Abstract
Background
Chronic cerebral hypoperfusion (CCH) is a critical pathophysiological mechanism underlying cerebral small vessel disease (CSVD). Accumulating evidence have demonstrated that resident pericytes and deposit extracellular matrix (ECM) and play a key role in mediating fibrosis in hypoxic changes. Edaravone dexborneol (EDB) is known to target multiple pathways involved in fibrosis.
Methods
We constructed the CCH mouse models that were subjected to either PBS or EDB at different concentrations. Measures of cognitive function, neuronal damage, white matter lesion (WML), the fibrous profiles of pericytes and ECM protein were investigated to assess the effect of EDB. RNA sequencing of OGD in pericytes was performed to identify a key signaling pathway.
Results
We observed that both medium and high concentrations of EDB could ameliorate CCH-induced cognitive impairment and emotional disorders. Neuronal damage in cortical layer and hippocampus and WML in corpus callosum were improved by EDB, which was consistent with the tends of fibrous pericytes and ECM proteins in these regions. RNA sequencing suggested that TGF-β1/IL-11 plays an important role in mechanism of pericytes fibrosis. Subsequently, the results of sequencing were confirmed in both cellular and mouse model.
Conclusions
Our findings reveal the role of pericyte-mediated fibrosis in depositing ECM in the pathogenesis of CSVD. EDB could improve symptoms and the underlying pathogenesis of CCH mice and decrease the expression of the fibrous profiles of pericytes and ECM proteins, which may be regulated by TGF-β1/ IL-11. EDB treatment, targeting pericytes fibrosis, may be a novel therapeutic strategy for CSVD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-025-06157-3.
Keywords: Edaravone dexborneol, Cerebral small vessel disease, Pericyte, Extracellular matrix, Fibrosis
Introduction
Cerebral small vessel disease (CSVD) is a leading cause of stroke and vascular cognitive impairment (VCI), and is the most common, chronic and progressive vascular disease in an aging society [1, 2]. Chronic cerebral hypoperfusion (CCH), resulting from either small vessel disease or critical arterial stenosis, causes oligodendrocyte death, white matter lesion (WML), and subsequent VCI [3, 4]. A rat model of bilateral common carotid artery occlusion (BCCAO) and a mouse model of bilateral carotid artery stenosis (BCAS) are used to induce cerebral hypoperfusion that has been developed for investigating chronic WML [5, 6]. The BCCAO surgery causes damage to the visual pathway, which can affect the results of behavioral tests. The BCAS model shows the memory deficit in frontal-subcortical circuits, but it cannot develop severe damage to the optic tract [7]. Therefore, we employed the BCAS model to induce CCH.
Extracellular matrix (ECM) is vital for maintaining the normal function of the brain vascular system, remodeling of synapses, and controlling the movement of interstitial fluid [8]. Both large and small vascular disease affect the cerebral ECM, but the major changes are observed in CSVD. CSVD studies have showed hypoxic changes in the blood vessels, which subsequently activated inflammatory cells surrounding the fibrotic vessels [9, 10]. Pericytes, which are one of the components in the blood-brain barrier (BBB), are the first cells to respond to brain hypoxia [11]. In addition, pericytes have been confirmed as a source of the fibrotic scars, depositing fibrous ECM proteins after separating from the vascular wall [12]. Accumulating evidence proves that resident pericytes originate from the neurovascular unit and deposit ECM that mediates fibrosis [13–15]. One of the vital processes in the fibrosis response is the activation of myofibroblast progenitors [16]. Pericytes are acknowledged as a cellular origin of fibrosis in the central nervous system (CNS) [14, 17]. Fibroblast-like cells express α-smooth muscle actin (α-SMA), which is the hallmark of pericyte-myofibroblast transition, and are responsible for depositing ECM as well as the extent of fibrosis [16]. Platelet-derived growth factor receptor β (PDGFRβ), a pericyte marker in the CNS, has been identified as a source of the fibrotic scar that deposits fibrous ECM proteins [18]. ECM proteins include collagen type I (COL1A1), collagen type IV, laminin, and fibronectin [19]. Moreover, vimentin can bind to cellular surfaces and the ECM, and the interaction between extracellular vimentin and cells can trigger the activation of fibroblasts into a fibrotic phenotype [20]. However, Roth, M. et al. found that parenchymal PDGFRβ+ pericytes are not the major contributors to ECM in the fibrotic scar [12]. Therefore, how brain pericytes fibrosis contributes to pathology of CSVD has not been elucidated. In this study, we explored the mechanism of pericytes fibrosis in CSVD using the CCH model.
Interleukin-11 (IL-11), a hematopoietic IL-6 family cytokine, is specifically expressed in fibroblasts, especially when activated, but is undetectable in most normal human tissues and cells [21]. IL-11 is a crucial pro-fibrosis factor that acts downstream of transforming growth factor β1 (TGFβ1), promoting fibroblast senescence and collagen expression [22]. Buizza, C. et al. found that IL-11 protein exclusively colocalizes with pericytes after stroke, which may have an important role in the early responses to hypoxia [23]. Interestingly, our study also detected that IL-11 and TGF-β1 may play a vital role in pericytes during oxygen-glucose deprivation (OGD), as revealed by RNA sequencing. TGF-β1 is central to fibrotic responses in the brain vasculature and increases the risk for CSVD [24]. The pericyte-myofibroblast transition and the development of tissue fibrosis are caused by TGF-β1/Smad3 pathway activation [25]. Based on the role of IL-11 in the fibrosis mechanism, we speculated that the fibrosis of pericytes in the ECM contributes to the pathology of CSVD and may be associated with TGF-β1/Smad3 activation and IL-11 release.
Edaravone dexborneol (EDB), a compound preparation approved in China, has been shown to improve 90-day functional outcome in patients with acute ischemic stroke [26]. EDB is composed of Edaravone and (+)-Borneol in a mass ratio of 4:1. Edaravone has been approved for its effects of clearing lipid peroxidation, ameliorating neurological impairment, regulating inflammation [27]. Recently, it was demonstrated that edaravone could attenuate early pulmonary fibrosis via amelioration of oxidative stress and the TGF-β1/SMAD3 pathway [28]. Borneol increased drug distribution into CNS by promoting the transports drugs across the BBB, implying a synergistic effect of therapy with edaravone [29]. To further investigate the effect and fibrotic mechanisms of EDB in CSVD, we utilized EDB to intervene in the CCH mouse model and evaluated cognitive function, neuronal damage, WML and pericyte fibrosis, which could provide a new therapeutic strategy for CSVD.
Methods
Animals and Ethics approval
C57BL/6j mice (male, 6–8 weeks, 21 ± 1.5 g) were purchased from GemPharmatech (China). Mice were housed under specific pathogen-free (SPF) conditions with a 12/12 h light-dark cycle at 22 ± 2 ℃ and 40–70% humidity in the Laboratory Animal Research Center of South China University of Technology. The animal experiments were approved by the Animal Care and Use Ethics Committee of the Laboratory Animal Research Center (No. 2019034) and the Research Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2019133A), in accordance with the Animal Research: Reporting in Vivo Experiments (ARRIVE) guidelines. Mice were euthanised under overdose anaesthesia with sodium pentobarbital (150 mg/kg, intraperitoneal injection) in this study.
Construction of the CCH model
In our study, CCH secondary to BCAS in a murine model was established as described previously [30, 31]. Briefly, mice were anaesthetized with 3% sodium pentobarbital with dose of 30 mg/kg of intraperitoneal injection and placed in the supine position. The common carotid artery (CCA) was exposed and carefully isolated from the vagus nerve. Then, two 4−0 wires were placed individually at the distal and proximal ends of the CCA. The artery was then gently lifted with silk sutures and placed between the metal microcoil (inner diameter of 0.18 mm, purchased from Wuxi SAMINI SPRING Co., Ltd.) positioned below the carotid bifurcation. The microcoil was twisted around the CCA by rotation. After 30 min, another microcoil was wrapped around the other CCA in the same manner. Finally, the skin was sutured and disinfected with 75% ethanol.
