Abstract
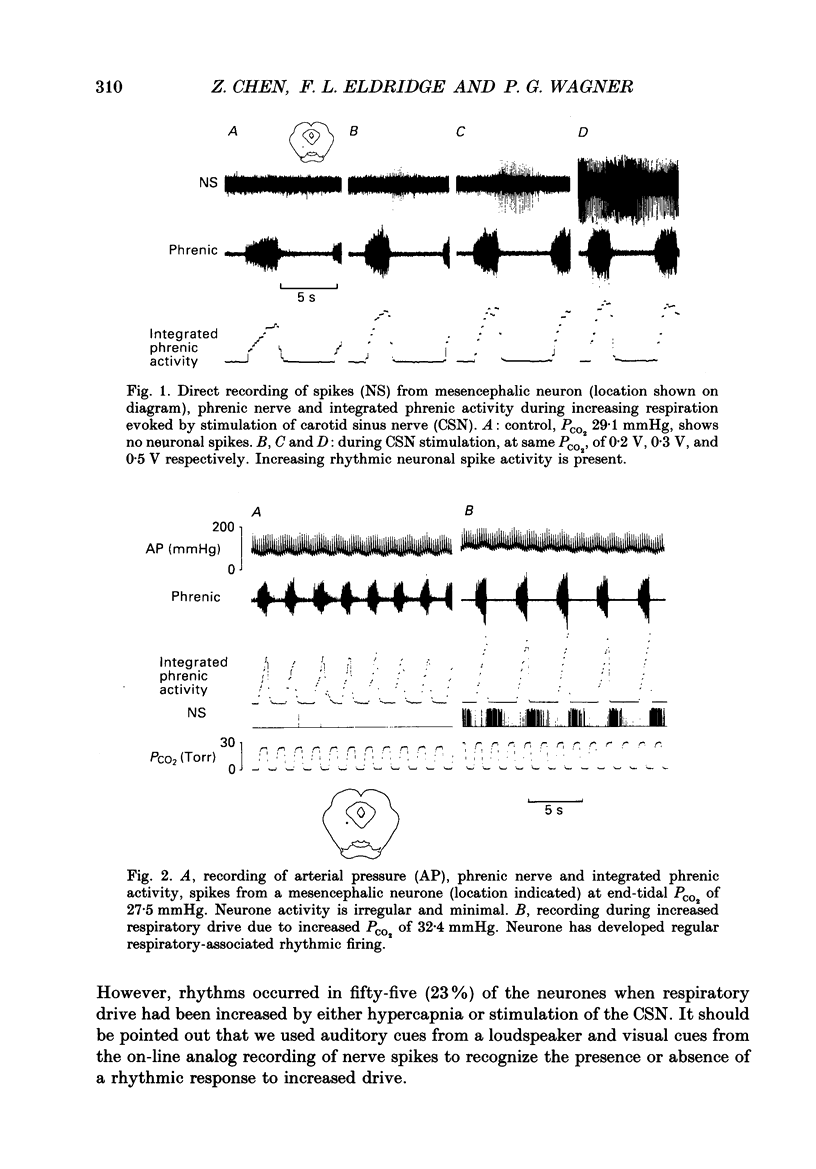
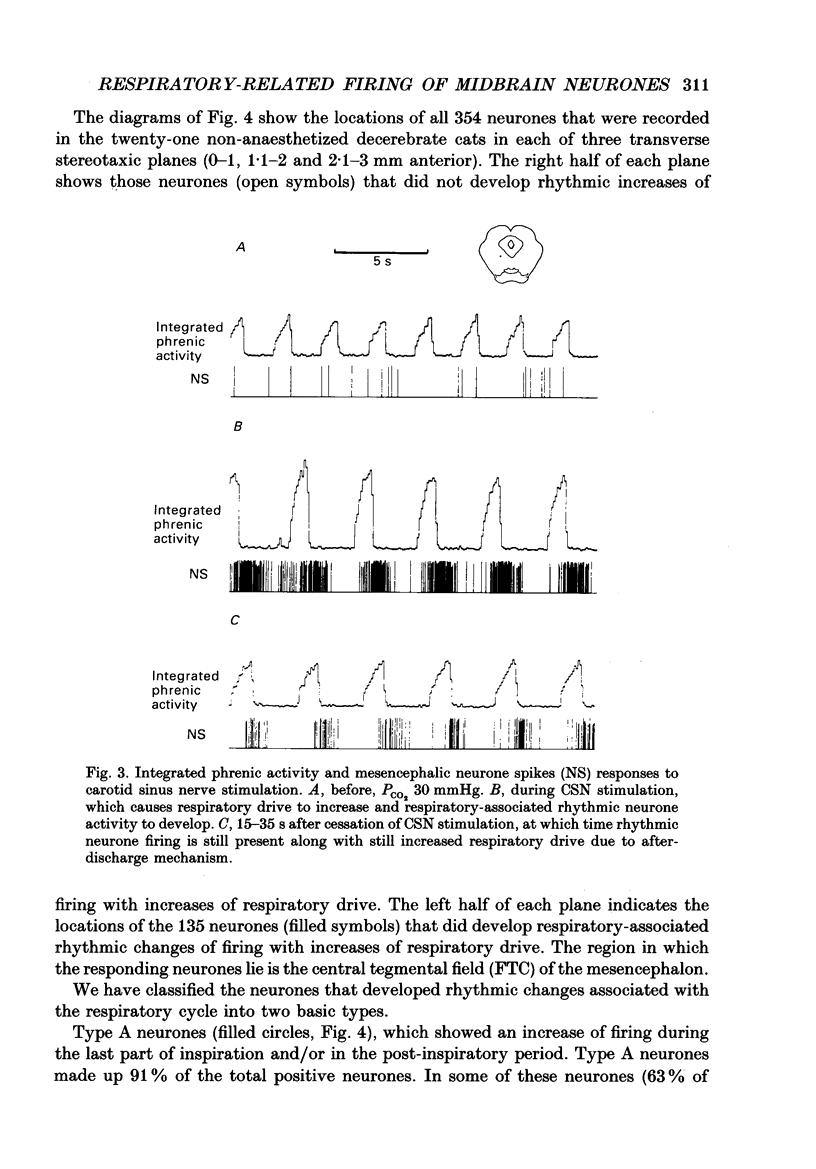
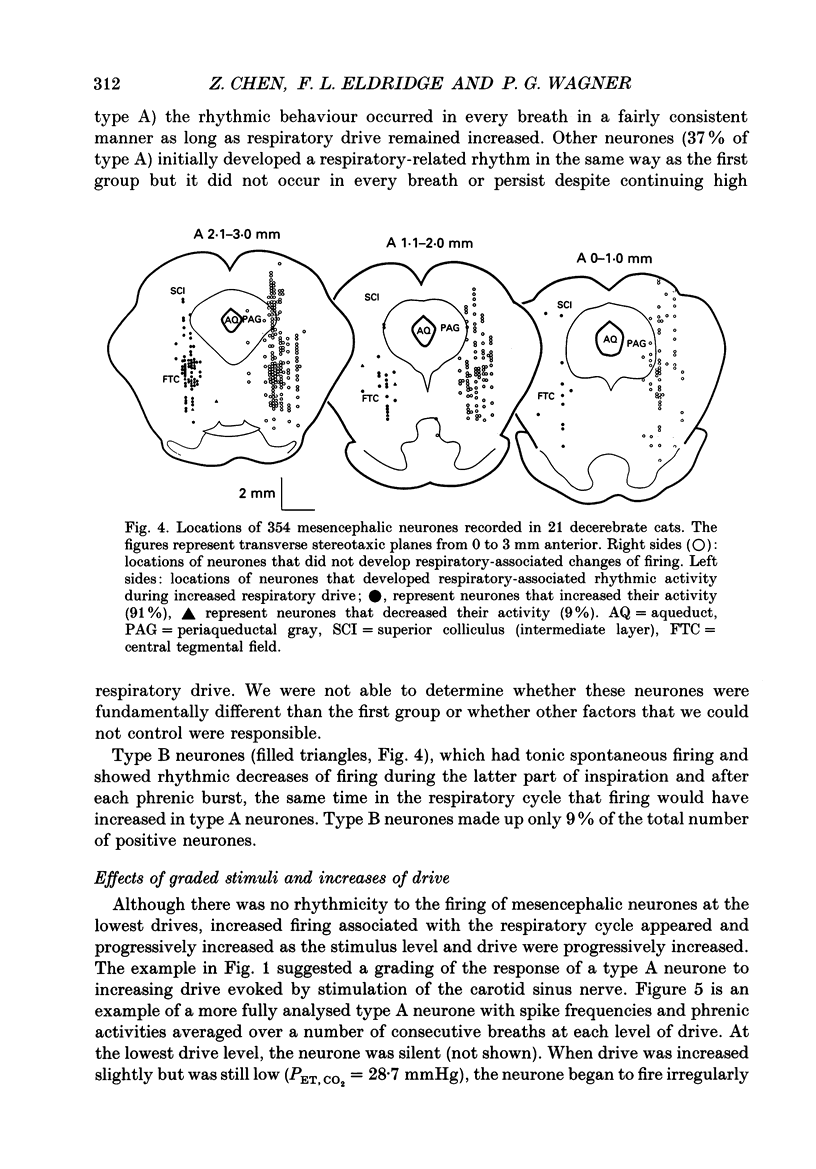
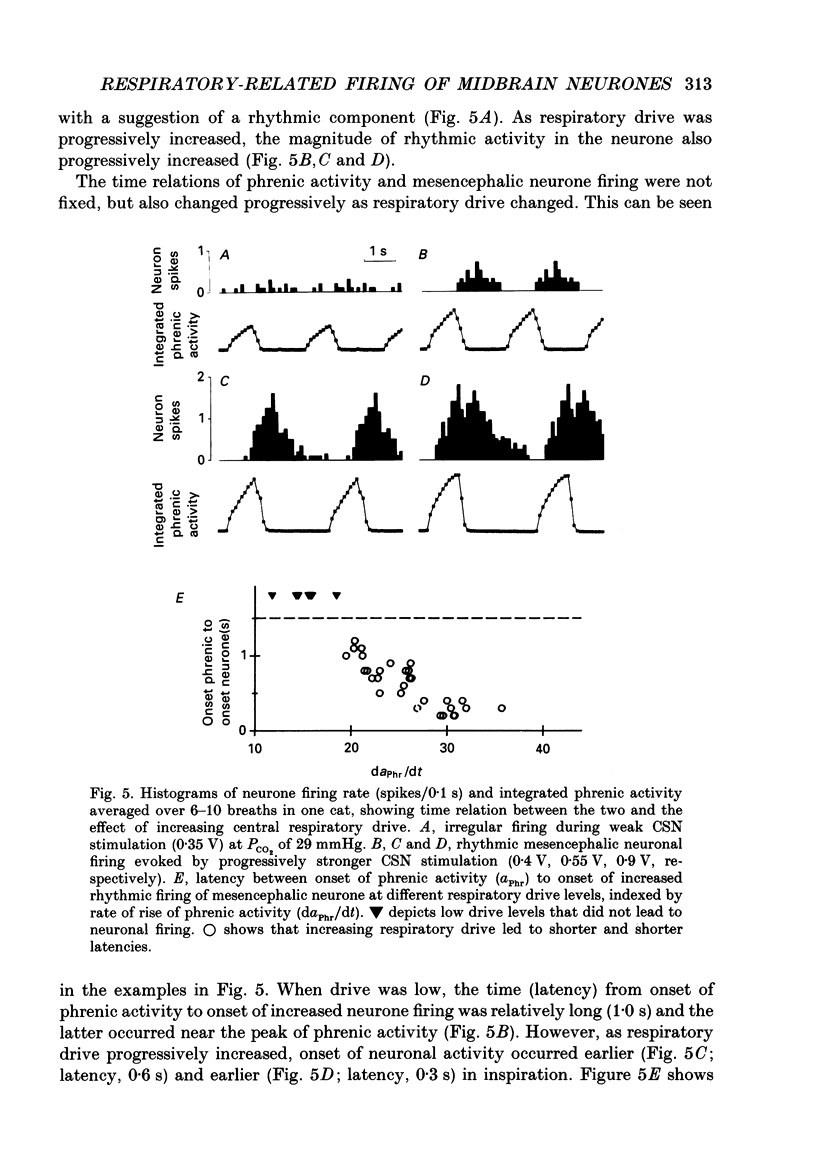
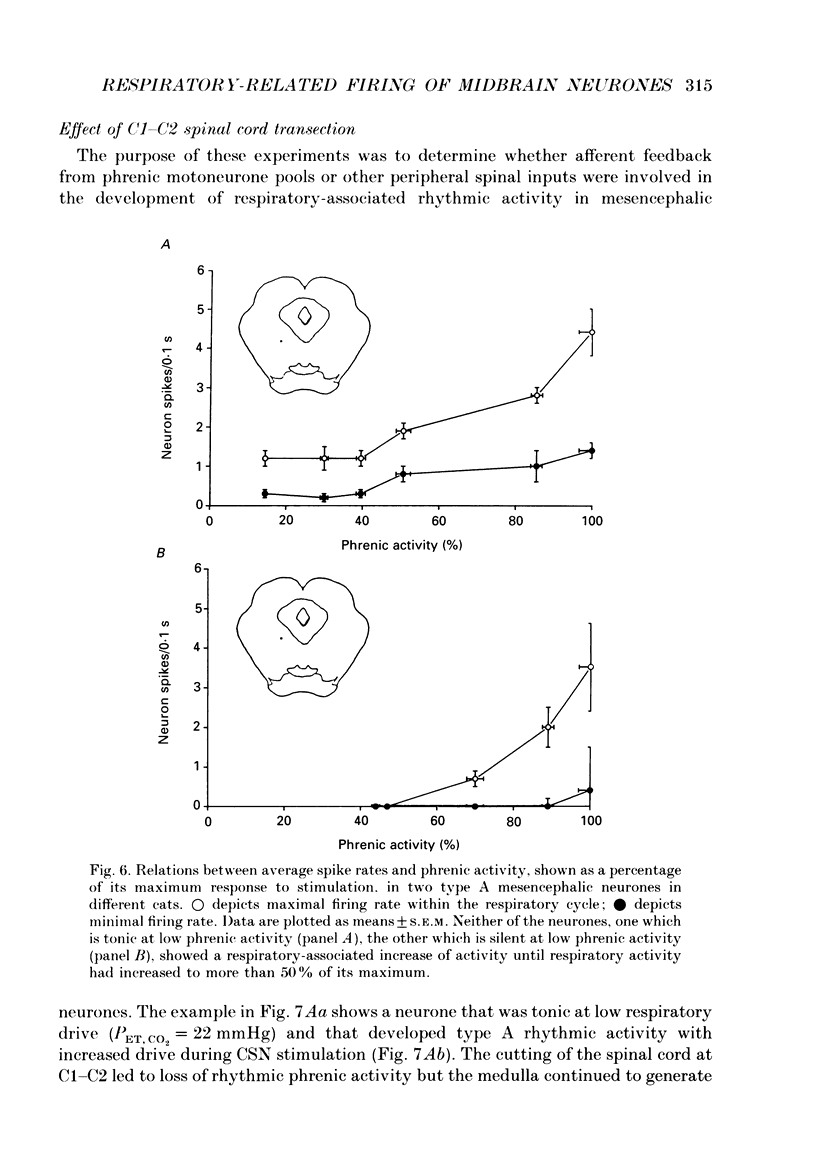
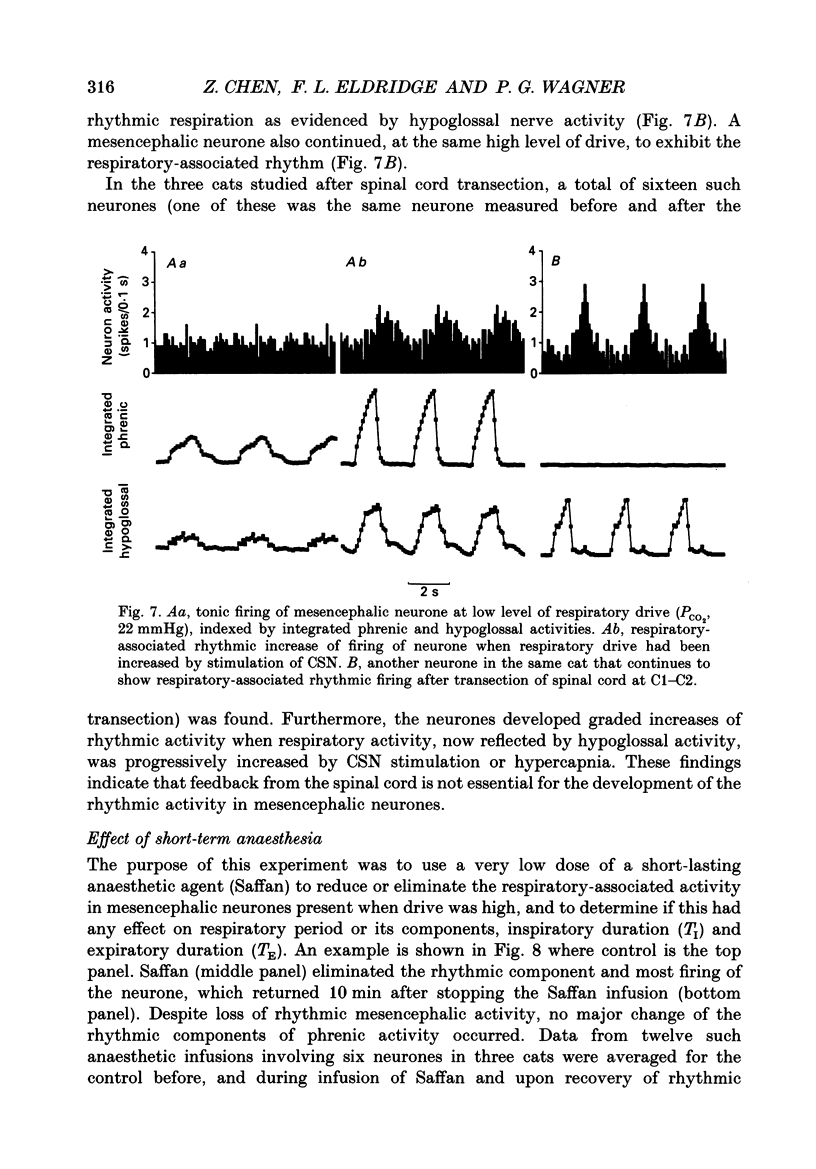
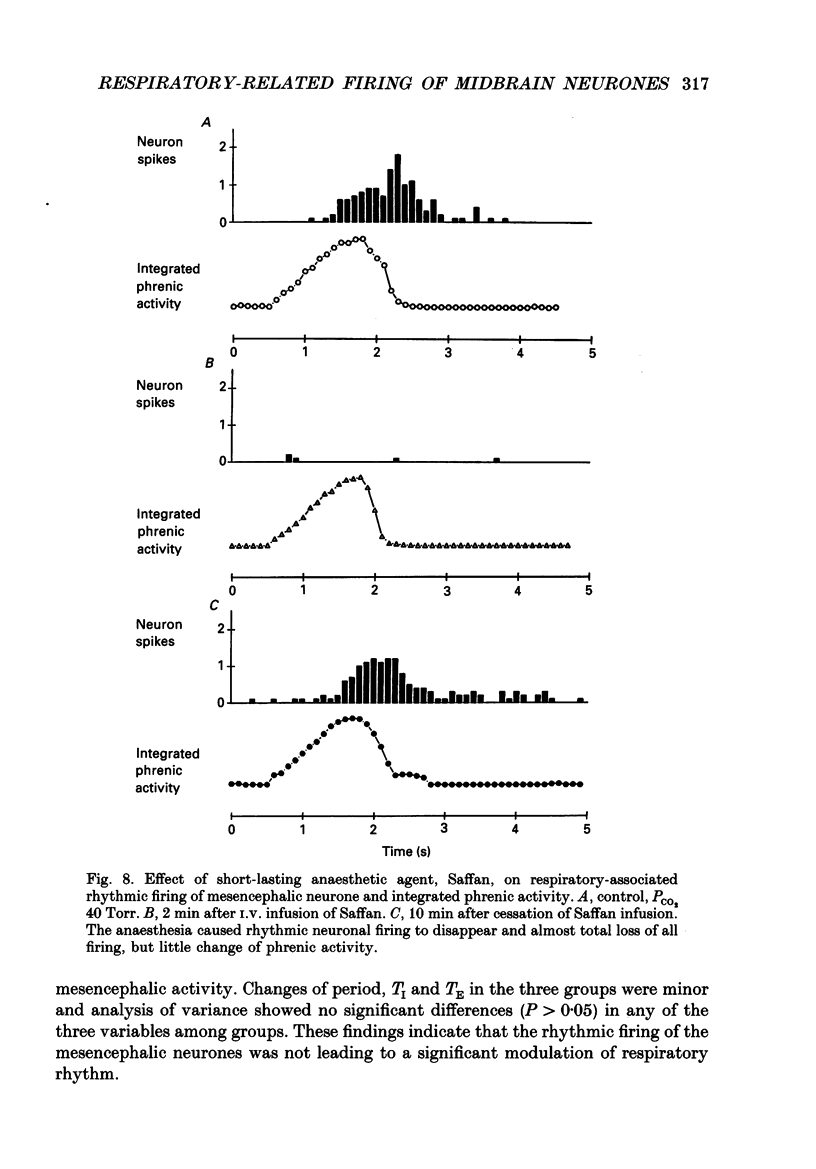
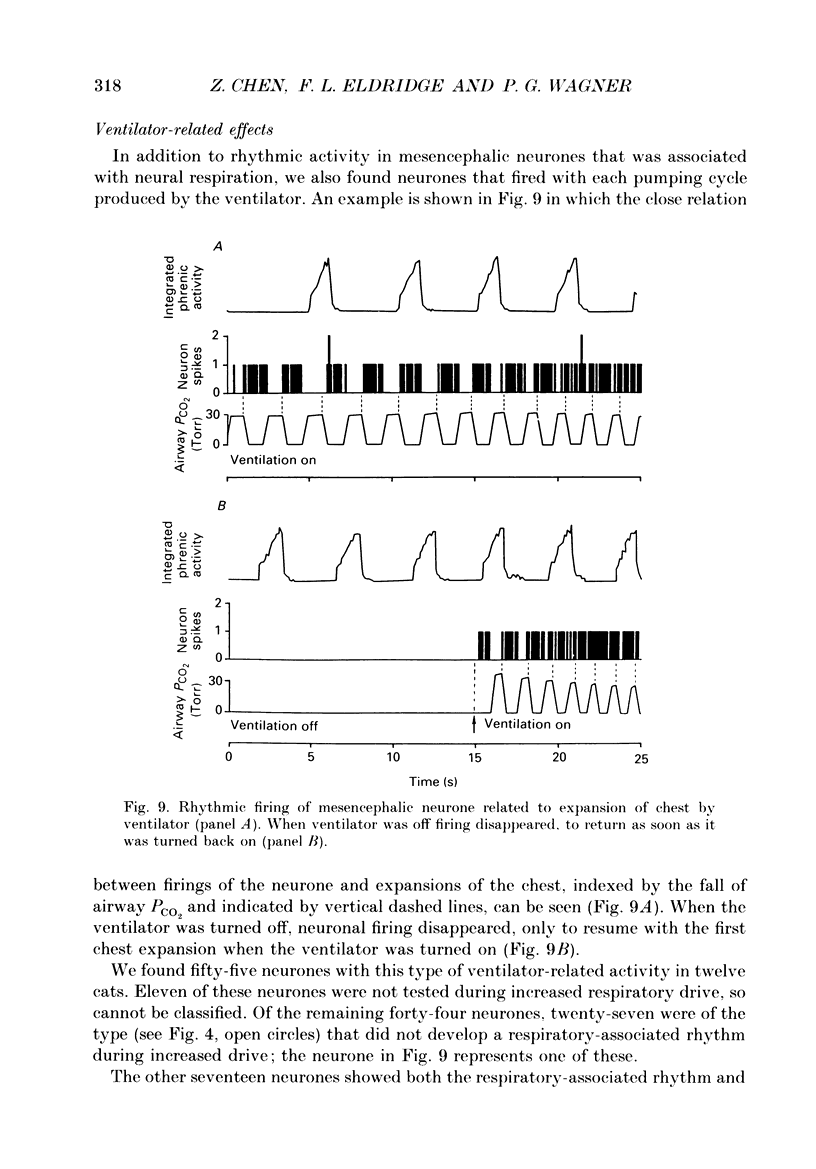
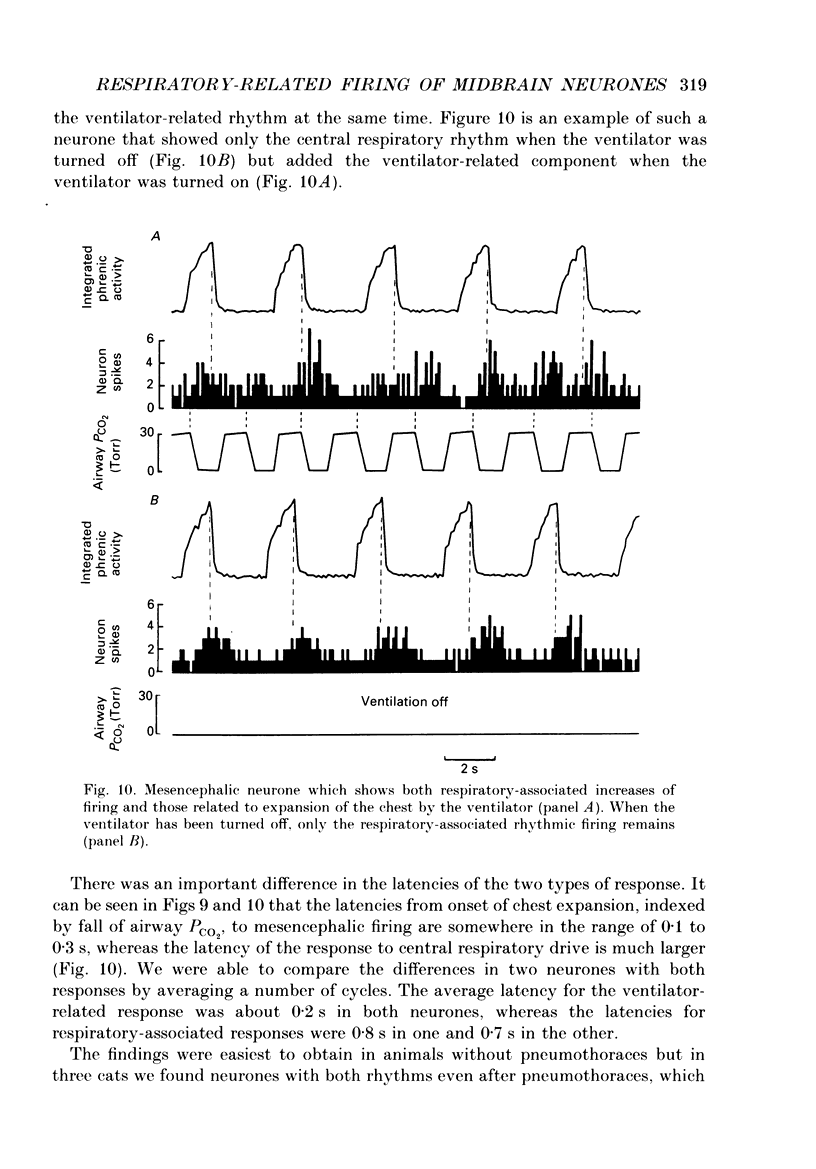
1. We recorded phrenic nerve activities and single unit firing of mesencephalic neurones in unanaesthetized supracollicularly decerebrated, paralysed and ventilated cats, in which vagi and carotid sinus nerves had been ablated. We made these measurements first at low levels of respiratory drive associated with normal PCO2 levels, then with increased respiratory drive and levels of phrenic activity produced by hypercapnia or by carotid sinus nerve stimulation. 