Abstract
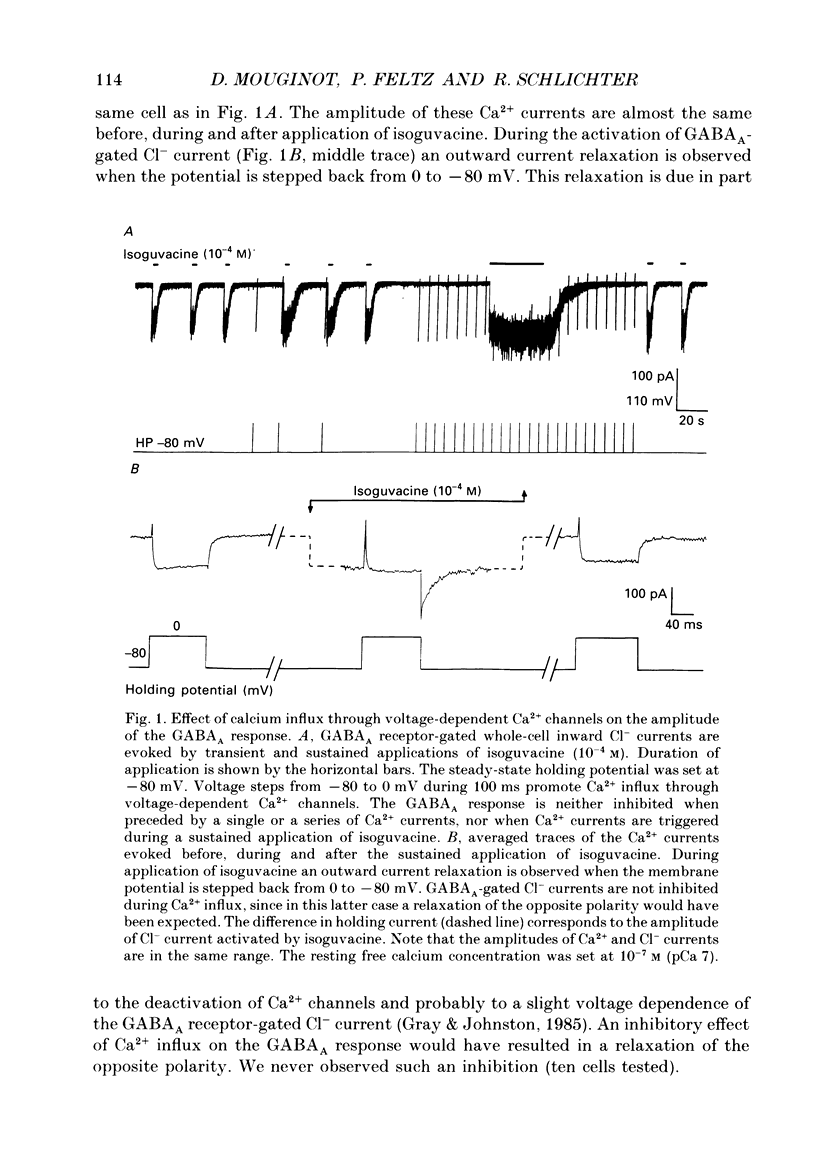
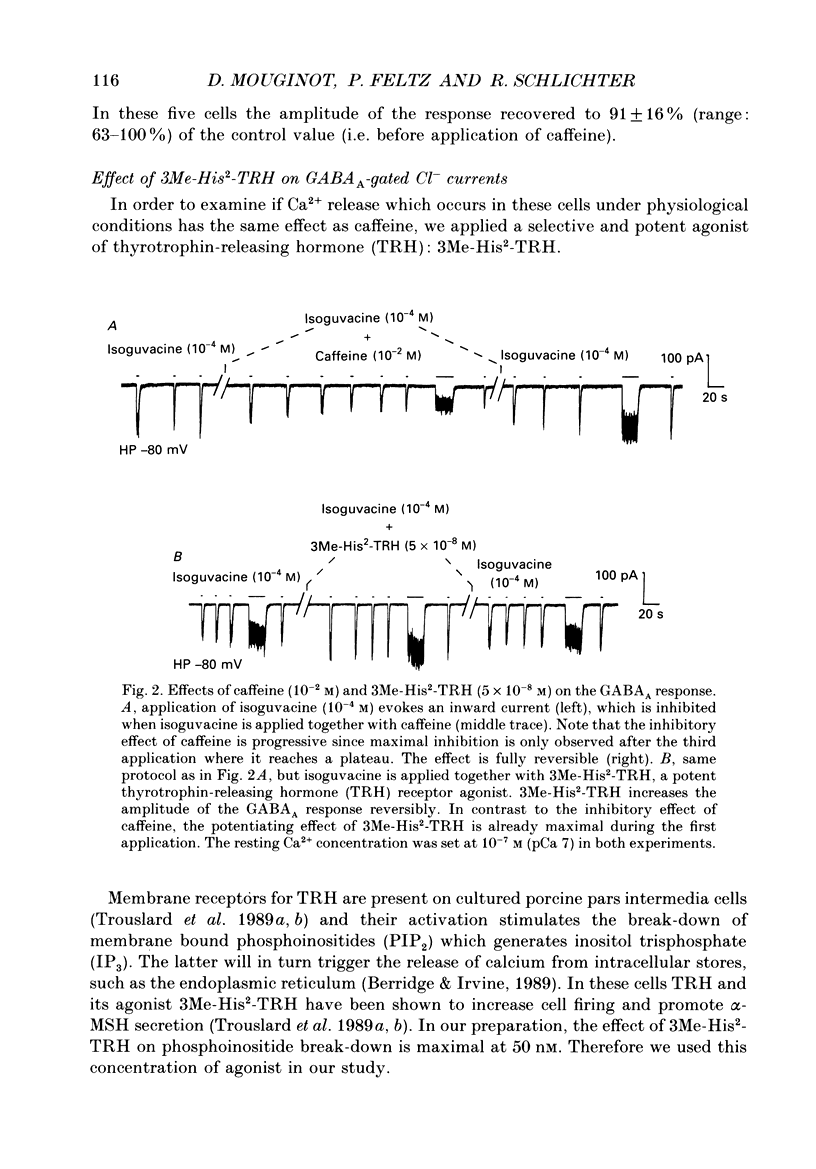
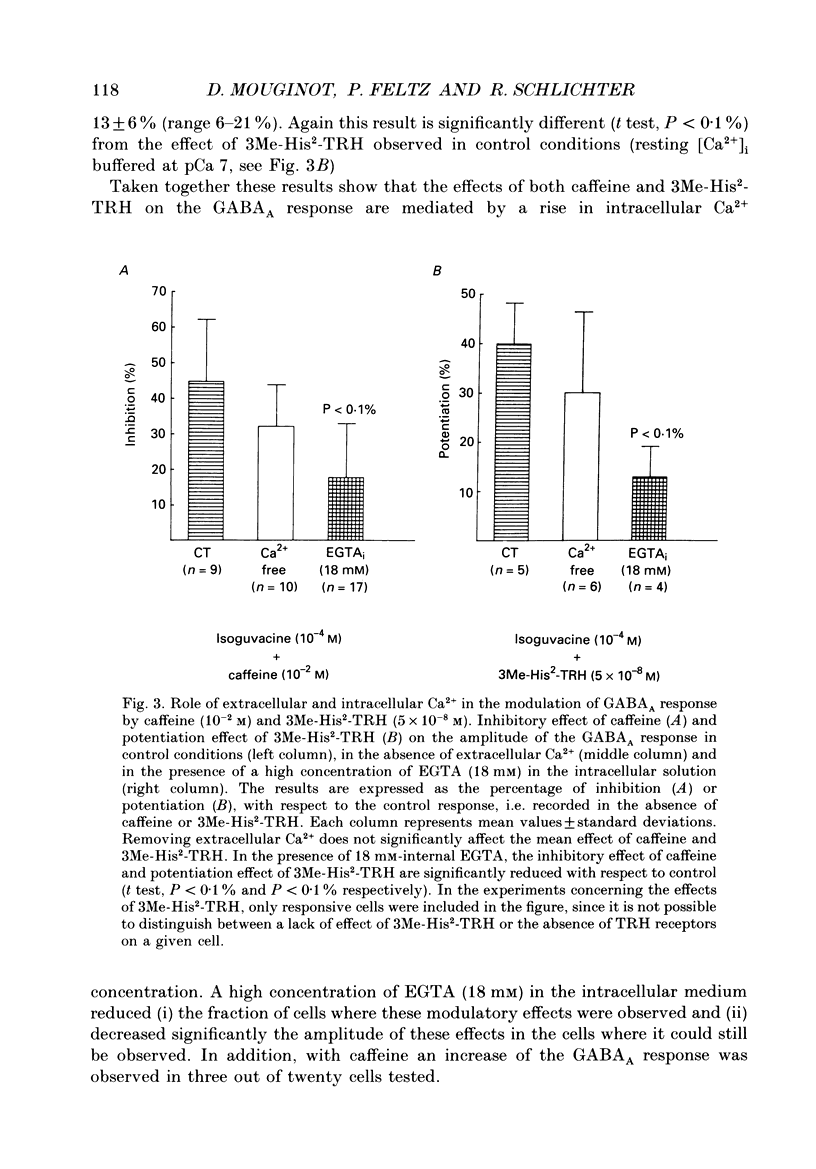
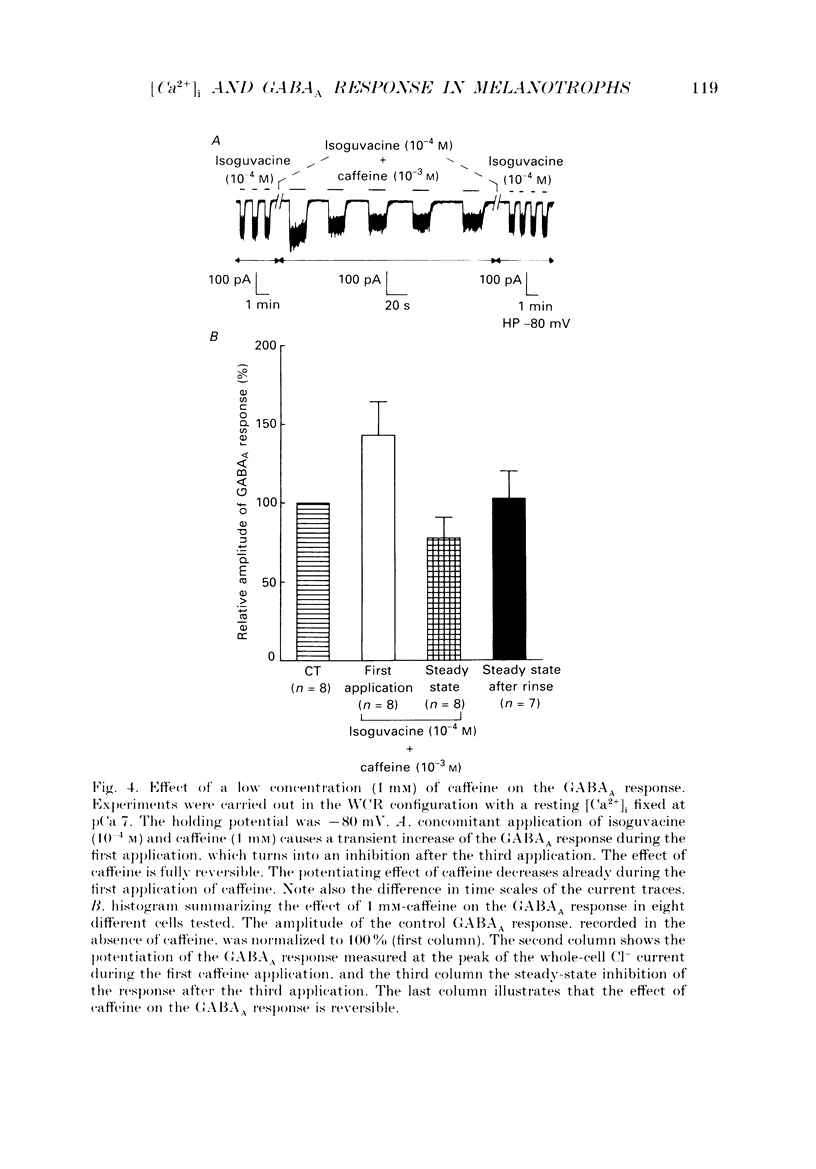
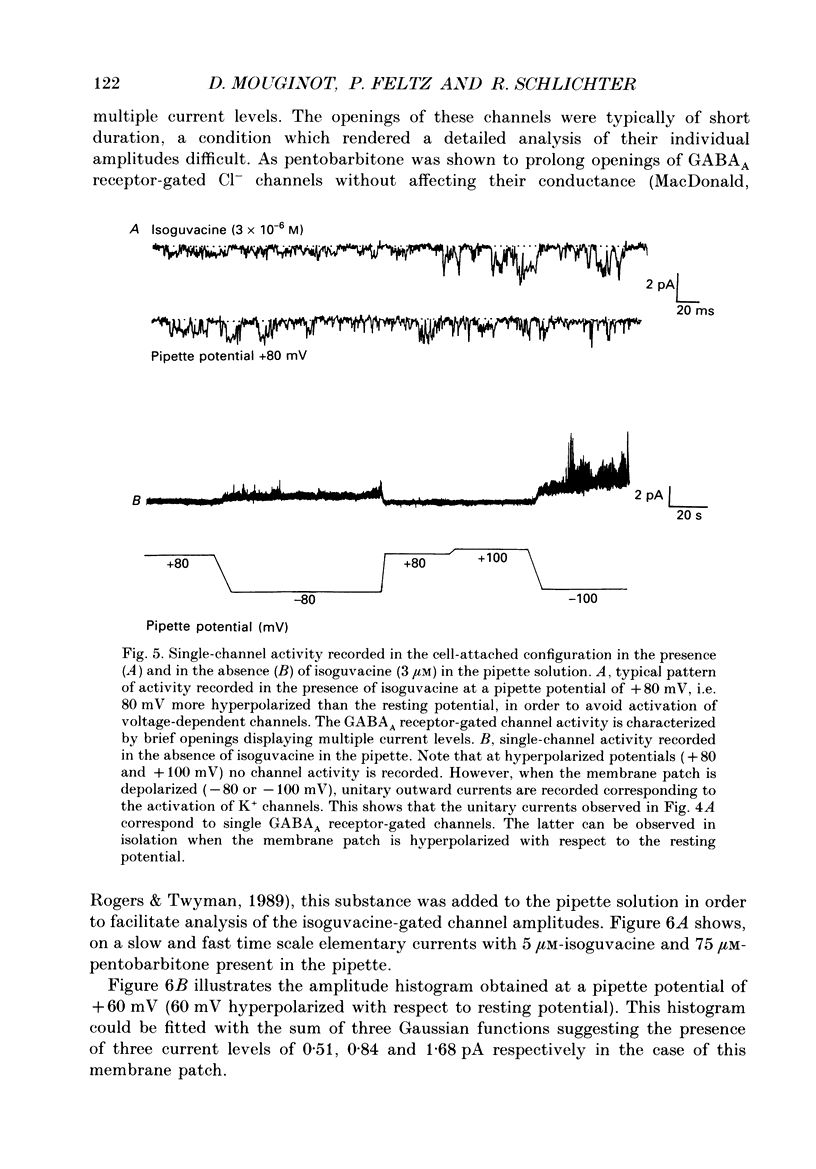
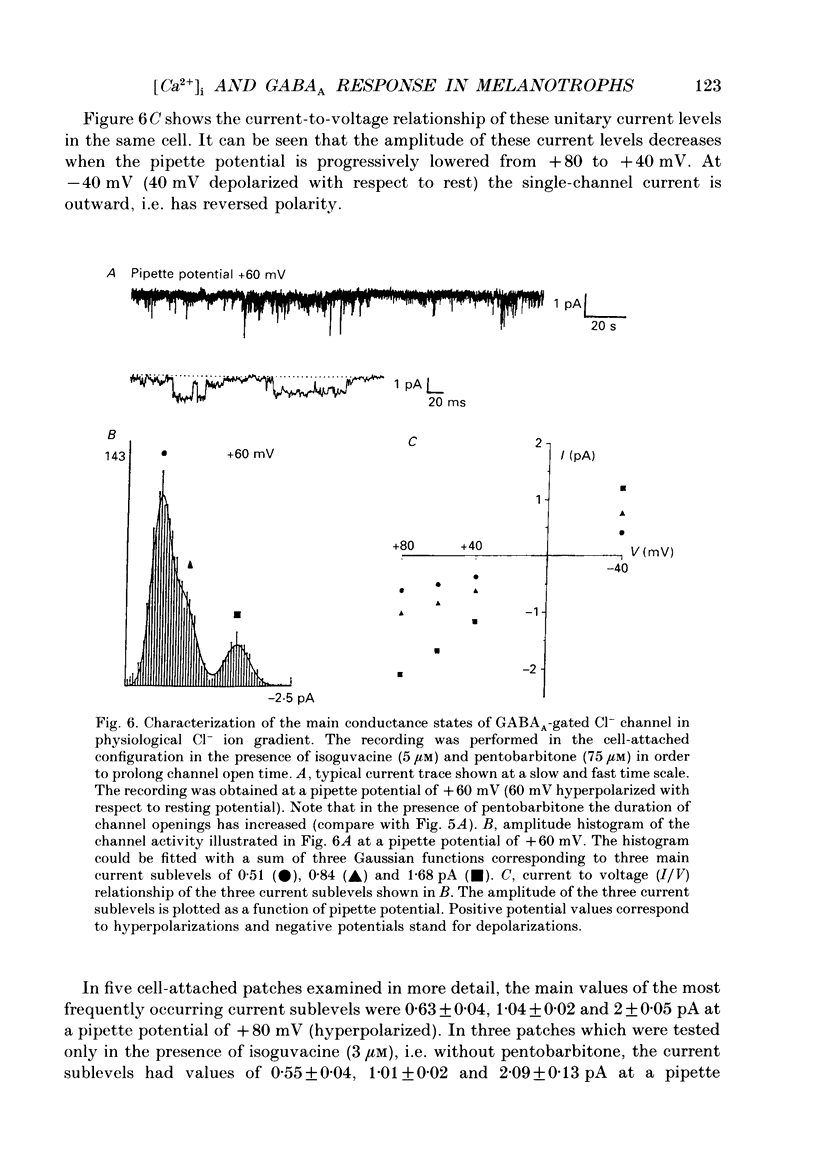
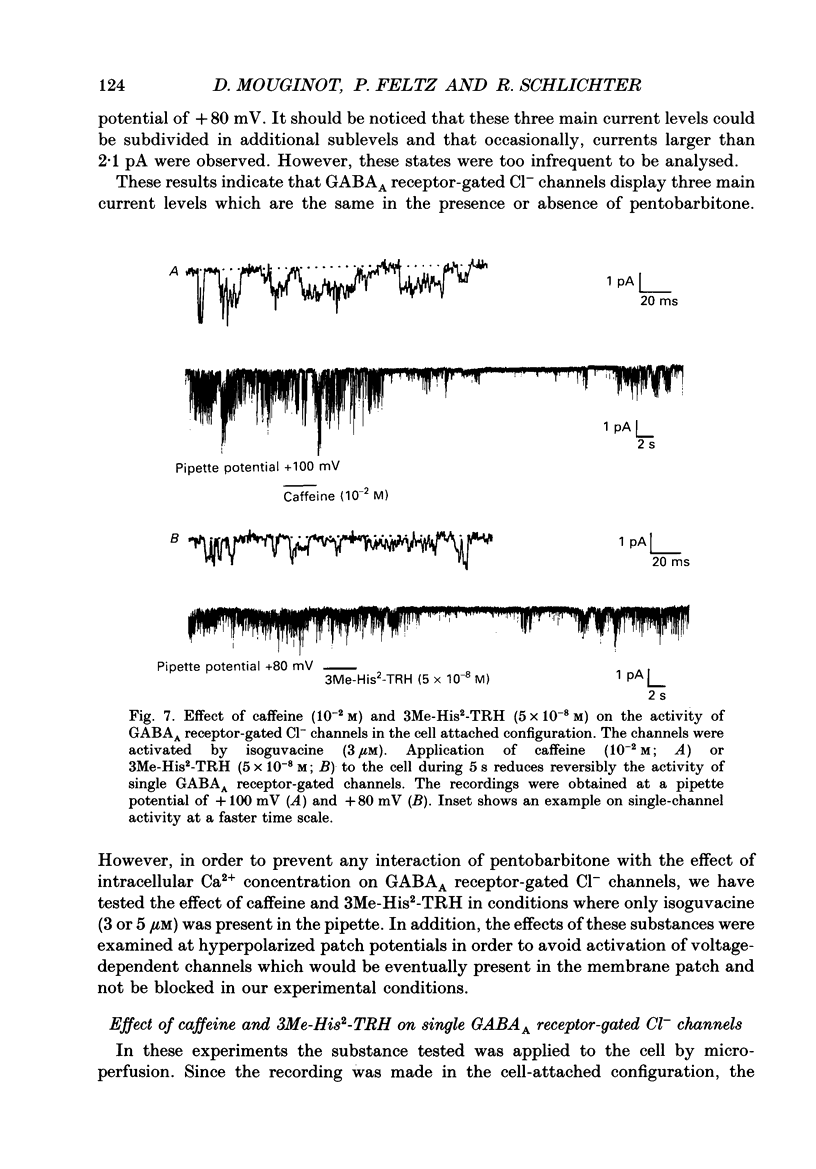
1. The modulatory role of intracellular Ca2+ concentration ([Ca2+]i) on gamma-aminobutyric acid type A (GABAA) receptor-gated Cl- currents was investigated in dialysed and intact cells of cultured porcine pituitary intermediate lobe (IL) cells using the patch-clamp technique. In order to isolate Ca2+ and Cl- currents all other membrane currents were blocked pharmacologically. Isoguvacine, a specific GABAA receptor agonist, was used to activate selectively GABAA receptor-mediated whole-cell and single-channel Cl- currents. 2. In the whole-cell recording (WCR) configuration inward Ca2+ currents triggered before and/or during the application of isoguvacine (100 microM), did not inhibit the GABAA receptor-mediated response. This lack of effect of calcium currents was obtained in all situations tested, i.e. when the intracellular Ca2+ concentration was only weakly buffered (0.5 mM-EGTA in the pipette solution), not buffered at all (no EGTA added to the pipette solution) or when the resting [Ca2+]i was buffered at 10(-7) M (pCa 7) with internal EGTA. 3. At pCa 7, simultaneous application of isoguvacine (100 microM) and caffeine (10 mM) resulted in a 47 +/- 15% reduction of the whole-cell GABAA response. In the same conditions, a ten times lower concentration of caffeine (1 mM), induced a transient increase of the GABAA response which turned into a steady-state inhibition during the subsequent applications. 