Abstract
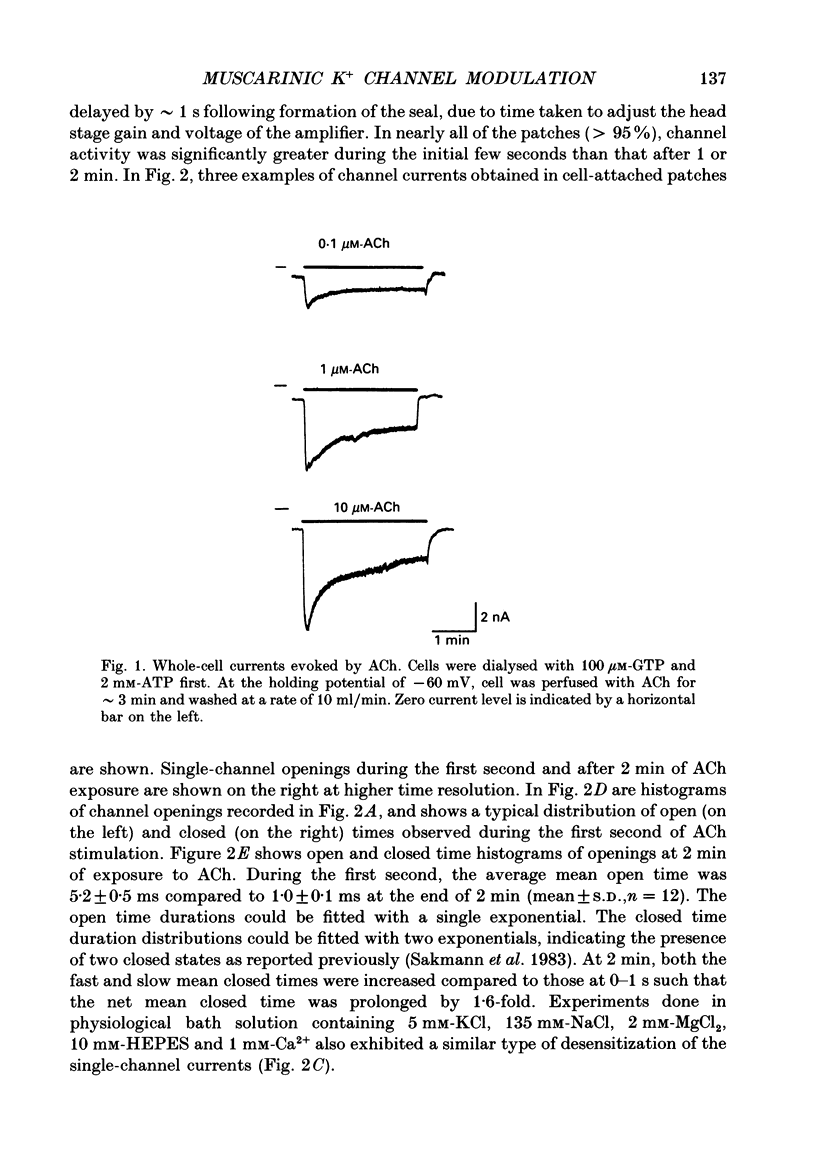
1. In voltage-clamped whole cells dialysed with GTP, extracellular application of ACh elicits an inwardly rectifying K+ current which subsequently decreases to a steady-state level well below the maximally induced current (desensitization). The mechanism of desensitization of the acetylcholine (ACh)-activated K+ channel current was studied in rat neonatal atrial cells at the single-channel level using the patch-clamp technique. 2. In cell-attached patches with ACh in the pipette, a similar pattern of K+ channel current desensitization was present. Single-channel analyses revealed that the initial rapid decrease in channel activity was associated with progressive shortening of the mean open time (tau o) and prolongation of the mean closed time (tau c) of the K+ channel. 3. In excised, inside-out patches with ACh in the pipette, GTP activated K+ channels with a tau o of approximately 1.0 ms. Addition of ATP to the cytosolic surface resulted in progressive increases in tau o (from 1 to 5 ms) and channel activity. These changes are similar but opposite in direction to those observed during the early phase of ACh-induced channel desensitization in cell-attached patches. 