Animal grouping and treatment
The EDB (Jiangsu Simcere Pharmaceutical Research Co., Ltd.) was a combination of edaravone 12.5 mg and dexborneol 2.5 mg, and the final EDB concentration was 2.5 mg/mL. The EDB solution was freshly prepared prior to use. EDB or saline was administered once daily (9:00–11:00 am) for 4 weeks, starting on the day after surgery. The mice were randomly divided into five groups: (i) sham group (sham-operated mice), (ii) CCH model group (BCAS surgery), and (iii) drug group (EDB-treated model mice); low concentration group (CCH + EDB 3 mg/kg i.p.); medium concentration group (CCH + EDB 6 mg/kg i.p.); high concentration group (CCH + EDB 12 mg/kg i.p.).
Cerebral blood flow (CBF) measurement
CBF in mice was evaluated using a two-dimensional laser speckle contrast analysis system (RWD RFLSI Pro Plus, China), as described previously [31]. Briefly, mice were anesthetized and the skull surface was exposed. The probe was positioned over the raw speckle images of regions of interest (ROIs) covering the parietal lobe in each hemisphere. Regional CBF (rCBF) values were recorded in both parietal lobes at baseline, 1 day, 14 days, and 30 days after surgery. The percent change in rCBF at each time point was analyzed by comparing the mean intensity with the baseline. The mice’s body temperature was maintained at 36.5 ± 0.5 ℃ using a heating pad during CBF measurement.
Magnetic resonance imaging (MRI)
We used DTI data to assess the loss of myelin fibers [32]. After neurological behavioral testing, mice were anesthetized with 3% isoflurane in a mixture of 70% N2O and 30% O2. MRI was performed using a Biospex 9.4 T/30 mm vertical bore small animal MRI scanner (Bruker Biospin, Germany) equipped with a large aperture small animal dielectric properties tomography (EPT) and a German iThera Medical photoacoustic tomography system. After positioning and pilot scans, T2-weighted images (T2WI) were acquired using a Rapid Acquisition with Relaxation Enhancement (RARE) sequence with the following parameters Echo Time/Repetition Time (TE/TR) = 24/4000 ms, averages = 3, matrix size = 180 × 180, 20 slices with a slice thickness of 0.7 mm, RARE factor = 8, and a field of view (FOV) of 16 × 16 mm. Corpus callosum signal abnormalities (degree of continuity) were identified as injuries on T2-weighted images by an expert in small animal MRI imaging. A whole-brain diffusion tensor imaging (DTI) dataset was acquired using a multislice spin-echo sequence with five reference and 30 non-collinear diffusion-weighted images with the following parameters TR = 3000 ms, TE = 20.2 ms, gradient directions = 90, diffusion gradient on time = 2.8 ms, field of view (FOV) = 20 × 20 mm [2], slice thickness = 0.7 mm and matrix size = 180 × 112. After acquiring five images with b = 0 s/mm2, b values of 1000 s/mm2 were acquired for each direction. MRI DTI data were analyzed using ParaVision 360 V2.0 and DSI Studio (http://dsistudio.labsolver.org/). Regions of interest (ROIs) were drawn from the hippocampus in a blinded fashion. Fractional anisotropy (FA), axonal diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD) values were determined for each ROI from identical consecutive sections of DTI images.
Behavioral tests
Morris water maze (MWM)
At 28–30 days after surgery, the spatial learning and memory function of the mice was evaluated using the MWM test (n = 9). The MWM is a circular white pool (diameter: 120 cm, height: 60 cm) filled with water (temperature:22–24℃) and divided into four quadrants with visual cues. A white platform (diameter: 9 cm) was placed in the center of the target quadrant and fixed approximately 1 cm below the water surface. The video-tracking system SuperMaze (Bitebio, China) recorded the tracks and the time taken by the mice to find the platform. For the place navigation test conducted over the first four days, each mouse was placed in the water from one of the quadrants. Mice were required to find the platform within 60s and stay on the platform for an additional 60s. Each mouse underwent four trainings sessions. On the fifth day, during the spatial probe trial, the platform was removed, and the diagonal quadrant of the previous platform location was selected as the starting point. The mean speed, escape latency, number of platform crossings, and time spent in the target quadrant around the platform were recorded within 60s.
Open field test (OFT)
The OFT is used to assess locomotor activity and anxiety-like behavior. Each mouse was placed in the center of an open chamber (50 × 50 × 50 cm). The movement trajectories of the mice were recorded for 5 min by a camera above the chamber. Both the total distance travelled and the time spent in the center area and corner zones were analyzed using Supermaze software (Shanghai, China).
Pole test
The pole test is used to evaluate motor coordination in mouse CCH models. Each mouse was placed at the top of a 50 cm pole and underwent three trials from top to bottom trials after training. The total descent time was recorded, and the average value was calculated.
Rotarod test
The rotarod test was performed to assess motor learning and coordination. Each mouse was trained to run at a speed of 10 rpm for 5 min over three consecutive days. On the test day, each mouse ran at speeds ranging from 4 to 40 rpm over 5 min, and the latency to fall (s) was recorded.
Immunohistochemistry staining
After behavioral testing, mice were anesthetized and perfused with 0.9% saline followed by 4% paraformaldehyde (PFA) via left ventricular perfusion. Brains were fixed with 4% PFA for 24 h, followed by gradient dehydration with 15% and 30% sucrose for 24 h until the brain tissue settled to the bottom of the EP tube. Brain tissues were embedded in OCT compound and sectioned at 20 μm (coronal sections) for immunohistochemical analysis using a cryo-microtome (CM3050S, Leica, Germany). Brain sections were washed in PBS, then treated with TritonX-100 permeabilization buffer for 15 min at room temperature (RT). The antigen was regenerated using endogenous peroxidase blockers for 15 min at RT. The sections were blocked with 10% normal goat serum for 1 h at RT and then incubated with primary antibodies NeuN (1:200, ab177487, abcam) overnight at 4 ℃. After washing in PBS, immunoreactions were incubated with secondary antibodies (VECTASTAIN Elite ABC Kit Peroxidase, Vector, PK-6100) and visualized with Vector DAB Peroxidase Substrate (Vector, SK-4100). Images were captured using a Leica DM IL LED inverted microscope, followed by image analysis using ImageJ software.
Immunofluorescence staining
The slices were washed in PBS and then permeabilized using 0.5% Triton-X100 for 30 min, and subsequently blocked with 10% normal goat serum for 1 h at RT. Following this, they were incubated at 4℃ overnight with primary antibodies: anti-PDGFRβ (1:200, 14–1402-82, Invitrogen), anti-α-SMA (1:300, 67735-1, Proteintech), anti-Vimentin (1:200, 22031-1, Proteintech), anti-COL1A1 (1:300, WL0088, Wanleibio). After washing, sections were incubated with secondary antibodies: Goat Anti-Rabbit IgG Alexa Fluor® 555 (abcam, ab150078, 1:1000) and Goat Antimouse IgG Alexa Fluor®488 (abcam, ab150113, 1:1000) for 1 h at RT. The negative control was processed without primary antibodies. Then, nuclei were stained with DAPI (Bestbio, BB-4401, 1:100) for 15 min at RT. Images were captured using a Leica DM IL LED inverted microscope or a laser-scanning confocal microscope (Leica, Germany). Fluorescent signals were quantified using ImageJ software. The colocalization was quantified using Fiji software, and the Pearson’s R value was calculated to assess the correlations of co-labeled intensities.