4. At pCa 7, when isoguvacine (100 microM) was applied together with 3Me-His2-TRH (50 nM), a potent analogue of the calcium-recruiting thyrotrophin-releasing hormone, the GABAA receptor-gated Cl- current was increased by 40 +/- 8%. In the absence of the Ca2+ chelator EGTA in the pipette solution, either potentiating or inhibitory effects of 3Me-His2-TRH on the GABAA response were observed. 5. If a high concentration (18 mM) of the calcium chelator EGTA was included in the pipette solution, caffeine and 3Me-His2-TRH had markedly lower effects on the GABAA response than those observed at pCa 7, suggesting that the effect of both substances was mediated by an increase in [Ca2+]i. 6. In the absence of extracellular Ca2+, the effects of caffeine and 3Me-His2-TRH were not significantly different from those obtained in the presence of Ca2+ (5 mM), suggesting that Ca2+ influx was not the major route for increasing [Ca2+]i. 7. In the cell-attached (CA) configuration, the presence of isoguvacine (3-5 microM) in the pipette solution triggered the opening of channels displaying multiple current levels.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgarten H. G., Björklund A., Holstein A. F., Nobin A. Organization and ultrastructural identification of the catecholamine nerve terminals in the neural lobe and pars intermedia of the rat pituitary. Z Zellforsch Mikrosk Anat. 1972;126(4):483–517. doi: 10.1007/BF00306908. [DOI] [PubMed] [Google Scholar]
