepress silencing when overexpressed. We found that Rhp6 active-site mutants were unable to disrupt silencing when overexpressed, arguing against a direct titration model in the case of Rhp6. Indirect titration might be another mechanism by which disruption of silencing occurs (Fig. 9D). In this model, ubiquitin modification of chromatin components or histones normally targets silencing factors to their site of action and directs the assembly of higher-order chromatin structures. Overexpression of ubiquitin-conjugating enzymes leads to excessive ubiquitination and to the titration of silencing factors. Finally (Fig. 9E), increased ubiquitination might directly prevent the formation of heterochromatin. Our results globally support the last two models, the small reduction of silencing observed in the absence of Rhp6 arguing in favor of the model in Fig. 9D in the case of Rhp6. Additional experiments are needed to identify the substrates and elucidate the mechanisms of action of UbcP3, Ubc15, and Rhp6.
FIG. 9.
Ubiquitin-conjugating enzymes and silencing: models. (A) Transcriptionally silenced region. Silencing factors, represented by objects of various shapes, are associated with chromatin (bar). (B to E) Models for the disruption of silencing by the overexpression of ubiquitin-conjugating enzymes (Ubc). The models are discussed in the text. Ub, ubiquitin.
Acknowledgments
We thank Takashi Toda for providing a ubc2::kanr strain; Bruce Edgar and Chris Norbury for the cDNA library; Pernilla Bjerling for strain Hu52; Janne Verhein-Hansen for technical assistance; and Stanley Brown, Karl Ekwall, Benoit Arcangioli, Richard Egel, and members of the Institute of Molecular Biology for comments and suggestions.
Our work was supported by a grant from the Novo Nordisk Foundation to G.T. and by a CRC grant to J.M.M. (SP2396/0201).
REFERENCES
- 1.Allshire, R. C., J. P. Javerzat, N. J. Redhead, and G. Cranston. 1994. Position effect variegation at fission yeast centromeres. Cell 76:157-169. [DOI] [PubMed] [Google Scholar]
- 2.Allshire, R. C. 1996. Transcriptional silencing in the fission yeast: a manifestation of higher order chromosome structure and function, p. 443-466. In V. E. A. Russo, R. A. Martienssen, and A. D. Riggs (ed.), Epigenetic mechanisms of gene regulation. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 3.Ayoub, N., I. Goldshmidt, R. Lyakhovetsky, and A. Cohen. 2000. A fission yeast repression element cooperates with centromere-like sequences and defines a mat silent domain boundary. Genetics 156:983-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailly, V., J. Lamb, P. Sung, S. Prakash, and L. Prakash. 1994. Specific complex formation between yeast RAD6 and RAD18 proteins: a potential mechanism for targeting RAD6 ubiquitin-conjugating activity to DNA damage sites. Genes Dev. 8:811-820. [DOI] [PubMed] [Google Scholar]
- 5.Bannister, A. J., P. Zegerman, J. F. Partridge, E. A. Miska, J. O. Thomas, R. C. Allshire, and T. Kouzarides. 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410:120-124. [DOI] [PubMed] [Google Scholar]
- 6.Beach, D., L. Rodgers, and J. Gould. 1985. ran1+ controls the transition from mitotic division to meiosis in fission yeast. Curr. Genet. 10:297-311. [DOI] [PubMed] [Google Scholar]
- 7.Bjerling, P. 1998. Analysing Clr2 as a trans-acting factor, and exploring the range of cis-mediated effects beyond mat3-M. Ph.D. thesis. University of Copenhagen, Copenhagen, Denmark.
- 8.Borgstrom, B. 1995. Characterization of the Schizosaccharomyces pombe rik1 gene. Ph.D. thesis. University of Copenhagen, Copenhagen, Denmark.