Luxol fast blue (LFB) myelin staining
CCH-induced white matter damages were evaluated using the LFB Myelin stain kit (G3245, Solarbio) following the manufacturer’s instructions. Specifically, the sections were incubated with LFB solution overnight at RT. Then, the sections were washed with 95% ethanol and rinsed with distilled water, and were transferred into the LFB differentiation solution for 15s, followed by 70% ethanol staining for 30s. Next, slices were immerged in the tar violet staining solution staining 30–40 s, dehydrated with 95% and 100% ethanol, and cleared with xylene. The severity of white matter lesion was graded as 0 (normal), 1 (disarrangement of nerve fibers), 2 (formation of marked vacuoles), and 3 (disappearance of myelinated fibers).
Cell culture
Pericyte isolation
Pericyte isolation has been described in our previous study [33]. Briefly, the meninges of neonatal mouse brains were removed under a dissection microscope. The brains were mechanically minced and the homogenate was digested in DMEM containing 1.05 mg/mL type II collagenase and 58.5 U/mL type I DNase for 10 min at room temperature. Then, the reaction was stopped and the cells were centrifuged. The resuspended cells were transferred to T25 flasks in low-glucose DMEM supplemented with 20% FBS, 1×penicillin/streptomycin, ITS (insulin transferrin sodium selenite supplement), 100 µg/ mL heparin and 1× smooth muscle growth supplement. Cells were split cells at 90–95% confluence using 0.25% trypsin-EDTA. Then, the cell suspension was stored in a T25 flask to settle for 1 h. Adherent cells were discarded, and the cell suspension was moved to a new flask at 37 ℃ and 5% CO2. This digestion process was repeated three times.
Oxygen-glucose deprivation (OGD) and EDB treatment
Pericytes underwent glucose and oxygen deprivation followed to mimic the environment of cerebral chronic ischemia in vitro. In short, pericytes were cultured in glucose-free medium (Gibco) in a hypoxia chamber (Thermo Fisher Scientific) with 5% O2 and 5% CO2 for 12 h, 24 h and 48 h. The cell culture media was then replaced with normal culture medium, and the cells were incubated under normal conditions. Cells were divided into five groups: a control group, an OGD group, an OGD + EDB low concentration group (10 µM), an OGD + EDB medium concentration group (50 µM), an OGD + EDB high concentration group (100 µM). The control group was concurrently cultured in normal circumstances with normal medium.
RNA sequencing
Total RNA was extracted from primary pericytes (1 × 107 cells per sample) treated with control and OGD using TRIzol. All samples were analyzed for RNA concentration, the RIN/RON ratio and the 28/18S ratio using a Fragment Analyzer, and qualified for cDNA library construction and sequencing. RNA sequencing was performed using the BGISEQ platform with paired-end 150 bp reads by Beijing Genomics Institute (BGI). After data filtering, 95.55% of the reads were mapped to the genome of Mus_musculus genome (NCBI, GCF_000001635.26_GRCm38.p6). A total of 4657 differentially expressed genes (DEGs) were identified using DESeq2 with a Q value ≤ 0.05. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and Gene Ontology (GO) terms of annotated DEGs were performed using the Dr.Tom system developed by BGI Genomics.
Western blotting
Cells were lysed with RIPA buffer (MCE, HY-K1001) containing phosphatase inhibitor cocktail (MCE, HY-K0021) and protease inhibitor cocktail (MCE, HY-K0010). The supernatants were collected after centrifugation at 12,000 g for 15 min at 4 ℃. The brain was separated into the cortical layer, corpus callosum and hippocampus, and was lysed on ice in RIPA buffer containing both phosphatase inhibitor and protease inhibitor cocktail. Samples were sonicated and centrifuged at 15,000 g at 4 ℃ for 30 min, with the remaining supernatant collected. The protein concentration was determined using a BCA kit (Beyotime, P0010S). The protein samples were subjected to SDS-PAGE and transferred to PVDF membranes (Merck Millipore, Immobilon-P). Membranes were blocked with 5% nonfat milk or 5% BSA in TBST for 2 h at RT. After washing with TBS, the membranes were incubated with primary antibodies: anti-PDGFRβ (1:1000, 3169T, CST), anti-α-SMA (1:1000, 67735-1, Proteintech), anti-Vimentin (1:1000, 22031-1, Proteintech), anti-COL1A1 (1:1000, WL0088, Wanleibio), anti-TGFβ1 (1:1000, WL02998, Wanleibio), anti-pSMAD3 (1:1000, 9520T, CST), anti-SMAD3 (1:1000, 9523T, CST), anti-IL-11 (1:1000, A1902, Abclonal), β-actin (Proteintech, 66009-1-Ig, 1:5000). Next, the membranes were incubated HRP-linked secondary antibodies: Goat Anti-Rabbit IgG(H + L), HRP conjugate (Proteintech, SA00001-2, 1:10000), HRP-conjugated Affinipure Goat Anti-Mouse IgG(H + L) (Proteintech, SA00001-1, 1:10000) for 2 h at RT. After washing with TBST, the membranes bands were visualized using Clarity™ Western ECL Substrate (Bio-Rad, 1705060). Quantitative image analysis was performed using Image J.
Statistical analysis
Data were presented as the mean ± SD and analyzed using GraphPad Prism 9 software. Differences between groups were determined using Student’s t-tests (two groups) and one-way ANOVA (at least three groups) followed by Tukey’s post hoc test. The neurological deficit score and the behavioral test were analyzed by two-way repeated measures ANOVA followed by Tukey’s post hoc test. A p-value < 0.05 was considered statistically significant.
Results
EDB ameliorated CCH-induced cognitive impairment and emotional disorders
Firstly, we measured the rCBF values using laser speckle contrast analysis. The sham mice showed no significant changes in rCBF over 30 days post-surgery. In contrast, the CCH mice displayed a 42% reduction in rCBF at the onset of surgery, which gradually recovered to 68% of the baseline by 30 days post-surgery. The EDB-treated CCH mice showed a trend toward less rCBF reduction at 14 days and 30 days after surgery compared to the CCH mice, but this difference was not statistical significance (Figure S1A, B).
CCH caused vascular dementia-like deficits. We evaluated the effect of EDB on CCH-induced spatial learning memory impairment using the Morris water maze test. CCH mice had longer escape latencies to the platform and less time in the target quadrant than control mice. In contrast, the latency time and the number of platform crossings were decreased in the medium or high concentration EDB-treated mice. The CCH group crossed the original platform position less frequently than the sham group. There was no significant difference in swimming speed among groups, which excluded the effect of swimming ability on cognitive evaluation (Fig. 1A–E). In addition, the emotional disorders were performed by the open field test. Our results showed that the mice in the CCH, the low-EDB and high-EDB groups spent more time on the edge of the open field and made fewer entries into the center of the open field than the sham group. The total distance of the CCH and low-EDB groups was shorter than that of the sham group, but the med-EDB exhibited an increased total distance compared with the CCH (Fig. 1F–K). These results suggest that the medium or high concentration EDB ameliorate CCH-induced cognitive impairment and emotional disorders.
Fig. 1.
