Abstract
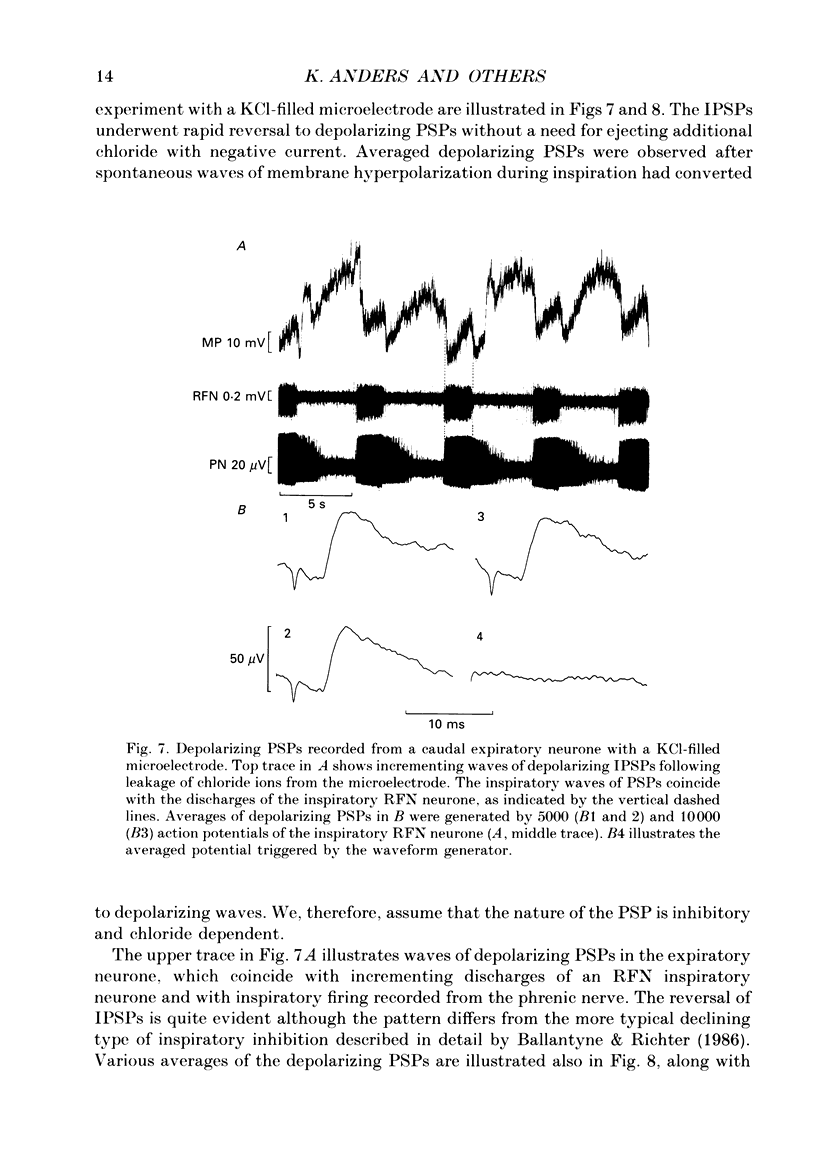
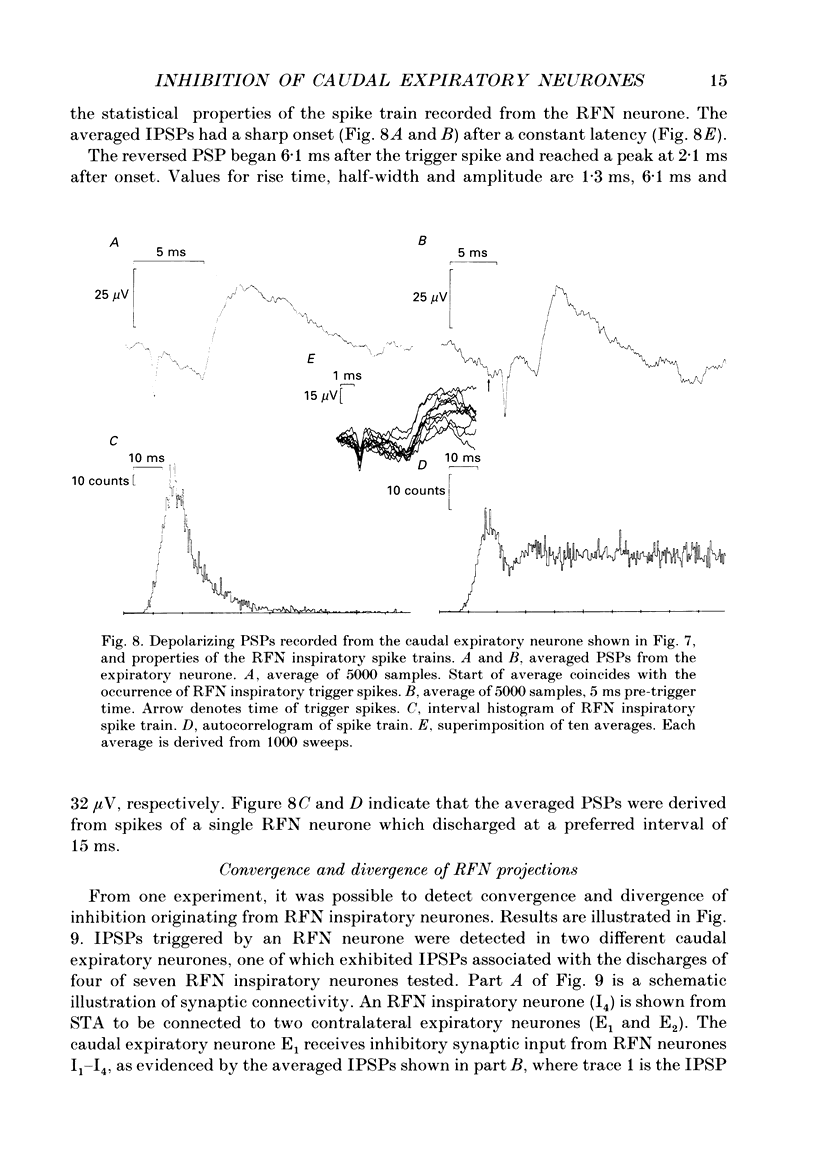
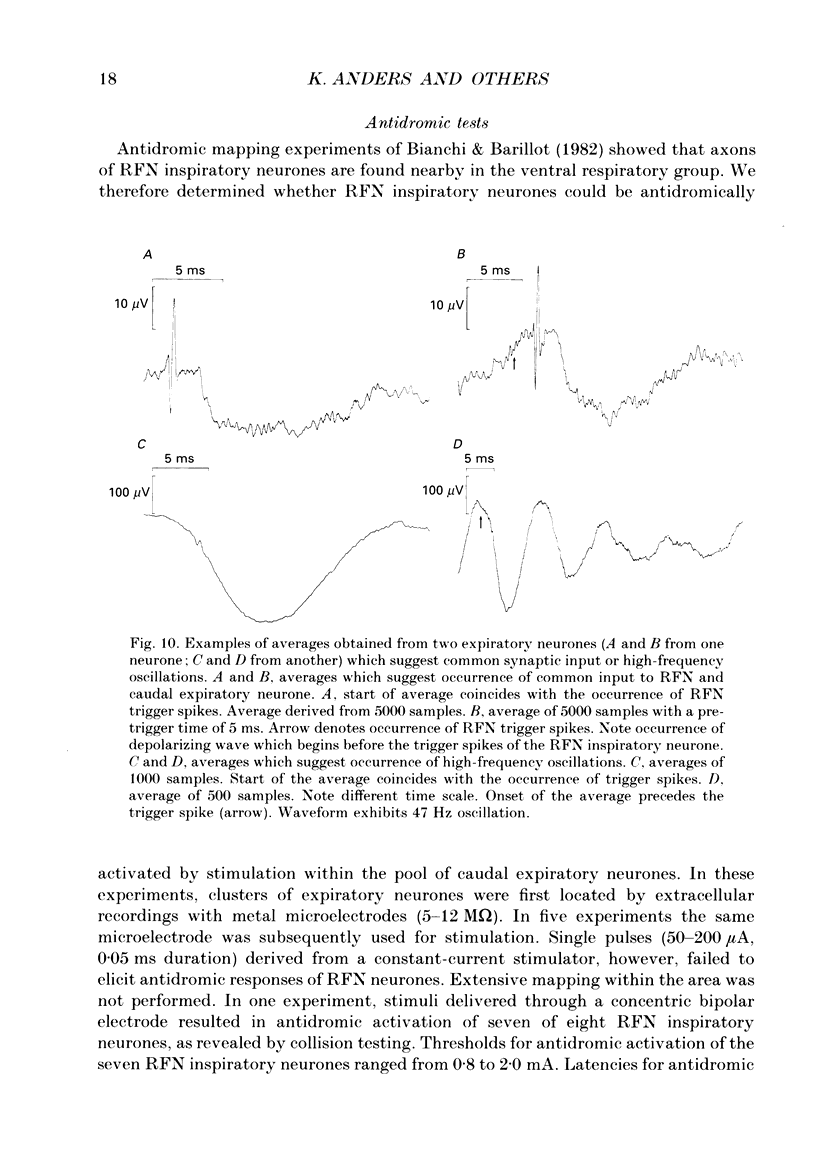
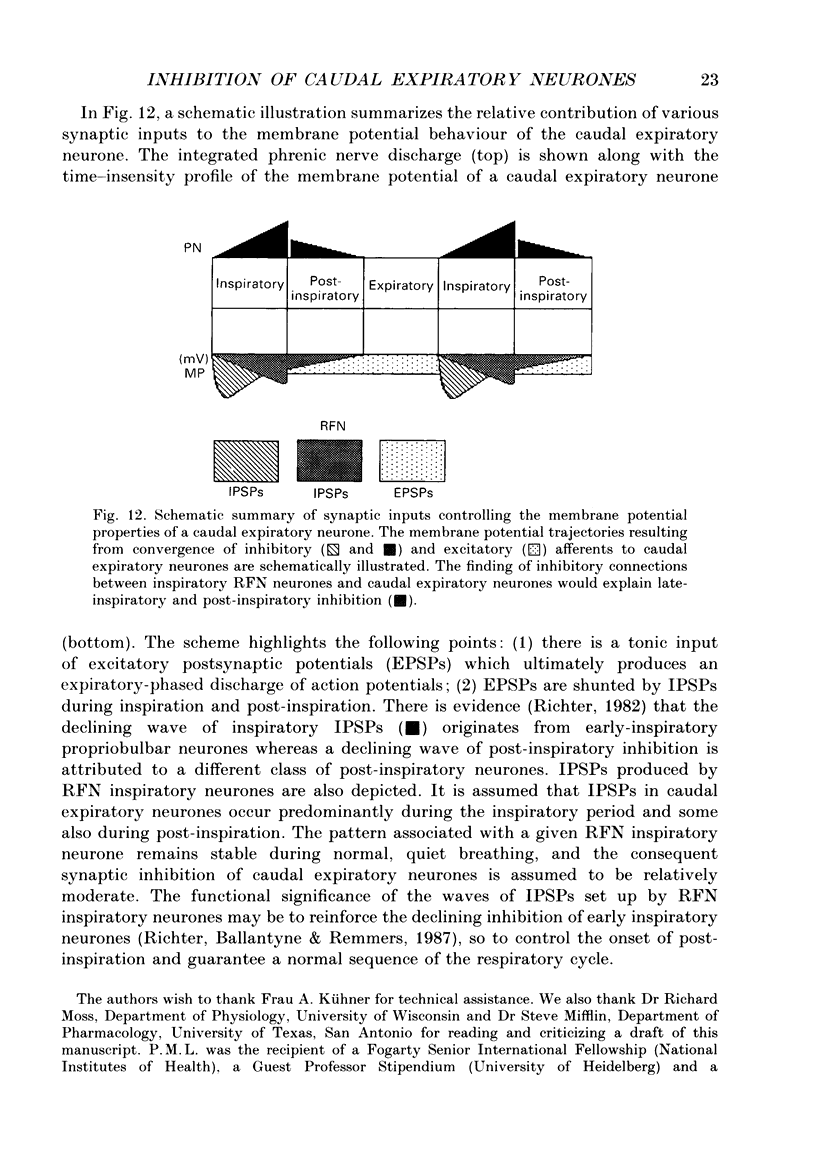
1. Comparisons between the spike discharge of inspiratory neurons within the retrofacial area (RFN), and the membrane potential of expiratory neurones within the caudal medulla were made in pentobarbitone-anaesthetized, vagotomized, artificially ventilated cats. Spike-triggered averaging (STA) of synaptic potentials, triggered by the discharge of inspiratory RFN neurones, was utilized to test for synaptic connectivity. 2. Eighty-nine neurons with respiratory-phased discharge patterns were recorded in the vicinity of the RFN. Fifty-four neurones discharged at or slightly before the onset of the inspiratory burst activity of the phrenic nerve and continued firing throughout inspiration. Two continued to fire during post-inspiration. Forty-five of fifty-four inspiratory RFN neurones exhibited incrementing discharge patterns, six discharged with a plateau pattern, while only three neurones had a decrementing discharge pattern. 3. The membrane potential trajectories of caudal expiratory neurones revealed a typical wave of early inspiratory hyperpolarization. Occasionally, a second wave of hyperpolarization occurred during late inspiration, in conjunction with increased phrenic nerve activity. 4. Spike-triggered averaging revealed averaged inhibitory postsynaptic potentials (IPSPs), indicative of inhibitory synaptic connections, between eight and sixty-three pairs of RFN inspiratory and caudal expiratory neurones. 5. Inhibitory postsynaptic potentials detected by STA exhibited a relatively long latency and a slow time course. The IPSPs began, on average, 3.8 ms after an RFN action potential. The rise times, half-widths and durations of IPSPs were longer than expected for a monosynaptic somal input from myelinated axons of inspiratory RFN neurones. It is suggested that an inhibitory relay neurone in the immediate vicinity of the expiratory neurones is activated by a collateral of the RFN inspiratory neurone. 