2. We found that at least a quarter of the neurones in the central tegmental field of the mesencephalon, which were irregularly tonic or silent at low respiratory drives, developed a rhythmic increase of firing associated with each respiration. There appeared to be a threshold at about 50% of maximum respiratory activity, below which the respiratory-associated rhythm did not occur. Above this level, neuronal firing increased in graded fashion with increasing magnitude of respiratory activity. The latency from onset of phrenic activity to onset of increased neuronal firing was quite long (1.0 s) at drives just above the threshold but shortened to as little as 0.3 s as drive increased towards its maximum. 3. Cutting the spinal cord at C1-C2 had no effect on the ability of increased respiratory activity to generate a respiratory-associated rhythm in mesencephalic neurones. 4. Short-lasting anaesthesia with the agent Saffan caused mesencephalic neurones to lose the respiratory-associated rhythm with little change in phrenic activity and no change in respiratory cycle timing. 5. We also found a mesencephalic response to ventilator-induced chest expansion. The latency of the response from onset of expansion, indexed by fall of airway PCO2, to onset of neurone firing was shorter (0.2 s) than that found with the respiratory-associated rhythm. In seventeen neurones we found both the respiratory-associated rhythm and the independent ventilator-associated rhythm. 6. We interpret our findings to show that the respiratory-associated rhythmic firing of midbrain neurones is not primarily involved in generation or modulation of the motor function of the respiratory oscillator. We