EDB ameliorated CCH-induced cognitive impairment and emotional disorders. A–E The Morris water maze was shown as representative traces (A) and recorded as escape latency (B), mean speed, * symbol represents statistically significant differences (p < 0.05) in each model group compared with sham group, # symbol represents statistically significant differences (p < 0.05) in CCH-med EDB group compared with CCH group, & symbol represents statistically significant differences (p < 0.05) in CCH-high EDB group compared with CCH group. C Time in the target quadrant around the platform (D), and number of platform crossings (E). n = 9 mice per group. F–K The open field test was shown as representative traces (F) and recorded as total distance (G), center time (H), corner time (I), number of entries into center area (J), and number of entries into outside area (K). n = 9 mice per group; Data were presented as mean ± SD. ns p>0.05, *p<0.05, **p<0.01, ***p<0.001. One-way ANOVA with Tukey’s multiple comparison (C–D and G–K), or Two-Way Repeated-Measures ANOVA with Tukey’s test (B), or Kruskal Wallis test (E)
In addition, we detected the motor deficits using the pole and rotarod tests. There were no significant changes in the total descent time in the pole test and the latency to fall in the rotarod test between groups. These results indicated that CCH pathology did not lead to significant motor deficits (Figure S1C–D).
EDB ameliorated CCH-induced neuronal damage
We evaluated the effect of EDB treatment on CCH-induced neuronal cell death in the cortical and hippocampal regions using the neuronal marker NeuN. Our results showed that CCH mice exhibited a nearly 70% reduction in neuron in the hippocampal CA1 and a 50% reduction in the CA3 region compared to sham mice, which were involved in spatial learning and memory and hippocampal-dependent cognitive functions. In contrast, the medium or high concentration of EDB significantly ameliorated CCH-induced neuronal cell death in the hippocampus (Fig. 2A, B). There was no significant change in the hippocampal DG region between groups. Next, compared to sham brains, a 32% reduction in neurons was detected in the cortical layers V of the CCH brains. In contrast, the high EDB-treated mice significantly improved CCH-induced neuronal cell death in cortical layers V (Fig. 2C, D). Collectively, these data indicate that the medium or high concentration of EDB reduces neuronal cell death in specific cortical and hippocampal regions, which likely prevents CCH-induced neuronal damage.
Fig. 2.
EDB ameliorated CCH-induced neuronal damage. A Representative NeuN immunohistochemical staining of hippocampus. The scale bar denotes 100–50 μm. B Quantification of NeuN + cells in the hippocampus (CA1, CA3, DG). n = 5 mice per group. C Representative NeuN immunohistochemical staining of the cortical layers. The scale bar denotes 100–20 μm. D Quantification of NeuN + cells in the cortical layers (II/III, V, VI). n = 5 mice per group. Data were presented as mean ± SD. ns p>0.05, *p<0.05, **p<0.01, ***p<0.001. One-way ANOVA with Tukey’s multiple comparison test (B and D)
EDB attenuated CCH-induced white matter tract damage
To detect the mechanisms of neuronal protection in the EDB-treated mice, brain MRI studies were performed from these five groups of mice (sham, CCH, CCH + low EDB, CCH + med EDB and CCH + high EDB). We used DTI metrics to measure and analyze white matter tracts in the corpus callosum (CC) and external capsule (EC). The CCH group showed an average of 22.92% reduction in the mean FA values in CC and 25.87% reduction in EC compared to the sham group (Fig. 3A, B). In contrast, the treatment of med-EDB and high-EDB significantly improved FA values in CC and EC compared to the CCH mice (Fig. 3A, B). In addition, the corpus callosum was evaluated for WML by KB staining. Compared with the sham group, the CCH group significantly increased the grade scores of white matter demyelination. In contrast, the medium EDB-treated mice displayed a 48% reduction in grade scores compared to the CCH mice (Fig. 3C, D). Taken together, these results indicate that the medium or high concentration EDB ameliorated CCH-induced white matter demyelination.
Fig. 3.
EDB attenuated CCH-induced white matter tract damage. A Representative DEC maps showing corpus callosum (CC) and external capsule (EC) for DTI analysis. B Quantitative analysis of mean FA values in CC and EC. n = 3 control group or 4 mice experimental group. C Representative corpus callosum LFB myelin staining showing corpus callosum. The scale bar denotes 100–20 μm. D Quantitative analysis of white matter injury score. n = 6 mice per group. E–FRepresentative DTI (E) and DEC maps (F) showing hippocampus for DTI analysis. G–J Quantitative analysis of mean FA values (G), MD values (H), AD values (I) and RD values (J) in hippocampus. n = 3 control group or 4 mice experimental group. Data were presented as mean ± SD. ns p>0.05, *p<0.05, **p<0.01, ***p<0.001. One-way ANOVA with Tukey’s multiple comparison test (B and G–J), or Kruskal Wallis test (D)
We found significant NeuN-positive neuron loss in the hippocampus of CCH mice, and the EDB treatment significantly improved this loss. Next, DTI metrics measurements demonstrated that the hippocampus of the CCH mice exhibited significantly lower FA values but higher RD values compared to sham brains. Interestingly, the medium EDB-treated mice showed increasing FA and decreasing RD values in DTI compared to the CCH mice, which correlated with the change in cognition function (Fig. 3E–J).
EDB attenuated the fibrous profiles of pericytes and ECM proteins after CCH
We first examined the marker of pericyte-myofibroblast transition, α-SMA, and ECM proteins, COL1A1, by co-staining with immunofluorescence. Our results showed that α-SMA was co-stained with COL1A1 in the cortical layer, corpus callosum and hippocampus. In the cortical layer, corpus callosum and hippocampus, α-SMA expression in CCH mice was significantly higher than that in sham mice, which was consistent with the change in COL1A1. In contrast, the high EDB-treated mice showed a significant reduction in α-SMA expression compared to the CCH mice, but both the medium EDB and the high EDB treatments could decrease COL1A1 expression (Fig. 4 and Figure S2). The colocalization efficiency showed no significant changes between groups, indicating simultaneous change in pericyte-myofibroblast transition and fibrous ECM protein.
Fig. 4.
EDB attenuated α-SMA and COL1A1 in corpus callosum and hippocampus after CCH. A and C Representative images of α-SMA (green) and COL1A1 (red) immunostaining of corpus callosum (A) and hippocampus (C). Nuclei were stained with DAPI (blue). Scale bar = 62.2 μm. B and D Fluorescence intensity of α-SMA and COL1A1, and colocalization coefficient of α-SMA and COL1A1 in corpus callosum (B) and hippocampus (D). n = 5 mice per group. Data were presented as mean ± SD. ns p>0.05, *p<0.05, **p<0.01, ***p<0.001. One-way ANOVA with Tukey’s multiple comparison (B and D)
Next, the expression profiles of PDGFRβ and COL1A1 were co-stained to examine the interaction between PDGFRβ-positive cells and collagen. We observed that PDGFRβ+ pericytes were associated with the blood vessel wall and deposited COL1A1+ ECM. In the corpus callosum, there was a significant increase in the fluorescence density of PDGFRβ+ in the CCH groups compared with the sham groups, and the EDB treatment showed a trend of reduced PDGFRβ+ expression. Both the corpus callosum and hippocampus demonstrated that PDGFRβ expression in the CCH group significantly increased compared to the sham group, which was associated with changes in COL1A1. In contrast, the medium EDB or high EDB treatment significantly reduce PDGFRβ and COL1A1 expression. The colocalization efficient in the high EDB group was significantly less than that in the CCH group in the corpus callosum, without noting changes in the cortical layer and hippocampus. (Fig. 5 and Figure S2).
Fig. 5.