6. Retrofacial inspiratory neurones were antidromically activated only when high-intensity electrical stimulation was applied in the vicinity of caudal expiratory neurones. 7. The averaged IPSPs were preceded by diphasic and triphasic 'spike potentials'. The averaged spike potentials were highly entrained to the action potentials of RFN inspiratory neurones which triggered IPSPs. The spike potentials may be terminal potentials recorded from axons of RFN inspiratory neurones. 8. Evidence for convergence of synaptic inputs was obtained from STA tests in a caudal expiratory neurone receiving IPSPs from four RFN neurones. 9. The functional significance of this observation is discussed. We conclude that RFN inspiratory neurones exert a moderate inhibitory influence and act conjointly with other types of medullary inspiratory neurones.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton C. R., Kirkwood P. A. The effect of carbon dioxide on the tonic and the rhythmic discharges of expiratory bulbospinal neurones. J Physiol. 1979 Nov;296:291–314. doi: 10.1113/jphysiol.1979.sp013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D., Jordan D., Spyer K. M., Wood L. M. Synaptic rhythm of caudal medullary expiratory neurones during stimulation of the hypothalamic defence area of the cat. J Physiol. 1988 Nov;405:527–546. doi: 10.1113/jphysiol.1988.sp017346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D., Richter D. W. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J Physiol. 1986 Jan;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A. L., Barillot J. C. Respiratory neurons in the region of the retrofacial nucleus: pontile, medullary, spinal and vagal projections. Neurosci Lett. 1982 Aug 31;31(3):277–282. doi: 10.1016/0304-3940(82)90033-7. [DOI] [PubMed] [Google Scholar]