4. The effect of ATP on the channel kinetics was abolished in Mg(2+)-free solution AMP-PNP (adenylyl-imidodiphosphate, a non-hydrolysable analogue of ATP), ADP, CTP (cytidine triphosphate), ITP (inosine triphosphate) or UTP (uridine triphosphate) did not alter the channel kinetics, suggesting that the ATP effect on channel gating probably occurs via phosphorylation by a membrane-bound kinase. H-8 (an isoquinolinesulphonamide derivative which inhibits protein kinases A and C) failed to prevent the action of ATP on the channel. 5. The increases in tau o and channel activity produced by ATP could be completely reversed by an elevation of cytosolic [Ca2+] to 3 x 10(-5) M or above. 6. The effect of Ca2+ on the ATP-induced changes in channel kinetics was blocked by sodium vanadate, a general phosphatase inhibitor. Okadaic acid, an inhibitor of protein phosphatase 1 and 2A, did not block the Ca2+ effect. Calmodulin antagonists, N-(6-aminohexyl)-5-chloro-1-naphthalenesulphonamide (W-7), trifluoroperazine, and calmidazolium, partially blocked the effect of Ca2+. 7. Alkaline phosphatase (20 units/ml) reversed the ATP-induced increases in tau o and channel activity. These results suggest that the ACh-activated K+ channel can be modulated by phosphorylation and dephosphorylation.(ABSTRACT TRUNCATED AT 400 WORDS)