EDB attenuated PDGFRβ and COL1A1 in corpus callosum and hippocampus after CCH. A and C Representative images of PDGFRβ (green) and COL1A1 (red) immunostaining of corpus callosum (A) and hippocampus (C). Nuclei were stained with DAPI (blue). Scale bar = 62.2 μm. B and D Fluorescence intensity of PDGFRβ and COL1A1, and colocalization coefficient of PDGFRβ and COL1A1 in corpus callosum (B) and hippocampus (D). n = 5 mice per group. Data were presented as mean ± SD. ns p>0.05, *p<0.05, **p<0.01, ***p<0.001. One-way ANOVA with Tukey’s multiple comparison (B and D)
In addition, we extracted proteins from the cortical layer, corpus callosum and hippocampus, respectively, and detected the expression of α-SMA, PDGFRβ, and COL1A1 using western blotting. Consistent with immunofluorescence, the expression of the pericytes fibrous markers α-SMA or PDGFRβ was significantly higher in the CCH groups than in the sham group, and the medium or high EDB treatment could reverse this trend. Interestingly, ECM protein COL1A1 expression was not completely consistent with the immunofluorescence results. In the corpus callosum, COL1A1 expression showed a significant increase in CCH mice, and the high EDB-treated mice showed a trend of reduced expression, but this did not reach statistical significance (Figure S4). Taken together, these results reveal that the pericytes fibrous markers α-SMA and PDGFRβ were enhance in cortical layer, corpus callosum and hippocampus region after surgery, accompanied by simultaneous change in the fibrous ECM protein, which was alleviated by the medium or high concentration EDB treatment.
EDB attenuated the fibrous profiles of pericytes and vimentin after CCH
We subsequently analyzed the fibroblast markers vimentin, which can bind to cellular surfaces and the ECM, promoting fibroblasts to adopt a fibrotic phenotype. In the α-SMA and vimentin co-stained region, vimentin expression was detected and individually analyzed in the cortical layer, corpus callosum and hippocampus. In the corpus callosum, the fluorescence density of vimentin showed no significant change between groups. However, vimentin expression in CCH mice was higher than in sham mice and medium EDB-treated mice. In the cortical layer, we observed that the vimentin density was significantly stronger in CCH group than in the various concentration EDB-treated groups and the sham group. The colocalization efficiency showed no significant change between groups, indicating the simultaneous change in α-SMA and the fibroblast markers vimentin. (Fig. 6 and Figure S3).
Fig. 6.
EDB attenuated α-SMA and Vimentin in corpus callosum and hippocampus after CCH. A and C Representative images of α-SMA (green) and Vimentin (red) immunostaining of corpus callosum (A) and hippocampus (C). Nuclei were stained with DAPI (blue). Scale bar = 62.2 μm. B and D Fluorescence intensity of α-SMA and Vimentin, and colocalization coefficient of α-SMA and Vimentin in corpus callosum (B) and hippocampus (D). n = 5 mice per group. Data were presented as mean ± SD. ns p>0.05, *p<0.05, **p<0.01, ***p<0.001. One-way ANOVA with Tukey’s multiple comparison (B and D)
Moreover, the fluorescence density of vimentin was assessed in the PDGFRβ and vimentin co-stained region. As shown, the vimentin density in CCH mice was higher than that in the sham groups in the corpus callosum, but was reversed by treatment with medium or high concentration EDB, which differed from the findings in the α-SMA and vimentin co-stained region. In the cortical layer and hippocampus, the results of vimentin expression were consistent with those in the α-SMA and vimentin co-stained region, and the colocalization efficiency showed no significant change between groups (Fig. 7 and Figure S3).
Fig. 7.
EDB attenuated PDGFRβ and Vimentin in corpus callosum and hippocampus after CCH. A and C Representative images of PDGFRβ (green) and Vimentin (red) immunostaining of corpus callosum (A) and hippocampus (C). Nuclei were stained with DAPI (blue). Scale bar = 62.2 μm. B and D Fluorescence intensity of PDGFRβ and Vimentin, and colocalization coefficient of PDGFRβ and Vimentin in corpus callosum (B) and hippocampus (D). n = 5 mice per group. Data were presented as mean ± SD. ns p>0.05, *p<0.05, **p<0.01, ***p<0.001. One-way ANOVA with Tukey’s multiple comparison (B and D)
We further calculated the expression of vimentin in three key regions using western blotting. In agreement with the vimentin fluorescence test, the expression of vimentin showed a significant increase in CCH mice, and only medium concentration EDB treatment could significantly reduce vimentin protein levels in all three brain regions (Figure S4). Collectively, these data suggest that the different pericyte fibrous markers (α-SMA or PDGFRβ) in co-stained regions could yield different results regarding vimentin density, particularly in the corpus callosum, and that medium concentration EDB treatment may have the most pronounced effect on reducing pericytes fibrosis.
EDB regulated the fibrous profiles of pericytes through TGF-β1/SMAD3, IL-11 signaling
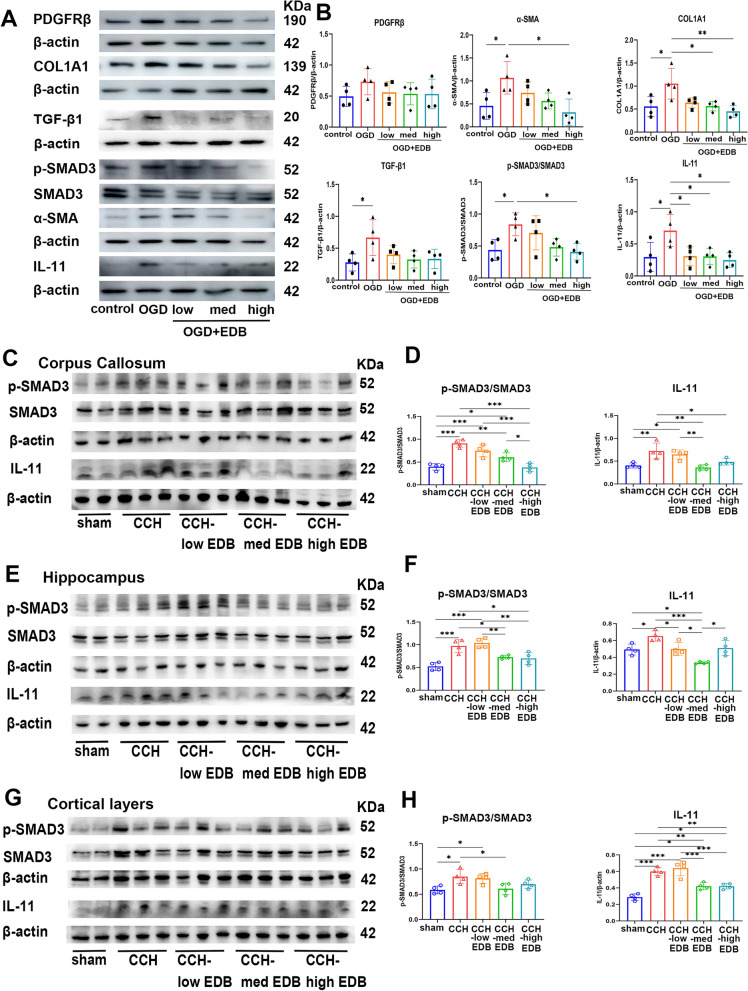
To further detect the fibrotic mechanisms of pericytes, we screened for key molecules using RNA sequencing by comparing primary pericytes with or without OGD intervention. A total of 435 differentially expressed genes (DEGs) were identified using DESeq2, with log2(FC) > 1 or < −1 and Q value ≤ 0.05; 305 genes were upregulated, and 130 were downregulated (Fig. 8A). The expression patterns of the top 20 upregulated and downregulated DEGs are shown in the heatmap (Figure S5A). Then, we analyzed the KEGG pathway based on the total DEGs to obtain the mainly enriched pathways in Phagosome (mmu04145), ECM-receptor interaction (mmu04512), Hypertrophic cardiomyopathy (mmu05410), Dilated cardiomyopathy (mmu05414), and Cytokine-cytokine receptor interaction (mmu04060) (Fig. 8B). We further separated the upregulated and downregulated DEGs for KEGG pathway enrichment analyses (Figure S5C and E). Our results showed that the upregulated DEGs were mainly enriched in the Phagosome (mmu04145), Viral protein interaction with cytokine and cytokine receptor (mmu04061) and Cytokine-cytokine receptor interaction (mmu04060). GO analysis of the total DEGs showed that they were mainly enriched in the extracellular space (GO:0005615), extracellular region (GO:0005576) and cell surface (GO:0009986) (Figure S5B). According to the enrichment of KEGG pathway, we chose the gene in Cytokine-cytokine receptor interaction, and the topological analysis of PPI node degrees identified interaction between TGF-β1 and IL-11 (Fig. 8C), which is consistent with KEGG pathway of the upregulated DEGs. Furthermore, we set a time gradient of OGD to confirm the optimal time for activated pericytes fibrosis and TGF-β1 pathway. Our results indicated that the levels of pericytes fibrous marker α-SMA and PDGFRβ were significantly enhanced in the OGD 12 h group compared with the control group, and ECM protein COL1A1exhibited simultaneous change at 12 h after OGD. Interestingly, we also observed that TGF-β1/SMAD3 and IL-11 in pericytes were significantly activated at OGD 12 h (Fig. 8D–J). Therefore, we chose 12 h OGD as the time point for subsequent EDB treatment.