- Bianchi A. L., Grélot L., Iscoe S., Remmers J. E. Electrophysiological properties of rostral medullary respiratory neurones in the cat: an intracellular study. J Physiol. 1988 Dec;407:293–310. doi: 10.1113/jphysiol.1988.sp017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagnat J., Jacquin T., Richter D. W. Voltage-dependent currents in neurones of the nuclei of the solitary tract of rat brainstem slices. Pflugers Arch. 1986 Apr;406(4):372–379. doi: 10.1007/BF00590939. [DOI] [PubMed] [Google Scholar]
- Cohen M. I., Feldman J. L. Discharge properties of dorsal medullary inspiratory neurons: relation to pulmonary afferent and phrenic efferent discharge. J Neurophysiol. 1984 Apr;51(4):753–776. doi: 10.1152/jn.1984.51.4.753. [DOI] [PubMed] [Google Scholar]
- Cohen M. I., Feldman J. L., Sommer D. Caudal medullary expiratory neurone and internal intercostal nerve discharges in the cat: effects of lung inflation. J Physiol. 1985 Nov;368:147–178. doi: 10.1113/jphysiol.1985.sp015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. I. Synchronization of discharge, spontaneous and evoked, between inspiratory neurons. Acta Neurobiol Exp (Wars) 1973;33(1):189–218. [PubMed] [Google Scholar]
- Dick T. E., Viana F., Berger A. J. Electrophysiological determination of the axonal projections of single dorsal respiratory group neurons to the cervical spinal cord of cat. Brain Res. 1988 Jun 28;454(1-2):31–39. doi: 10.1016/0006-8993(88)90800-1. [DOI] [PubMed] [Google Scholar]
- Ezure K., Manabe M. Decrementing expiratory neurons of the Bötzinger complex. II. Direct inhibitory synaptic linkage with ventral respiratory group neurons. Exp Brain Res. 1988;72(1):159–166. doi: 10.1007/BF00248511. [DOI] [PubMed] [Google Scholar]
- Fedorko L., Merrill E. G. Axonal projections from the rostral expiratory neurones of the Bötzinger complex to medulla and spinal cord in the cat. J Physiol. 1984 May;350:487–496. doi: 10.1113/jphysiol.1984.sp015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko L., Merrill E. G., Lipski J. Two descending medullary inspiratory pathways to phrenic motoneurones. Neurosci Lett. 1983 Dec 30;43(2-3):285–291. doi: 10.1016/0304-3940(83)90202-1. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Speck D. F. Interactions among inspiratory neurons in dorsal and ventral respiratory groups in cat medulla. J Neurophysiol. 1983 Feb;49(2):472–490. doi: 10.1152/jn.1983.49.2.472. [DOI] [PubMed] [Google Scholar]