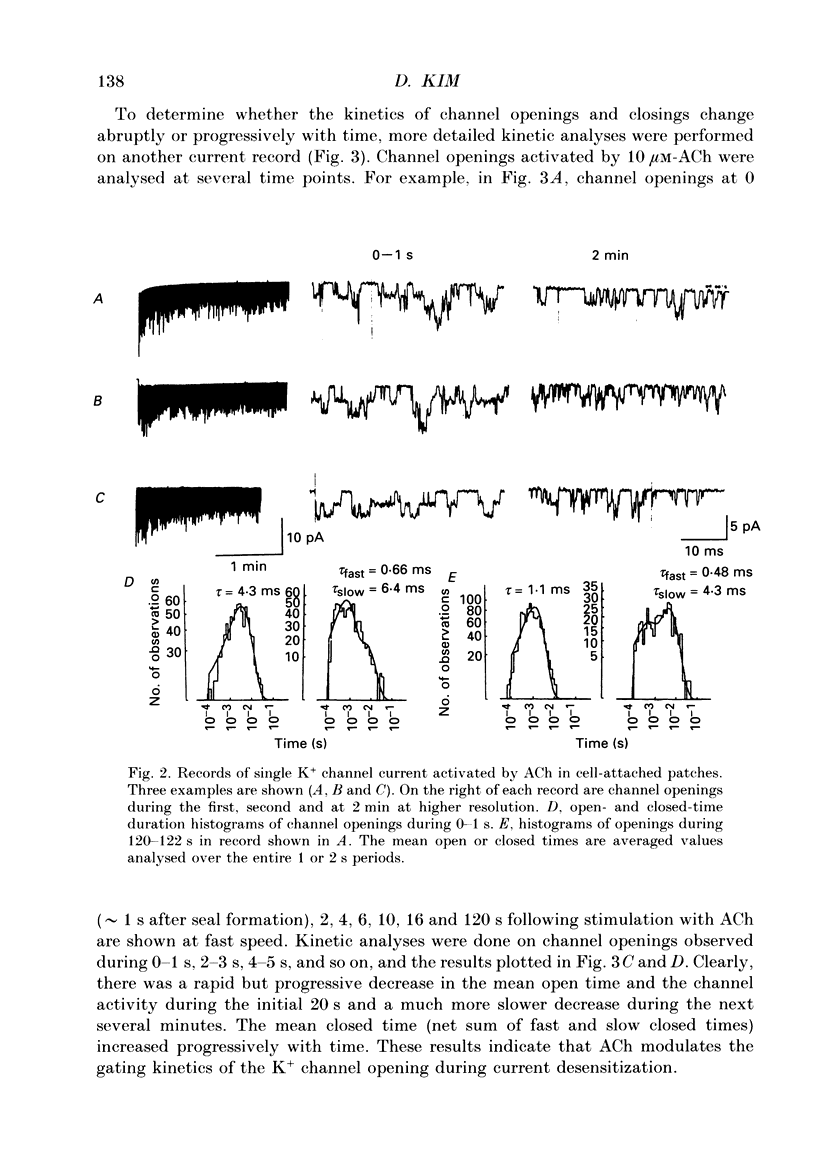
Full text
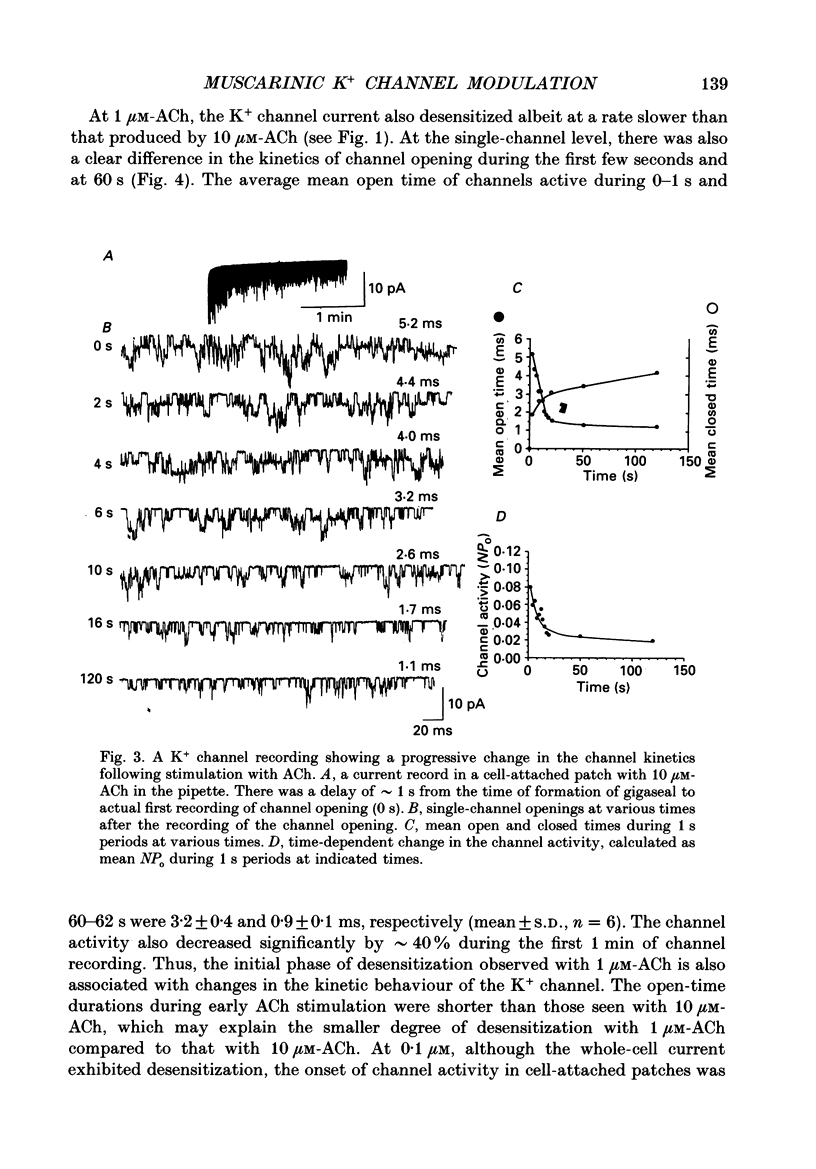
PDF



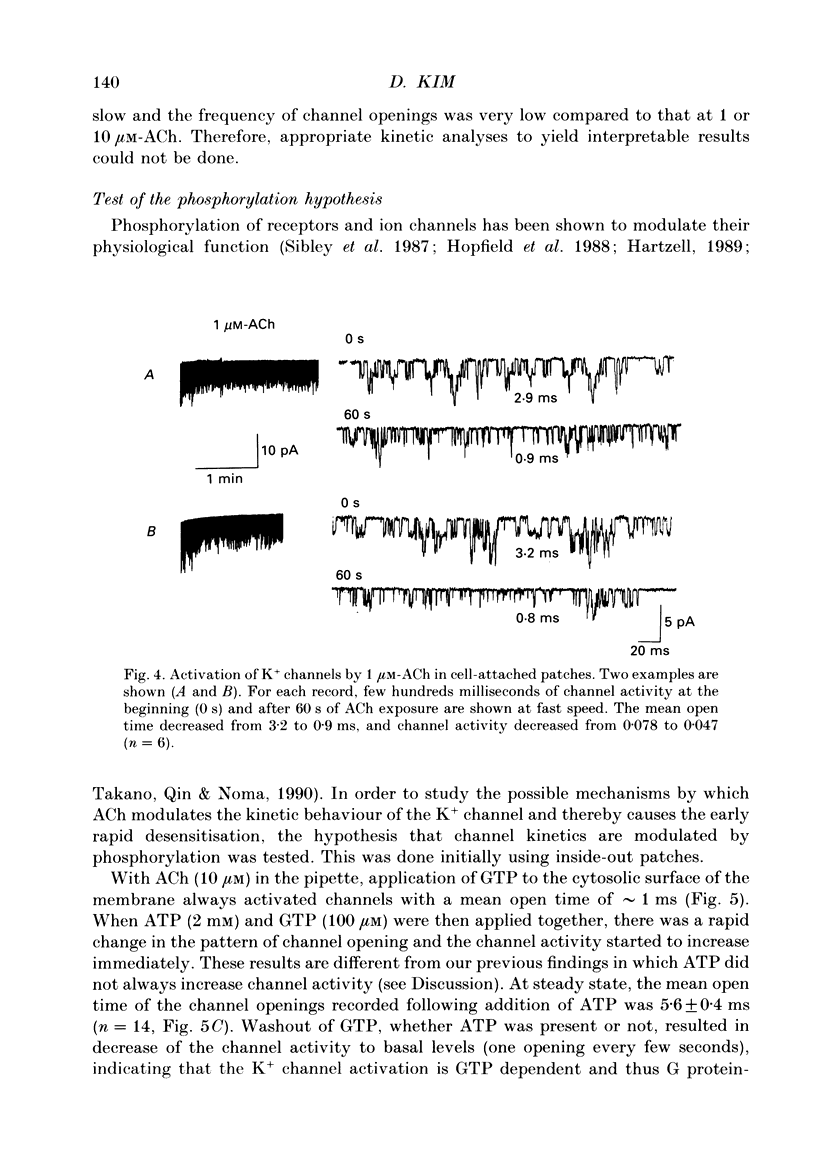
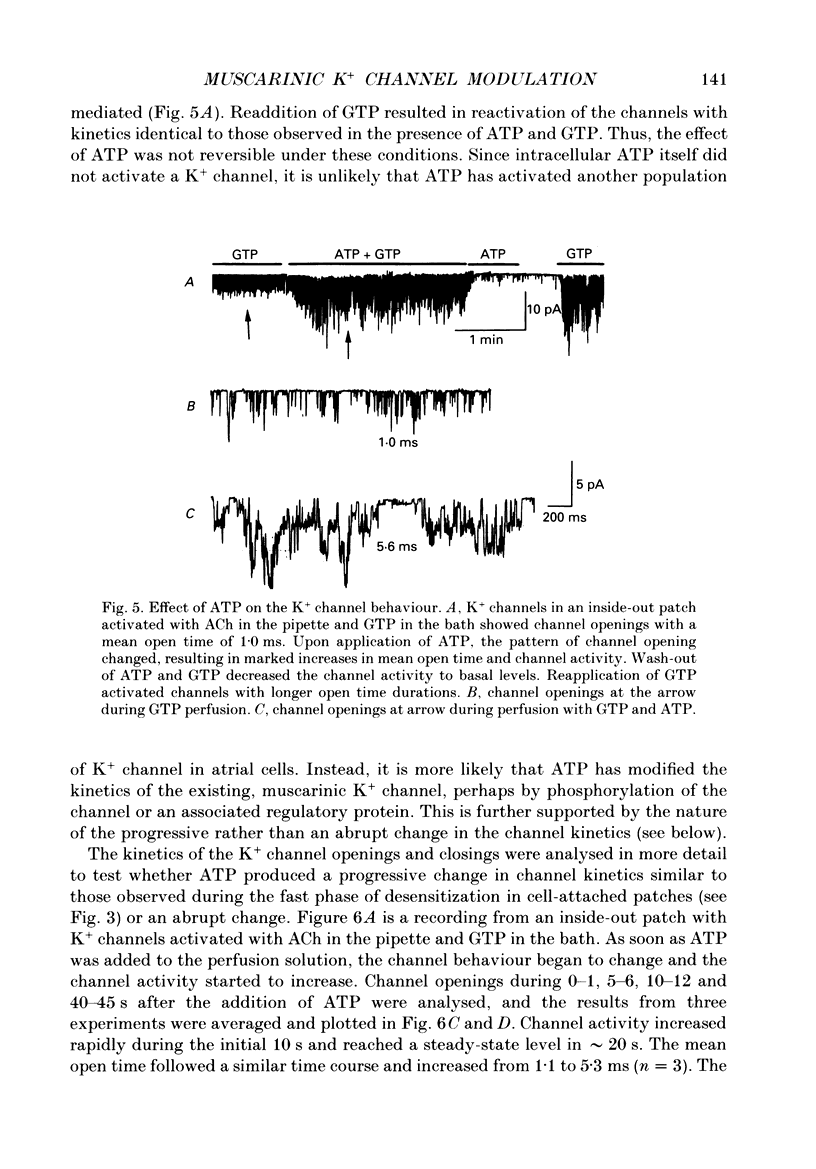
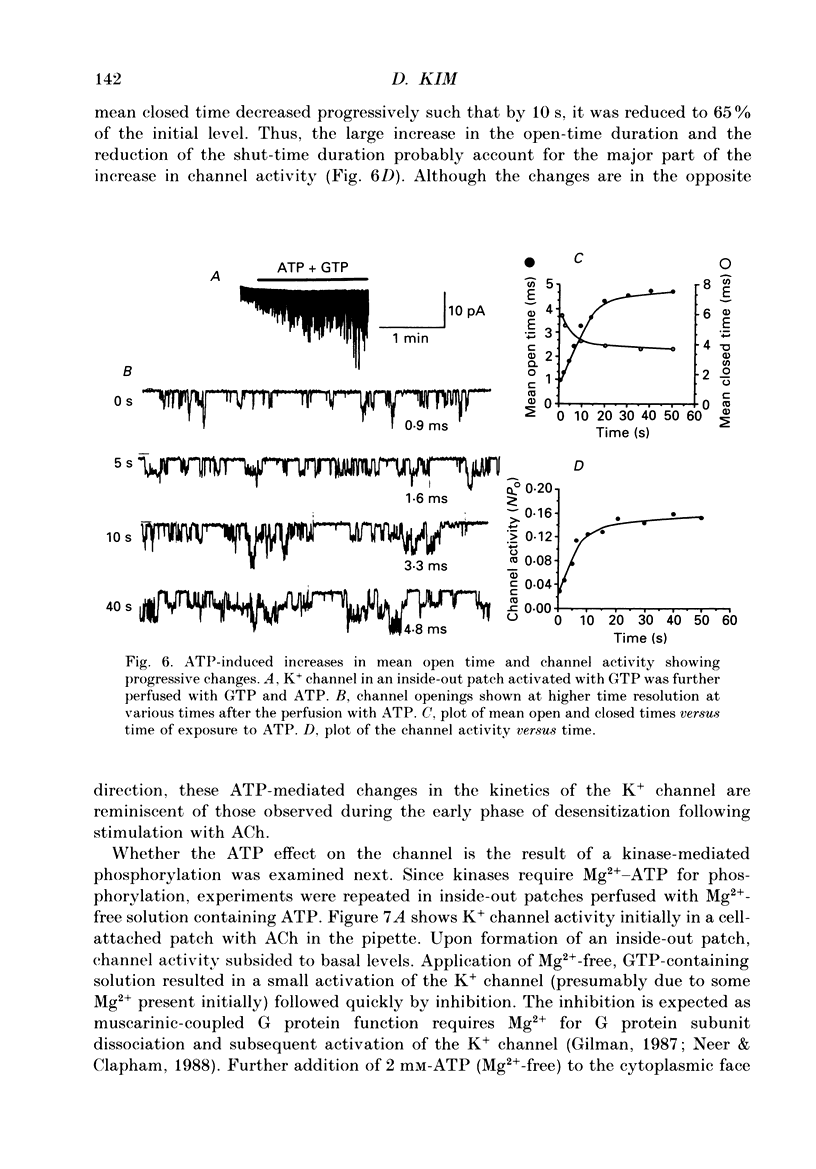
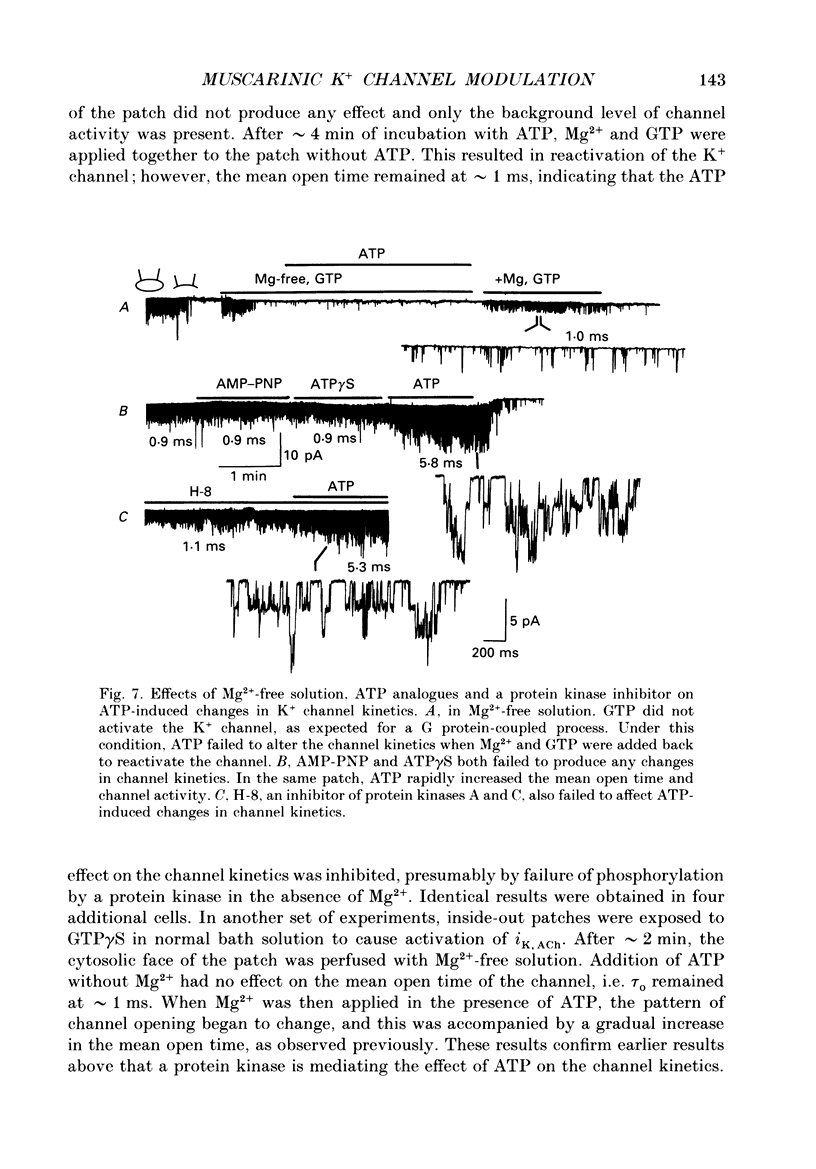
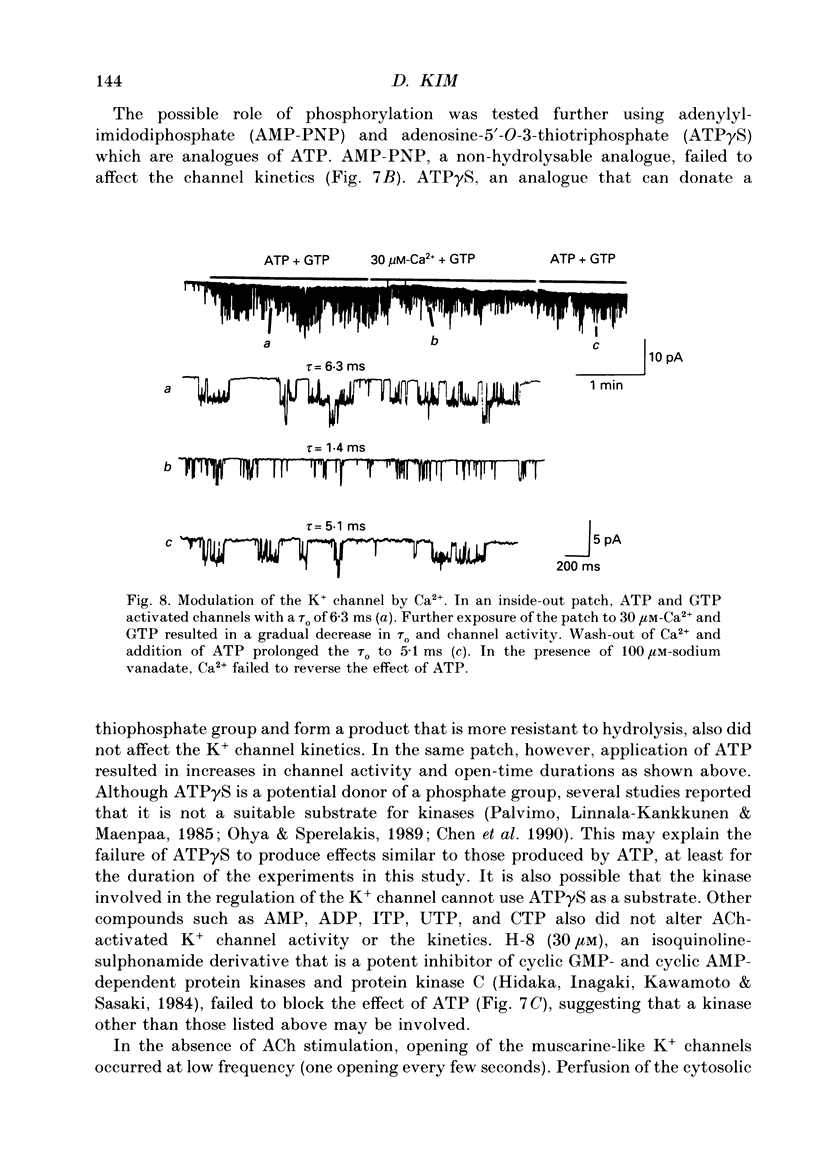







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine C. K., Bezanilla F. Phosphorylation modulates potassium conductance and gating current of perfused giant axons of squid. J Gen Physiol. 1990 Feb;95(2):245–271. doi: 10.1085/jgp.95.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic J. L., Stone W. C., Caron M. G., Lefkowitz R. J. Inhibition of the beta-adrenergic receptor kinase by polyanions. J Biol Chem. 1989 Apr 25;264(12):6707–6710. [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Carlson K. E., Brass L. F., Manning D. R. Thrombin and phorbol esters cause the selective phosphorylation of a G protein other than Gi in human platelets. J Biol Chem. 1989 Aug 5;264(22):13298–13305. [PubMed] [Google Scholar]