Fig. 8.
OGD promotes the fibrous profiles of pericytes and collagen expression through IL-11 signaling. A Total RNA extracted from pericytes with or without OGD was analyzed using RNA sequencing. Volcano plots of all the identified genes in the RNA sequencing with upregulated DEGs in red, downregulated DEGs in green, and non-DEGs in grey. B The top 40 of KEGG pathways based on the total DEGs. C The PPI of total DEGs in Cytokine-cytokine receptor interaction. D Representative western blot images of PDGFRβ, α-SMA, COL1A1, TGF-β1, p-SMAD3, IL-11 and individual β-actin with control, OGD 12 h, OGD 24 h, and OGD 48 h. E–J Quantification of PDGFRβ (E), α-SMA (F), COL1A1 (G), TGF-β1(b), p-SMAD3 (I), and IL-11 (J). β-actin served as a control. n = 4 mice per group. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. One-way ANOVA with Tukey’s multiple comparison (E–J)
As shown, the medium EDB or the high EDB treatment could significantly decrease the expression of both α-SMA and COL1A1, in vitro. However, PDGFRβ expression exhibited a decreasing trend after EDB treatment, but did not reach statistical significance. Additionally, the protein levels of p-SMAD3 and IL-11 were down-regulated in the EDB-treated group when compared to the CCH group (Fig. 9A, B).
Fig. 9.
EDB regulated the fibrous profiles of pericytes through TGF-β1/SMAD3, IL-11 signaling. A Representative western blot images of PDGFRβ, α-SMA, COL1A1, TGF-β1, p-SMAD3, IL-11 and individual β-actin with control, OGD, OGD + low EDB-treated, OGD + med EDB-treated, OGD + high EDB-treated. B Quantification of PDGFRβ, α-SMA, COL1A1, TGF-β1, p-SMAD3/SMAD3 and IL-11. β-actin served as a control. n = 4 mice per group. C–H Western blot analysis of protein levels TGF-β1, p-SMAD3, IL-11 and individual β-actin in control and model groups with corpus callosum (C–D), hippocampus (E–F) and cortical layer (G–H). Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. One-way ANOVA with Tukey’s multiple comparison (B, D, F and H)
Moreover, we examined the levels of TGF-β1/SMAD3 and IL-11 in cortical layer, corpus callosum and hippocampus, respectively. Both p-SMAD3 and IL-11 expression in CCH mice were significantly higher than in the sham group across three brain regions. In contrast, we detected reduced p-SMAD3 and IL-11, along with decreased pericytes fibrous markers and ECM protein expression, in the medium EDB or high EDB treated group. Notably, the medium concentration showed a more stable trend than the high group (Fig. 9C–H). Taken together, pericytes fibrosis in the ECM, which contributes to pathology of CSVD, is associated with TGF-β1/SMAD3 and IL-11 activation. Moreover, the medium concentration of EDB may regulate the fibrous profiles of pericytes through this pathway.
Discussion
CSVD typically manifests with lacunar infarcts and ischaemic white matter lesions (WML) [34]. The ECM has emerged as a key target relevant to the pathogenesis of CSVD due to its involvement in the mechanism of WML [35]. Pericyte-derived PDGFRβ-positive fibroblast-like cells was involved in the production of ECM proteins, which is crucial for fibrosis after spinal cord or brain injury [15]. However, it remains uncertain how pericytes fibrosis in the ECM contributes to pathology of CSVD. In this study, we constructed CCH models to observe the fibrous profiles of pericytes and ECM proteins. Subsequently, we added EDB to treat CCH mice and assessed its effect on improving pericytes fibrosis in the ECM.
Our results showed rCBF decreases and gradually recovers to near baseline, which are consistent with those reported by Bhatia [36]. The treatment with EDB did not significantly improve rCBF. However, some evidence showed that EDB improved rCBF in the ischemic hemisphere after stroke [37, 38]. Based on the change in rCBF in CCH mice, we found the rCBF was self-limited and gradually recovered after CCH surgery, thus the effect of EDB treatment on rCBF may not be prominent.
The strength of our study lies in our comprehensive approach, which included cognitive behavioral tests, neuronal damage, white matter microstructural analysis with MRI DTI, and evaluation of WML changes with LFB myelin staining. This multifaceted methodology allowed us to effectively evaluate the impact of EDB treatment. In our study, both medium and high concentration of EDB were found to ameliorate CCH-induced cognitive impairment and emotional disorders. Zhang et al. observed that EDB could improve VD rats’ learning and memory function using MWM test and neuronal damage in the hippocampal CA1 region [39]; however, they did not evaluate emotional disorders or the overall neuronal changes in cortical layer and hippocampus. In line with our findings, Du et al. also found significant neuronal damage in the hippocampal CA1 and CA3 regions induced by CCH, which affected spatial learning and memory in hippocampal-dependent cognitive functions [40]. Furthermore, Deng et al. investigated the effects of CCH on NeuN-positive cells in the cortex, which showed a significant decrease due to CCH-induced neurological injury [41]. We showed that neuronal damage was not apparent in the entire cortex, but was evident in cortical layers V. The neural terminals from the dorsal hippocampus CA1 form excitatory connection with layer 5 pyramidal neurons in the prelimbic area, contributing to neuropathic pain and short-term memory [42]. Edaravone has been shown to mitigate cerebral infarction induced anxiety and depressive-like behavior by impeding inflammation [43]. The medium concentration of EDB treatment was shown to alleviate anxiety and depressive-like behaviors in our study.
Next, white matter damage was evaluated by FA and RD values with DTI, where low FA and high RD values are indicative of white matter microstructural damage and demyelination, respectively [44, 45]. We observed a significant decrease in the FA value in CCH mice white matter, and a dramatic improvement was noted in medium or high EDB-treated mice. Edaravone administered improved the disruption of white matter integrity in the damaged corpus callosum of APP23 mice with CCH [46]. Subsequently, we observed hippocampal DTI indices and found a similar tend with the corpus callosum. Liu et al. note changes in FA and RD values in the hippocampus, and considered that astrogliosis contributes to these changes, while there was no significant change observed after inhibiting reactive astrocytes [31]. Recent evidence has shown that pericytes contribute to white matter damage in CSVD [47–49]. Therefore, we further investigated the mechanism by which pericytes deposit ECM, mediating fibrosis in the CCH model.