- HABER E., KOHN K. W., NGAI S. H., HOLADAY D. A., WANG S. C. Localization of spontaneous respiratory neuronal activities in the medulla oblongata of the cat: a new location of the expiratory center. Am J Physiol. 1957 Aug;190(2):350–355. doi: 10.1152/ajplegacy.1957.190.2.350. [DOI] [PubMed] [Google Scholar]
- Hilaire G., Monteau R., Bianchi A. L. A cross-correlation study of interactions among respiratory neurons of dorsal, ventral and retrofacial groups in cat medulla. Brain Res. 1984 Jun 4;302(1):19–31. doi: 10.1016/0006-8993(84)91281-2. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol. 1972 May;222(3):623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Proceedings: Monosynaptic excitation of thoracic expiratory motoneurones from lateral respiratory neurones in the medulla of the cat. J Physiol. 1973 Oct;234(2):87P–89P. [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Tuck D. L., Westgaard R. H. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:105–135. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter F., Richter D. W., Camerer H., Senekowitsch R. Morphological and electrical description of medullary respiratory neurons of the cat. Pflugers Arch. 1977 Nov 25;372(1):7–16. doi: 10.1007/BF00582200. [DOI] [PubMed] [Google Scholar]
- Lipski J., Merrill E. G. Electrophysiological demonstration of the projection from expiratory neurones in rostral medulla to contralateral dorsal respiratory group. Brain Res. 1980 Sep 22;197(2):521–524. doi: 10.1016/0006-8993(80)91140-3. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Fedorko L. Monosynaptic inhibition of phrenic motoneurons: a long descending projection from Bötzinger neurons. J Neurosci. 1984 Sep;4(9):2350–2353. doi: 10.1523/JNEUROSCI.04-09-02350.