- Behrends J. C., Maruyama T., Tokutomi N., Akaike N. Ca2+-mediated suppression of the GABA-response through modulation of chloride channel gating in frog sensory neurones. Neurosci Lett. 1988 Apr 12;86(3):311–316. doi: 10.1016/0304-3940(88)90502-2. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Björklund A., Falck B., Hromek F., Owman C., West K. A. Identification and terminal distribution of the tubero-hypophyseal monoamine fibre systems in the rat by means of stereotaxic and microspectrofluorimetric techniques. Brain Res. 1970 Jan 6;17(1):1–23. doi: 10.1016/0006-8993(70)90305-7. [DOI] [PubMed] [Google Scholar]
- Chen Q. X., Stelzer A., Kay A. R., Wong R. K. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990 Jan;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote T. E., Felder R., Kebabian J. W., Sekura R. D., Reisine T., Affolter H. U. D-2 dopamine receptor-mediated inhibition of pro-opiomelanocortin synthesis in rat intermediate lobe. Abolition by pertussis toxin or activators of adenylate cyclase. J Biol Chem. 1986 Apr 5;261(10):4555–4561. [PubMed] [Google Scholar]
- Demeneix B. A., Taleb O., Loeffler J. P., Feltz P. GABAA and GABAB receptors on porcine pars intermedia cells in primary culture: functional role in modulating peptide release. Neuroscience. 1986 Apr;17(4):1275–1285. doi: 10.1016/0306-4522(86)90094-1. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S. Calcium component to action potentials in rat pars intermedia cells. J Physiol. 1980 Dec;309:623–630. doi: 10.1113/jphysiol.1980.sp013530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey E. A., Cote T. E., Grewe C. W., Kebabian J. W. [3H]spiroperidol identifies a D-2 dopamine receptor inhibiting adenylate cyclase activity in the intermediate lobe of the rat pituitary gland. Endocrinology. 1982 Jun;110(6):1897–1904. doi: 10.1210/endo-110-6-1897. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C., Thaw C. Thyrotropin-releasing hormone (TRH) stimulates biphasic elevation of cytoplasmic free calcium in GH3 cells. Further evidence that TRH mobilizes cellular and extracellular Ca2+. Endocrinology. 1985 Feb;116(2):591–596. doi: 10.1210/endo-116-2-591. [DOI] [PubMed] [Google Scholar]
- Gray R., Johnston D. Rectification of single GABA-gated chloride channels in adult hippocampal neurons. J Neurophysiol. 1985 Jul;54(1):134–142. doi: 10.1152/jn.1985.54.1.134. [DOI] [PubMed] [Google Scholar]
- Gyenes M., Farrant M., Farb D. H. "Run-down" of gamma-aminobutyric acidA receptor function during whole-cell recording: a possible role for phosphorylation. Mol Pharmacol. 1988 Dec;34(6):719–723. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Inoue M., Oomura Y., Yakushiji T., Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986 Nov 13;324(6093):156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Blocking effects of cobalt and related ions on the gamma-aminobutyric acid-induced current in turtle retinal cones. J Physiol. 