- 9.Bradbury, E. M. 1992. Reversible histone modifications and the chromosome cell cycle. Bioessays 14:9-16. [DOI] [PubMed] [Google Scholar]
- 10.Brunner, D., and P. Nurse. 2000. CLIP170-like tip1p spatially organizes microtubular dynamics in fission yeast. Cell 102:695-704. [DOI] [PubMed] [Google Scholar]
- 11.Bryk, M., M. Banerjee, M. Murphy, K. E. Knudsen, D. J. Garfinkel, and M. J. Curcio. 1997. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 11:255-269. [DOI] [PubMed] [Google Scholar]
- 12.Chen, H. Y., J. M. Sun, Y. Zhang, J. R. Davie, and M. L. Meistrich. 1998. Ubiquitination of histone H3 in elongating spermatids of rat testes. J. Biol. Chem. 273:13165-13169. [DOI] [PubMed] [Google Scholar]
- 13.Davie, J. R., and L. C. Murphy. 1990. Level of ubiquitinated histone H2B in chromatin is coupled to ongoing transcription. Biochemistry 29:4752-4757. [DOI] [PubMed] [Google Scholar]
- 14.Edwards, K. A., R. A. Montague, S. Shepard, B. A. Edgar, R. L. Erikson, and D. P. Kiehart. 1994. Identification of Drosophila cytoskeletal proteins by induction of abnormal cell shape in fission yeast. Proc. Natl. Acad. Sci. USA 91:4589-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egel, R., M. Willer, S. Kjaerulff, J. Davey, and O. Nielsen. 1994. Assessment of pheromone production and response in fission yeast by a halo test of induced sporulation. Yeast 10:1347-1354. [DOI] [PubMed] [Google Scholar]
- 16.Eissenberg, J. C., and S. C. Elgin. 2000. The HP1 protein family: getting a grip on chromatin. Curr. Opin. Genet. Dev. 10:204-210. [DOI] [PubMed] [Google Scholar]
- 17.Ekwall, K., G. Cranston, and R. C. Allshire. 1999. Fission yeast mutants that alleviate transcriptional silencing in centromeric flanking repeats and disrupt chromosome segregation. Genetics 153:1153-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekwall, K., O. Nielsen, and T. Ruusala. 1991. Repression of a mating type cassette in the fission yeast by four DNA elements. Yeast 7:745-755. [DOI] [PubMed] [Google Scholar]
- 19.Ekwall, K., T. Olsson, B. M. Turner, G. Cranston, and R. C. Allshire. 1997. Transient inhibition of histone deacetylation alters the structural and functional imprint at fission yeast centromeres. Cell 91:1021-1032. [DOI] [PubMed] [Google Scholar]
- 20.Ekwall, K., and T. Ruusala. 1994. Mutations in rik1, clr2, clr3 and clr4 genes asymmetrically derepress the silent mating-type loci in fission yeast. Genetics 136:53-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enomoto, S., P. D. McCune-Zierath, M. Gerami-Nejad, M. A. Sanders, and J. Berman. 1997. RLF2, a subunit of yeast chromatin assembly factor-I, is required for telomeric chromatin function in vivo. Genes Dev. 11:358-370. [DOI] [PubMed] [Google Scholar]
- 22.Finley, D., and V. Chau. 1991. Ubiquitination. Annu. Rev. Cell Biol. 7:25-69. [DOI] [PubMed] [Google Scholar]
- 23.Forsburg, S. L. 1993. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 21:2955-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Game, J. C., and P. D. Kaufman. 1999. Role of Saccharomyces cerevisiae chromatin assembly factor-I in repair of ultraviolet radiation damage in vivo. Genetics 151:485-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grewal, S. I. 2000. Transcriptional silencing in fission yeast. J. Cell. Physiol. 184:311-318. [DOI] [PubMed] [Google Scholar]
- 26.Grewal, S. I., M. J. Bonaduce, and A. J. Klar. 1998. Histone deacetylase homologs regulate epigenetic inheritance of transcriptional silencing and chromosome segregation in fission yeast. Genetics 150:563-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grewal, S. I., and A. J. Klar. 1996. Chromosomal inheritance of epigenetic states in fission yeast during mitosis and meiosis. Cell 86:95-101. [DOI] [PubMed] [Google Scholar]
- 28.Haas, A., P. M. Reback, G. Pratt, and M. Rechsteiner. 1990. Ubiquitin-mediated degradation of histone H3 does not require the substrate-binding ubiquitin protein ligase, E3, or attachment of polyubiquitin chains. J. Biol. Chem. 265:21664-21669. [PubMed] [Google Scholar]