In the mechanism of fibrous profiles of pericytes, CCH mice showed a significantly higher expression of the fibrous profiles of pericytes, which were co-stained with the ECM protein COL1A1 in three regions. α-SMA is often absent in quiescent pericytes in normal CNS, but is readily expressed in pericytes under pathological conditions such as tumor, tissue fibrosis, and inflammation [50]. α-SMA+ cells associated with vessels gradually increased after stroke [51]. The levels of pericyte marker α-SMA was correlated with ECM proteins, playing a considerable role in physiology and pathology [52]. The density of PDGFRβ+ pericytes in the white matter correlated with myelin attenuation [49]. An ageing-related dementias study demonstrated increased expression of PDGFRβ+ pericytes, which were positively correlated with the basement membrane marker collagen IV [53]. PDGFRβ+ pericytes were co-stained with ECM protein in the peri-infarct areas, and was significantly attenuated after pMCAO in PDGFRβ+/− mice, indicating PDGFRβ+ cells may be responsible for the production of ECM proteins and participate in fibrosis of ischemic areas [54]. These findings Our findings suggest that α-SMA+/PDGFRβ+ pericytes may product ECM proteins to mediate fibrosis in CSVD.
Treatment with EDB could greatly improve the damage of pericytes after cerebral ischemia/reperfusion injury [55]. In subarachnoid hemorrhage, Edaravone treatment significantly ameliorated subsequent apoptosis of pericytes [56]. Our studies showed that the medium or high concentration EDB could attenuate the fibrous profiles of pericytes and ECM proteins, indicating a potentially therapeutic mechanism.
To further confirm the regulated pathways, we screened the TGF-β1 and IL-11 pathways along with increases in PDGFRβ, α-SMA, and COL1A1. Under hypoxia conditions, TGF-β1 increased the levels of α-SMA and other fibrosis markers in pericytes through SMAD signaling pathways [57]. In addition, the expression of TGF-β target IL-11 was exclusively colocalized with pericytes (PDGFRβ and CD13 expressing cells) after stroke, which may involve in fibrosis and inflammation [23]. Inhibition of TGF-β1/SMAD3/IL-11 signaling pathways may alleviates fibrosis and inflammation in systemic sclerosis [58]. We confirmed the similar tendency of TGF-β1/SMAD3 and IL-11 in CCH mice, which were improved simultaneously by EDB treatment. Therapeutic agents edaravone target various cytokines-associated signaling pathways, such as TGF-β/SMAD, NF-κB, to reduce the pathophysiology of stoke [59]. Therefore, we speculate that EDB-treated pericytes depositing ECM proteins to mediate fibrosis in CSVD may be regulated by the TGF-β1/SMAD3/IL-11 pathways. However, we did not use the inhibitor of TGF-β1/SMAD3/IL-11 to further confirm this pathway by EDB mediation, which will be validated in our subsequent research.
Conclusion
This study highlights the role of pericyte-mediated fibrosis in depositing ECM within the pathogenesis of CSVD. CCH induced neuronal damage in the cortical and hippocampal regions and white matter lesion formation in the corpus callosum, consistent with the changes in fibrous pericytes and ECM protein in these regions. EDB could improve the symptoms and pathogenesis of CCH mice and decrease the expression of the fibrous profiles of pericytes and ECM proteins, which may be regulated by TGF-β1/SMAD3/IL-11 pathways. EDB treatment, targeting IL-11-mediated fibrosis in pericytes may represent a new therapeutic strategy in CSVD.
Electronic supplementary material
Acknowledgements
Not applicable.
Author contributions
Q.R.D., L.J.W. and Y.Y.G. conceived and designed the experiments. Q.R.D., Z.Y.L., Y.X.X., H.H.F., L.Z., and J.X.L. prepared materials and performed the experiments. Q.R.D., K.N., P.K.H. G.X.M., and Y.H.Z. collected and analyzed the data. Q.R.D. wrote the original draft of the manuscript. Q.R.D., Z.Y.L., K.N., Y.Y.G., and L.J.W. reviewed and revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 82371249, 82101377, 82471257, 81974195); the Science and Technology Planning Project of Guangzhou (Nos. 202201000005); the Science and Technology Planning Project of Guangdong Province (No. 2020A0505140006); the NSFC Incubation Project of Guangdong Provincial People’s Hospital (No. KY0120220053); and the Guangdong Medical Research Foundation (Nos. A2021308 and A2022092); Guangdong Basic and Applied Basic Research Foundation (No. 2023A1515110937).
Data availability
RNA sequencing data has been deposited in the NCBI Sequence Read Archive (SRA) repository under project PRJNA1178705.
Declarations
Ethics approval and consent to participate
All animal experiments were approved by the Animal Care and Use Ethics Committee of Laboratory Animal Research Center (No. 2019034) and the Research Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2019133A).
Consent for publication
Not applicable.
Competing interests
All authors have declared that there are no competing interests.
Declaration of generative AI in scientific writing
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qingrui Duan and Zhiyang Liu contributed equally to this work.
Contributor Information
Kun Nie, Email: niekun@gdph.org.cn.
Yuyuan Gao, Email: gaoyuyuan@gdph.org.cn.
Lijuan Wang, Email: wanglijuan@gdph.org.cn.
References
- 1.van den Brink H, Doubal FN, Duering M. Advanced MRI in cerebral small vessel disease. Int J Stroke Off J Int Stroke Soc. 2023;18(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chojdak-Łukasiewicz J, Dziadkowiak E, Zimny A, Paradowski B. Cerebral small vessel disease: a review. Adv Clin Exp Med Off Organ Wroclaw Med Univ. 2021;30(3):349–56. [DOI] [PubMed] [Google Scholar]
- 3.Bhuiyan MIH, Habib K, Sultan MT, et al. SPAK inhibitor ZT-1a attenuates reactive astrogliosis and oligodendrocyte degeneration in a mouse model of vascular dementia. CNS Neurosci Ther. 2024;30(3):e14654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajeev V, Chai YL, Poh L, et al. Chronic cerebral hypoperfusion: a critical feature in unravelling the etiology of vascular cognitive impairment. Acta Neuropathol Commun. 2023;11(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia P, Kaur G, Singh N. Ozagrel a thromboxane A2 synthase inhibitor extenuates endothelial dysfunction, oxidative stress and neuroinflammation in rat model of bilateral common carotid artery occlusion induced vascular dementia. Vascul Pharmacol. 2021;137:106827. [DOI] [PubMed] [Google Scholar]
- 6.Li L, He G, Shi M, et al. Edaravone dexborneol ameliorates cognitive impairment by regulating the NF-κB pathway through AHR and promoting microglial polarization towards the M2 phenotype in mice with bilateral carotid artery stenosis (BCAS). Eur J Pharmacol. 2023;957:176036. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa H, Shindo A, Mizutani A, Tomimoto H, Lo EH, Arai K. A brief overview of a mouse model of cerebral hypoperfusion by bilateral carotid artery stenosis. J Cereb Blood Flow Metabol Off J Int Soc Cereb Blood Flow Metab. 2023;43(2suppl):18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoshneviszadeh M, Henneicke S, Pirici D, et al. Microvascular damage, neuroinflammation and extracellular matrix remodeling in Col18a1 knockout mice as a model for early cerebral small vessel disease. Matrix Biol J Int Soc Matrix Biol. 2024;128:39–64. [DOI] [PubMed] [Google Scholar]
- 9.Hainsworth AH, Markus HS, Schneider JA. Cerebral small vessel disease, hypertension, and vascular contributions to cognitive impairment and dementia. Hypertens (Dallas Tex : 1979). 2024;81(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy PAMAS. Regulation of Flt 1 splicing by fibronectin and integrin signaling during aging. Bethesda: NIH RePORTER; 2023. [Google Scholar]