1986 Apr;373:463–479. doi: 10.1113/jphysiol.1986.sp016058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Loeffler J. P., Demeneix B. A., Kley N. A., Höllt V. Dopamine inhibition of proopiomelanocortin gene expression in the intermediate lobe of the pituitary. Interactions with corticotropin-releasing factor and the beta-adrenergic receptors and the adenylate cyclase system. Neuroendocrinology. 1988 Feb;47(2):95–101. doi: 10.1159/000124898. [DOI] [PubMed] [Google Scholar]
- Louiset E., Cazin L., Lamacz M., Tonon M. C., Vaudry H. Dual effects of thyrotrophin-releasing hormone (TRH) on K+ conductance in frog pituitary melanotrophs. TRH-induced alpha-melanocyte-stimulating hormone release is not mediated through voltage-sensitive K+ channels. J Mol Endocrinol. 1989 Nov;3(3):207–218. doi: 10.1677/jme.0.0030207. [DOI] [PubMed] [Google Scholar]
- MacDonald R. L., Rogers C. J., Twyman R. E. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J Physiol. 1989 Oct;417:483–500. doi: 10.1113/jphysiol.1989.sp017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. Calcium signalling in neurons. Trends Neurosci. 1988 Oct;11(10):415–419. doi: 10.1016/0166-2236(88)90191-9. [DOI] [PubMed] [Google Scholar]