- 29.Halverson, D., M. Baum, J. Stryker, J. Carbon, and L. Clarke. 1997. A centromere DNA-binding protein from fission yeast affects chromosome segregation and has homology to human CENP-B. J. Cell Biol. 136:487-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 31.Henchoz, S., F. De Rubertis, D. Pauli, and P. Spierer. 1996. The dose of a putative ubiquitin-specific protease affects position-effect variegation in Drosophila melanogaster. Mol. Cell. Biol. 16:5717-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang, H., A. Kahana, D. E. Gottschling, L. Prakash, and S. W. Liebman. 1997. The ubiquitin-conjugating enzyme Rad6 (Ubc2) is required for silencing in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:6693-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanova, A. V., M. J. Bonaduce, S. V. Ivanov, and A. J. Klar. 1998. The chromo and SET domains of the Clr4 protein are essential for silencing in fission yeast. Nat. Genet. 19:192-195. [DOI] [PubMed] [Google Scholar]
- 34.Javerzat, J. P., G. Cranston, and R. C. Allshire. 1996. Fission yeast genes which disrupt mitotic chromosome segregation when overexpressed. Nucleic Acids Res. 24:4676-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Javerzat, J. P., G. McGurk, G. Cranston, C. Barreau, P. Bernard, C. Gordon, and R. Allshire. 1999. Defects in components of the proteasome enhance transcriptional silencing at fission yeast centromeres and impair chromosome segregation. Mol. Cell. Biol. 19:5155-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenuwein, T. 2001. Re-SET-ting heterochromatin by histone methyltransferases. Trends Cell Biol. 11:266-273. [DOI] [PubMed] [Google Scholar]
- 37.Kahana, A., and D. E. Gottschling. 1999. DOT4 links silencing and cell growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:6608-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufman, P. D., R. Kobayashi, and B. Stillman. 1997. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 11:345-357. [DOI] [PubMed] [Google Scholar]
- 39.Kelly, M., J. Burke, M. Smith, A. Klar, and D. Beach. 1988. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 7:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitamura, K., S. Katayama, S. Dhut, M. Sato, Y. Watanabe, M. Yamamoto, and T. Toda. 2001. Phosphorylation of Mei2 and Ste11 by Pat1 kinase inhibits sexual differentiation via ubiquitin proteolysis and 14-3-3 protein in fission yeast. Dev. Cell 1:389-399. [DOI] [PubMed] [Google Scholar]
- 41.Koepp, D. M., J. W. Harper, and S. J. Elledge. 1999. How the cyclin became a cyclin: regulated proteolysis in the cell cycle. Cell 97:431-434. [DOI] [PubMed] [Google Scholar]
- 42.Koken, M. H., J. W. Hoogerbrugge, I. Jasper-Dekker, J. de Wit, R. Willemsen, H. P. Roest, J. A. Grootegoed, and J. H. Hoeijmakers. 1996. Expression of the ubiquitin-conjugating DNA repair enzymes HHR6A and B suggests a role in spermatogenesis and chromatin modification. Dev. Biol. 173:119-132. [DOI] [PubMed] [Google Scholar]
- 43.Lachner, M., D. O'Carroll, S. Rea, K. Mechtler, and T. Jenuwein. 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410:116-120. [DOI] [PubMed] [Google Scholar]
- 44.Lorentz, A., K. Ostermann, O. Fleck, and H. Schmidt. 1994. Switching gene swi6, involved in repression of silent mating-type loci in fission yeast, encodes a homologue of chromatin-associated proteins from Drosophila and mammals. Gene 143:139-143. [DOI] [PubMed] [Google Scholar]
- 45.Matsui, S. I., B. K. Seon, and A. A. Sandberg. 1979. Disappearance of a structural chromatin protein A24 in mitosis: implications for molecular basis of chromatin condensation. Proc. Natl. Acad. Sci. USA 76:6386-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maundrell, K. 1990. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J. Biol. Chem. 265:10857-10864. [PubMed] [Google Scholar]
- 47.Maundrell, K. 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123:127-130. [DOI] [PubMed] [Google Scholar]
- 48.Meeks-Wagner, D., and L. H. Hartwell. 1986. Normal stoichiometry of histone dimer sets is necessary for high fidelity of mitotic chromosome transmission. Cell 44:43-52. [DOI] [PubMed] [Google Scholar]