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill E. G., Lipski J. Inputs to intercostal motoneurons from ventrolateral medullary respiratory neurons in the cat. J Neurophysiol. 1987 Jun;57(6):1837–1853. doi: 10.1152/jn.1987.57.6.1837. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Lipski J., Kubin L., Fedorko L. Origin of the expiratory inhibition of nucleus tractus solitarius inspiratory neurones. Brain Res. 1983 Mar 14;263(1):43–50. doi: 10.1016/0006-8993(83)91198-8. [DOI] [PubMed] [Google Scholar]
- Meyers D. E., Snow P. J. Distribution of activity in the spinal terminations of single hair follicle afferent fibers to somatotopically identified regions of the cat spinal cord. J Neurophysiol. 1986 Oct;56(4):1022–1038. doi: 10.1152/jn.1986.56.4.1022. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A. The effect of carbon dioxide on the membrane potential of medullary respiratory neurons. Brain Res. 1974 Jul 26;75(2):345–349. doi: 10.1016/0006-8993(74)90759-8. [DOI] [PubMed] [Google Scholar]
- Otake K., Sasaki H., Mannen H., Ezure K. Morphology of expiratory neurons of the Bötzinger complex: an HRP study in the cat. J Comp Neurol. 1987 Apr 22;258(4):565–579. doi: 10.1002/cne.902580407. [DOI] [PubMed] [Google Scholar]
- Rapoport S., Susswein A., Uchino Y., Wilson V. J. Synaptic actions of individual vestibular neurones on cat neck motoneurones. J Physiol. 1977 Nov;272(2):367–382. doi: 10.1113/jphysiol.1977.sp012049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. W., Ballantyne D., Remmers J. E. The differential organization of medullary post-inspiratory activities. Pflugers Arch. 1987 Nov;410(4-5):420–427. doi: 10.1007/BF00586520. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Takakusaki K., Ohta Y., Mori S. Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp Brain Res. 1989;74(1):11–23. doi: 10.1007/BF00248276. [DOI] [PubMed] [Google Scholar]
- Watt D. G., Stauffer E. K., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. J Neurophysiol. 1976 Nov;39(6):1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]