believe, instead, that these neurones are part of a sensory pathway conveying information about the magnitude of central neural respiratory drive, as well as spinally transmitted information from receptors in the chest wall, to thalamus and cortex. We suggest that the sensation ultimately generated may be that of 'air hunger' or dyspnoea.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banzett R. B., Lansing R. W., Reid M. B., Adams L., Brown R. 'Air hunger' arising from increased PCO2 in mechanically ventilated quadriplegics. Respir Physiol. 1989 Apr;76(1):53–67. doi: 10.1016/0034-5687(89)90017-0. [DOI] [PubMed] [Google Scholar]
- Bertrand F., Hugelin A., Vibert J. F. Quantitative study of anatomical distribution of respiration related neurons in the pons. Exp Brain Res. 1973 Feb 28;16(4):383–399. doi: 10.1007/BF00233430. [DOI] [PubMed] [Google Scholar]
- Davenport P. W., Friedman W. A., Thompson F. J., Franzén O. Respiratory-related cortical potentials evoked by inspiratory occlusion in humans. J Appl Physiol (1985) 1986 Jun;60(6):1843–1848. doi: 10.1152/jappl.1986.60.6.1843. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P. Central neural respiratory drive and afterdischarge. Respir Physiol. 1980 Apr;40(1):49–63. doi: 10.1016/0034-5687(80)90004-3. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. Relationship between phrenic nerve activity and ventilation. Am J Physiol. 1971 Aug;221(2):535–543. doi: 10.1152/ajplegacy.1971.221.2.535. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. Relationship between respiratory nerve and muscle activity and muscle force output. J Appl Physiol. 1975 Oct;39(4):567–574. doi: 10.1152/jappl.1975.39.4.567. [DOI] [PubMed] [Google Scholar]