- Nemeth E. F., Taraskevich P. S., Douglas W. W. Cytosolic Ca2+ in melanotrophs: pharmacological insights into regulatory influences of electrical activity and ion channels. Endocrinology. 1990 Feb;126(2):754–758. doi: 10.1210/endo-126-2-754. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Mugnaini E., Tappaz M. L., Weise V. K., Dahl A. L., Schmechel D. E., Kopin I. J. Central GABAergic innervation of neurointermediate pituitary lobe: biochemical and immunocytochemical study in the rat. Proc Natl Acad Sci U S A. 1982 Jan;79(2):675–679. doi: 10.1073/pnas.79.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Multiple effects of caffeine on contraction and cytosolic free Ca2+ levels in vascular smooth muscle of rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):443–448. doi: 10.1007/BF00172125. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Wollheim C. B. Thyrotropin-releasing hormone increases cytosolic free Ca2+ in clonal pituitary cells (GH3 cells): direct evidence for the mobilization of cellular calcium. J Cell Biol. 1984 Jul;99(1 Pt 1):83–87. doi: 10.1083/jcb.99.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer A., Kay A. R., Wong R. K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988 Jul 15;241(4863):339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Slater N. T., ten Bruggencate G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature. 1987 Apr 16;326(6114):698–701. doi: 10.1038/326698a0. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Wong R. K. GABAA responses in hippocampal neurons are potentiated by glutamate. Nature. 1989 Jan 12;337(6203):170–173. doi: 10.1038/337170a0. [DOI] [PubMed] [Google Scholar]