- Carmeliet E., Mubagwa K. Desensitization of the acetylcholine-induced increase of potassium conductance in rabbit cardiac Purkinje fibres. J Physiol. 1986 Feb;371:239–255. doi: 10.1113/jphysiol.1986.sp015971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q. X., Stelzer A., Kay A. R., Wong R. K. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990 Jan;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Kim D. G protein activation mechanisms of the cardiac K+ channel, iK.ACh. Soc Gen Physiol Ser. 1989;44:55–68. [PubMed] [Google Scholar]
- Codina J., Yatani A., Grenet D., Brown A. M., Birnbaumer L. The alpha subunit of the GTP binding protein Gk opens atrial potassium channels. Science. 1987 Apr 24;236(4800):442–445. doi: 10.1126/science.2436299. [DOI] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cohen P. Protein phosphorylation and hormone action. Proc R Soc Lond B Biol Sci. 1988 Jul 22;234(1275):115–144. doi: 10.1098/rspb.1988.0040. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gundersen R. E., Devreotes P. N. In vivo receptor-mediated phosphorylation of a G protein in Dictyostelium. Science. 1990 May 4;248(4955):591–593. doi: 10.1126/science.2110382. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Heidbüchel H., Callewaert G., Vereecke J., Carmeliet E. ATP-dependent activation of atrial muscarinic K+ channels in the absence of agonist and G-nucleotides. Pflugers Arch. 1990 Apr;416(1-2):213–215. doi: 10.1007/BF00370246. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Ho A. K., Ling Q. L., Duffield R., Lam P. H., Wang J. H. Phosphorylation of brain muscarinic receptor: evidence of receptor regulation. Biochem Biophys Res Commun. 1987 Feb 13;142(3):911–918. doi: 10.1016/0006-291x(87)91500-2. [DOI] [PubMed] [Google Scholar]
- Hopfield J. F., Tank D. W., Greengard P., Huganir R. L. Functional modulation of the nicotinic acetylcholine receptor by tyrosine phosphorylation. Nature. 1988 Dec 15;336(6200):677–680. doi: 10.1038/336677a0. [DOI] [PubMed] [Google Scholar]
- Jalife J., Hamilton A. J., Moe G. K. Desensitization of the cholinergic receptor at the sinoatrial cell of the kitten. Am J Physiol. 1980 Apr;238(4):H439–H448. doi: 10.1152/ajpheart.1980.238.4.H439. [DOI] [PubMed] [Google Scholar]
- Kim D. Beta-adrenergic regulation of the muscarinic-gated K+ channel via cyclic AMP-dependent protein kinase in atrial cells. Circ Res. 1990 Nov;67(5):1292–1298. doi: 10.1161/01.res.67.5.1292. [DOI] [PubMed] [Google Scholar]