- Kastella K. G., Spurgeon H. A., Weiss G. K. Respiratory-related neurons in anterior hypothalamus of the cat. Am J Physiol. 1974 Sep;227(3):710–713. doi: 10.1152/ajplegacy.1974.227.3.710. [DOI] [PubMed] [Google Scholar]
- Kukorelli T., Naményi J., Adám G. Visceral afferent projection areas in the cortex. II. Representation of the carotid sinus receptor area. Acta Physiol Acad Sci Hung. 1969;36(3):261–263. [PubMed] [Google Scholar]
- Kumagai H., Sakai F., Sakuma A., Hukuhara T. Relationship between activity of respiratory center and EEG. Prog Brain Res. 1966;21:98–111. doi: 10.1016/s0079-6123(08)62973-8. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L., Kiley J. P. Oscillations of medullary extracellular fluid pH caused by breathing. Respir Physiol. 1984 Feb;55(2):193–203. doi: 10.1016/0034-5687(84)90022-7. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E. Neural respiratory and circulatory interaction during chemoreceptor stimulation and cooling of ventral medulla in cats. J Physiol. 1986 Jan;370:217–231. doi: 10.1113/jphysiol.1986.sp015931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netick A., Orem J. Erroneous classification of neuronal activity by the respiratory modulation index. Neurosci Lett. 1981 Feb 6;21(3):301–306. doi: 10.1016/0304-3940(81)90221-4. [DOI] [PubMed] [Google Scholar]
- Orem J., Netick A. Characteristics of midbrain respiratory neurons in sleep and wakefulness in the cat. Brain Res. 1982 Jul 29;244(2):231–241. doi: 10.1016/0006-8993(82)90082-8. [DOI] [PubMed] [Google Scholar]
- Preiss G., Kirchner F., Polosa C. Patterning of sympathetic preganglionic neuron firing by the central respiratory drive. Brain Res. 1975 Apr 11;87(2-3):363–374. doi: 10.1016/0006-8993(75)90434-5. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Mercer R. R., Eldridge F. L. Servo control of end-tidal CO2 in paralyzed animals. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):133–136. doi: 10.1152/jappl.1978.45.1.133. [DOI] [PubMed] [Google Scholar]
- Syka J., Popelár J., Radil-Weiss T. Comparison of spontaneous activity of mesencephalic reticular neurones in the waking state and during pentobarbital anaesthesia. Physiol Bohemoslov. 1977;26(1):21–30. [PubMed] [Google Scholar]
- Vibert J. F., Caille D., Bertrand F., Gromysz H., Hugelin A. Ascending projection from the respiratory centre to mesencephalon and diencephalon. Neurosci Lett. 1979 Jan;11(1):29–33. doi: 10.1016/0304-3940(79)90051-x. [DOI] [PubMed] [Google Scholar]