- Taleb O., Loeffler J. P., Trouslard J., Demeneix B. A., Kley N., Hollt V., Feltz P. Ionic conductances related to GABA action on secretory and biosynthetic activity of pars intermedia cells. Brain Res Bull. 1986 Nov;17(5):725–730. doi: 10.1016/0361-9230(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Taleb O., Trouslard J., Demeneix B. A., Feltz P., Bossu J. L., Dupont J. L., Feltz A. Spontaneous and GABA-evoked chloride channels on pituitary intermediate lobe cells and their internal Ca requirements. Pflugers Arch. 1987 Aug;409(6):620–631. doi: 10.1007/BF00584663. [DOI] [PubMed] [Google Scholar]
- Taleb O., Trouslard J., Demeneix B. A., Feltz P. Characterization of calcium and sodium currents in porcine pars intermedia cells. Neurosci Lett. 1986 May 6;66(1):55–60. doi: 10.1016/0304-3940(86)90165-5. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Pharmacological and ionic features of gamma-aminobutyric acid receptors influencing electrical properties of melanotrophs isolated from the rat pars intermedia. Neuroscience. 1985 Jan;14(1):301–308. doi: 10.1016/0306-4522(85)90179-4. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Hirning L. D., Miller R. J. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol Pharmacol. 1988 Nov;34(5):664–673. [PubMed] [Google Scholar]