- 49.Moazed, D., and D. Johnson. 1996. A deubiquitinating enzyme interacts with SIR4 and regulates silencing in S. cerevisiae. Cell 86:667-677. [DOI] [PubMed] [Google Scholar]
- 50.Montelone, B. A., S. Prakash, and L. Prakash. 1981. Recombination and mutagenesis in rad6 mutants of Saccharomyces cerevisiae: evidence for multiple functions of the RAD6 gene. Mol. Gen. Genet. 184:410-415. [DOI] [PubMed] [Google Scholar]
- 51.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 52.Mueller, R. D., H. Yasuda, C. L. Hatch, W. M. Bonner, and E. M. Bradbury. 1985. Identification of ubiquitinated histones 2A and 2B in Physarum polycephalum. Disappearance of these proteins at metaphase and reappearance at anaphase. J. Biol. Chem. 260:5147-5153. [PubMed] [Google Scholar]
- 53.Murray, A. 1995. Cyclin ubiquitination: the destructive end of mitosis. Cell 81:149-152. [DOI] [PubMed] [Google Scholar]
- 54.Nakayama, J., A. J. Klar, and S. I. Grewal. 2000. A chromodomain protein, Swi6, performs imprinting functions in fission yeast during mitosis and meiosis. Cell 101:307-317. [DOI] [PubMed] [Google Scholar]
- 55.Nakayama, J., J. C. Rice, B. D. Strahl, C. D. Allis, and S. I. Grewal. 2001. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292:110-113. [DOI] [PubMed] [Google Scholar]
- 56.Nakayama, J., R. C. Allshire, A. J. Klar, and S. I. Grewal. 2001. A role for DNA polymerase alpha in epigenetic control of transcriptional silencing in fission yeast. EMBO J. 20:2857-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nickel, B. E., C. D. Allis, and J. R. Davie. 1989. Ubiquitinated histone H2B is preferentially located in transcriptionally active chromatin. Biochemistry 28:958-963. [DOI] [PubMed] [Google Scholar]
- 58.Nickel, B. E., and J. R. Davie. 1989. Structure of polyubiquitinated histone H2A. Biochemistry 28:964-968. [DOI] [PubMed] [Google Scholar]
- 59.Noma, K., C. D. Allis, and S. I. Grewal. 2001. Transitions in distinct histone h3 methylation patterns at the heterochromatin domain boundaries. Science 293:1150-1155. [DOI] [PubMed] [Google Scholar]
- 60.Olsson, T. G., R. A. Silverstein, K. Ekwall, and P. Sunnerhagen. 1999. Transient inhibition of histone deacetylase activity overcomes silencing in the mating-type region in fission yeast. Curr. Genet. 35:82-87. [DOI] [PubMed] [Google Scholar]
- 61.Osaka, F., H. Seino, T. Seno, and F. Yamao. 1997. A ubiquitin-conjugating enzyme in fission yeast that is essential for the onset of anaphase in mitosis. Mol. Cell. Biol. 17:3388-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rea, S., F. Eisenhaber, D. O'Carroll, B. D. Strahl, Z. W. Sun, M. Schmid, S. Opravil, K. Mechtler, C. P. Ponting, C. D. Allis, and T. Jenuwein. 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406:593-599. [DOI] [PubMed] [Google Scholar]
- 63.Reynolds, P., M. H. Koken, J. H. Hoeijmakers, S. Prakash, and L. Prakash. 1990. The rhp6+ gene of Schizosaccharomyces pombe: a structural and functional homolog of the RAD6 gene from the distantly related yeast Saccharomyces cerevisiae. EMBO J. 9:1423-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robzyk, K., J. Recht, and M. A. Osley. 2000. Rad6-dependent ubiquitination of histone H2B in yeast. Science 287:501-504. [DOI] [PubMed] [Google Scholar]
- 65.Roest, H. P., J. van Klaveren, J. de Wit, C. G. van Gurp, M. H. Koken, M. Vermey, J. H. van Roijen, J. W. Hoogerbrugge, J. T. Vreeburg, W. M. Baarends, D. Bootsma, J. A. Grootegoed, and J. H. Hoeijmakers. 1996. Inactivation of the HR6B ubiquitin-conjugating DNA repair enzyme in mice causes male sterility associated with chromatin modification. Cell 86:799-810. [DOI] [PubMed] [Google Scholar]
- 66.Rose, M., F. Winston, and P. Hieter. 1990. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 67.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 68.Seeger, M., C. Gordon, K. Ferrell, and W. Dubiel. 1996. Characteristics of 26 S proteases from fission yeast mutants, which arrest in mitosis. J. Mol. Biol. 263:423-431. [DOI] [PubMed] [Google Scholar]