- Kim D., Lewis D. L., Graziadei L., Neer E. J., Bar-Sagi D., Clapham D. E. G-protein beta gamma-subunits activate the cardiac muscarinic K+-channel via phospholipase A2. Nature. 1989 Feb 9;337(6207):557–560. doi: 10.1038/337557a0. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Ito H., Sugimoto T., Shimizu T., Miki I., Ui M. Arachidonic acid metabolites as intracellular modulators of the G protein-gated cardiac K+ channel. Nature. 1989 Feb 9;337(6207):555–557. doi: 10.1038/337555a0. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Acetylcholine activation of K+ channels in cell-free membrane of atrial cells. Am J Physiol. 1986 Sep;251(3 Pt 2):H681–H684. doi: 10.1152/ajpheart.1986.251.3.H681. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflugers Arch. 1987 Oct;410(3):227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- Kwatra M. M., Benovic J. L., Caron M. G., Lefkowitz R. J., Hosey M. M. Phosphorylation of chick heart muscarinic cholinergic receptors by the beta-adrenergic receptor kinase. Biochemistry. 1989 May 30;28(11):4543–4547. doi: 10.1021/bi00437a005. [DOI] [PubMed] [Google Scholar]
- Kwatra M. M., Hosey M. M. Phosphorylation of the cardiac muscarinic receptor in intact chick heart and its regulation by a muscarinic agonist. J Biol Chem. 1986 Sep 25;261(27):12429–12432. [PubMed] [Google Scholar]
- Kwatra M. M., Leung E., Maan A. C., McMahon K. K., Ptasienski J., Green R. D., Hosey M. M. Correlation of agonist-induced phosphorylation of chick heart muscarinic receptors with receptor desensitization. J Biol Chem. 1987 Dec 5;262(34):16314–16321. [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Sperelakis N. Modulation of single slow (L-type) calcium channels by intracellular ATP in vascular smooth muscle cells. Pflugers Arch. 1989 Jul;414(3):257–264. doi: 10.1007/BF00584624. [DOI] [PubMed] [Google Scholar]
- Palvimo J., Linnala-Kankkunen A., Mäenpä P. H. Thiophosphorylation and phosphorylation of chromatin proteins from calf thymus in vitro. Biochem Biophys Res Commun. 1985 Jan 16;126(1):103–108. doi: 10.1016/0006-291x(85)90577-7. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Pyne N. J., Murphy G. J., Milligan G., Houslay M. D. Treatment of intact hepatocytes with either the phorbol ester TPA or glucagon elicits the phosphorylation and functional inactivation of the inhibitory guanine nucleotide regulatory protein Gi. FEBS Lett. 1989 Jan 16;243(1):77–82. doi: 10.1016/0014-5793(89)81221-9. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Salata J. J., Jalife J. "Fade" of hyperpolarizing responses to vagal stimulation at the sinoatrial and atrioventricular nodes of the rabbit heart. Circ Res. 1985 May;56(5):718–727. doi: 10.1161/01.res.56.5.718. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987 Mar 27;48(6):913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Sweetnam P. M., Lloyd J., Gallombardo P., Malison R. T., Gallager D. W., Tallman J. F., Nestler E. J. Phosphorylation of the GABAa/benzodiazepine receptor alpha subunit by a receptor-associated protein kinase. J Neurochem. 1988 Oct;51(4):1274–1284. doi: 10.1111/j.1471-4159.1988.tb03097.x. [DOI] [PubMed] [Google Scholar]
- Takano M., Qin D. Y., Noma A. ATP-dependent decay and recovery of K+ channels in guinea pig cardiac myocytes. Am J Physiol. 1990 Jan;258(1 Pt 2):H45–H50. doi: 10.1152/ajpheart.1990.258.1.H45. [DOI] [PubMed] [Google Scholar]
- Wolf H., Hofmann F. Purification of myosin light chain kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5852–5855. doi: 10.1073/pnas.77.10.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]