- Thayer S. A., Perney T. M., Miller R. J. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1988 Nov;8(11):4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiko S. A., Taraskevich P. S., Douglas W. W. GABA acts directly on cells of pituitary pars intermedia to alter hormone output. Nature. 1983 Feb 24;301(5902):706–707. doi: 10.1038/301706a0. [DOI] [PubMed] [Google Scholar]
- Trouslard J., Demeneix B. A., Feltz P. Spontaneous spiking activities of porcine pars intermedia cells: effects of thyrotropin-releasing hormone. Neuroendocrinology. 1989 Jul;50(1):33–43. doi: 10.1159/000125199. [DOI] [PubMed] [Google Scholar]
- Trouslard J., Loeffler J. P., Demeneix B. A., Feltz P. Thyrotropin-releasing hormone stimulates porcine melanotrope cells in primary culture. Neurosci Lett. 1989 Mar 27;98(2):234–239. doi: 10.1016/0304-3940(89)90516-8. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier J., Burgus R. Synthetic TRF (thyrotropin releasing factor) analogues. II. pGlu-N3imMe-His-Pro-NH2: a synthetic analogue with specific activity greater than that of TRF2. Endocrinology. 1971 Dec;89(6):1485–1488. doi: 10.1210/endo-89-6-1485. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Hökfelt T., Wu J. Y. GABA neuron systems in hypothalamus and the pituitary gland. Immunohistochemical demonstration using antibodies against glutamate decarboxylase. Neuroendocrinology. 1982 Feb;34(2):117–125. doi: 10.1159/000123288. [DOI] [PubMed] [Google Scholar]
- Zhang Z. W., Feltz P. Nicotinic acetylcholine receptors in porcine hypophyseal intermediate lobe cells. J Physiol. 1990 Mar;422:83–101. doi: 10.1113/jphysiol.1990.sp017974. [DOI] [PMC free article] [PubMed] [Google Scholar]