- 69.Singer, M. S., A. Kahana, A. J. Wolf, L. L. Meisinger, S. E. Peterson, C. Goggin, M. Mahowald, and D. E. Gottschling. 1998. Identification of high-copy disruptors of telomeric silencing in Saccharomyces cerevisiae. Genetics 150:613-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh, J., V. Goel, and A. J. Klar. 1998. A novel function of the DNA repair gene rhp6 in mating-type silencing by chromatin remodeling in fission yeast. Mol. Cell. Biol. 18:5511-5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spencer, V. A., and J. R. Davie. 1999. Role of covalent modifications of histones in regulating gene expression. Gene 240:1-12. [DOI] [PubMed] [Google Scholar]
- 72.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41-45. [DOI] [PubMed] [Google Scholar]
- 73.Sun, Z. W., and M. Hampsey. 1999. A general requirement for the Sin3-Rpd3 histone deacetylase complex in regulating silencing in Saccharomyces cerevisiae. Genetics 152:921-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sung, P., S. Prakash, and L. Prakash. 1988. The RAD6 protein of Saccharomyces cerevisiae polyubiquitinates histones, and its acidic domain mediates this activity. Genes Dev. 2:1476-1485. [DOI] [PubMed] [Google Scholar]
- 75.Sung, P., S. Prakash, and L. Prakash. 1990. Mutation of cysteine-88 in the Saccharomyces cerevisiae RAD6 protein abolishes its ubiquitin-conjugating activity and its various biological functions. Proc. Natl. Acad. Sci. USA 87:2695-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sung, P., S. Prakash, and L. Prakash. 1991. Stable ester conjugate between the Saccharomyces cerevisiae RAD6 protein and ubiquitin has no biological activity. J. Mol. Biol. 221:745-749. [DOI] [PubMed] [Google Scholar]
- 77.Swerdlow, P. S., T. Schuster, and D. Finley. 1990. A conserved sequence in histone H2A which is a ubiquitination site in higher eucaryotes is not required for growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 10:4905-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szankasi, P., W. D. Heyer, P. Schuchert, and J. Kohli. 1988. DNA sequence analysis of the ade6 gene of Schizosaccharomyces pombe. J. Mol. Biol. 204:917-925. [DOI] [PubMed] [Google Scholar]
- 79.Thon, G., K. P. Bjerling, and I. S. Nielsen. 1999. Localization and properties of a silencing element near the mat3-M mating-type cassette of Schizosaccharomyces pombe. Genetics 151:945-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thon, G., A. Cohen, and A. J. Klar. 1994. Three additional linkage groups that repress transcription and meiotic recombination in the mating-type region of Schizosaccharomyces pombe. Genetics 138:29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thon, G., and T. Friis. 1997. Epigenetic inheritance of transcriptional silencing and switching competence in fission yeast. Genetics 145:685-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thon, G., and A. J. Klar. 1992. The clr1 locus regulates the expression of the cryptic mating-type loci of fission yeast. Genetics 131:287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thon, G., and J. Verhein-Hansen. 2000. Four chromo-domain proteins of Schizosaccharomyces pombe differentially repress transcription at various chromosomal locations. Genetics 155:551-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thon, G., K. P. Bjerling, C. M. Bünner, and J. Verhein-Hansen. Expression-state boundaries in the mating-type region of fission yeast. Genetics, in press. [DOI] [PMC free article] [PubMed]
- 85.Varshavsky, A. 1997. The ubiquitin system. Trends Biochem. Sci. 22:383-387. [DOI] [PubMed] [Google Scholar]
- 86.Verkade, H. M., T. Teli, L. V. Laursen, J. M. Murray, and M. J. O'Connell. 2001. A homologue of the Rad18 postreplication repair gene is required for DNA damage responses throughout the fission yeast cell cycle. Mol. Gen. Genet. 265:993-1003. [DOI] [PubMed] [Google Scholar]
- 87.Wu, J., and M. Grunstein. 2000. 25 years after the nucleosome model: chromatin modifications. Trends Biochem. Sci. 25:619-623. [DOI] [PubMed] [Google Scholar]
- 88.Xin, H., W. Lin, W. Sumanasekera, Y. Zhang, X. Wu, and Z. Wang. 2000. The human RAD18 gene product interacts with HHR6A and HHR6B. Nucleic Acids Res. 28:2847-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]