- 11.Carlsson R, Enström A, Paul G. Molecular regulation of the response of brain pericytes to hypoxia. Int J Mol Sci. 2023;24(6):5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth M, Enström A, Aghabeick C, Carlsson R, Genové G, Paul G. Parenchymal pericytes are not the major contributor of extracellular matrix in the fibrotic scar after stroke in male mice. J Neurosci Res. 2020;98(5):826–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang AC, Vest RT, Kern F, et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature. 2022;603(7903):885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dias DO, Kalkitsas J, Kelahmetoglu Y, et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun. 2021;12(1):5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura K, Ago T. Pericyte-mediated molecular mechanisms underlying tissue repair and functional recovery after ischemic stroke. J Atheroscler Thromb. 2023;30(9):1085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong Y, Wei B, Wang W, et al. Single-cell RNA-Sequencing identifies bone marrow-derived progenitor cells as a main source of Extracellular Matrix-Producing cells across multiple organ-based Fibrotic diseases. Int J Biol Sci. 2024;20(13):5027–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holl D, Göritz C. Decoding fibrosis in the human central nervous system. Am J Physiol Cell Physiol. 2023;325(6):C1415–20. [DOI] [PubMed] [Google Scholar]
- 18.Rentsch NH, Rust R. Scary’ pericytes: the fibrotic scar in brain and spinal cord lesions. Trends Neurosci. 2022;45(1):6–7. [DOI] [PubMed] [Google Scholar]
- 19.Graf F, Horn P, Ho AD, Boutros M, Maercker C. The extracellular matrix proteins type I collagen, type III collagen, fibronectin, and laminin 421 stimulate migration of cancer cells. FASEB J Off Publ Federat Am Soc Exp Biol. 2021;35(7):e21692. [DOI] [PubMed] [Google Scholar]
- 20.Bucki R, Iwamoto DV, Shi X, et al. Extracellular vimentin is sufficient to promote cell attachment, spreading, and motility by a mechanism involving N-acetyl glucosamine-containing structures. J Biol Chem. 2023;299(8):104963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Reilly S. Interleukin-11 and its eminent role in tissue fibrosis: a possible therapeutic target. Clin Exp Immunol. 2023;214(2):154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song T, Gu Y, Hui W, Yang X, Liu Y, Chen X. Oxygen-glucose deprivation promoted fibroblast senescence and collagen expression via IL11. Int J Mol Sci. 2022;23(20):12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buizza C, Enström A, Carlsson R, Paul G. The transcriptional landscape of pericytes in acute ischemic stroke. Translational Stroke Res. 2024;15(4):714–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato T, Sekine Y, Nozaki H, et al. Excessive production of transforming growth factor β1 causes mural cell depletion from cerebral small vessels. Front Aging Neurosci. 2020;12:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y, Su H, Zeng J, et al. Integrin β8 prevents pericyte-myofibroblast transition and renal fibrosis through inhibiting the TGF-β1/TGFBR1/Smad3 pathway in diabetic kidney disease. Trans Res J Lab Clin Med. 2024;265:36–50. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Wang A, Meng X, et al. Edaravone Dexborneol versus Edaravone alone for the treatment of acute ischemic stroke: a phase III, randomized, double-blind, comparative trial. Stroke. 2021;52(3):772–80. [DOI] [PubMed] [Google Scholar]
- 27.Dang R, Wang M, Li X, et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J Neuroinflamm. 2022;19(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Q, Liang Q, Gao S, et al. Development of therapeutic drugs for idiopathic pulmonary fibrosis. Chin J New Drugs. 2021;30(14):1274–81. [Google Scholar]
- 29.Xie Q, Li J, Dong T, et al. Neuroprotective effects of synthetic borneol and natural borneol based on the neurovascular unit against cerebral ischaemic injury. J Pharm Pharmacol. 2022;74(2):236–49. [DOI] [PubMed] [Google Scholar]
- 30.Liu Q, Shkirkova K, Lamorie-Foote K, et al. Air Pollution Particulate Matter exposure and chronic cerebral hypoperfusion and measures of White Matter Injury in a murine model. Environ Health Perspect. 2021;129(8):87006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q, Bhuiyan MIH, Liu R, et al. Attenuating vascular stenosis-induced astrogliosis preserves white matter integrity and cognitive function. J Neuroinflamm. 2021;18(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newville J, Howard TA, Chavez GJ, Valenzuela CF, Cunningham LA. Persistent myelin abnormalities in a third trimester-equivalent mouse model of fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2022;46(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan Q, Zhang Q, Nie K, et al. Myo1d promotes alpha-synuclein transfer from brain microvascular endothelial cells to pericytes through tunneling nanotubes. iScience. 2023;26(8):107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu LY, Chai YL, Cheah IK, et al. Blood-based biomarkers of cerebral small vessel disease. Ageing Res Rev. 2024;95:102247. [DOI] [PubMed] [Google Scholar]
- 35.Rajeev V, Fann DY, Dinh QN, et al. Intermittent fasting attenuates Hallmark Vascular and neuronal pathologies in a mouse model of vascular cognitive impairment. Int J Biol Sci. 2022;18(16):6052–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatia K, Kindelin A, Nadeem M, et al. Complement C3a receptor (C3aR) mediates vascular dysfunction, hippocampal Pathology, and cognitive impairment in a mouse model of VCID. Translational Stroke Res. 2022;13(5):816–29. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Zhang X, Zhang C, et al. Edaravone dexborneol downregulates neutrophil extracellular trap expression and ameliorates blood-brain barrier permeability in acute ischemic stroke. Mediators Inflamm. 2022;2022:3855698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Wang L, Zhu B, et al. A comparative study of the neuroprotective effects of dl-3-n-butylphthalide and edaravone dexborneol on cerebral ischemic stroke rats. Eur J Pharmacol. 2023;951:175801. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Xiao Y, Liu H, et al. Edaravone Dexborneol alleviates neuroinflammation by reducing Neuroglial Cell Proliferation and suppresses neuronal Apoptosis/Autophagy in vascular dementia rats. Neurochem Res. 2023;48(10):3113–28. [DOI] [PubMed] [Google Scholar]
- 40.Du Y, He J, Xu Y, et al. SIRT6 prevent chronic cerebral hypoperfusion induced cognitive impairment by remodeling mitochondrial dynamics in a STAT5-PGAM5-Drp1 dependent manner. J Translational Med. 2024;22(1):788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng S, Gao Y, Lv M, et al. I-C-F-6 attenuates chronic cerebral hypoperfusion-induced neurological injury in mice by modulating microglia polarization. Naunyn Schmiedebergs Arch Pharmacol. 2024;397(6):3917–28. [DOI] [PubMed] [Google Scholar]
- 42.Han S, Ren J, Li Z, Wen J, Jiang B, Wei X. Deactivation of dorsal CA1 pyramidal neurons projecting to medial prefrontal cortex contributes to neuropathic pain and short-term memory impairment. Pain. 2024;165(5):1044–59. [DOI] [PubMed] [Google Scholar]
- 43.Wang TS, Jing LJ. Therapeutic effect and psychological impact of aspirin plus edaravone on patients with cerebral infarction. World J Psychiatry. 2024;14(5):644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurata S, Nishitani S, Kawata NYS, et al. Diffusion tensor imaging of white-matter structural features of maltreating mothers and their associations with intergenerational chain of childhood abuse. Sci Rep. 2024;14(1):5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faraji R, Ganji Z, Zamanpour SA, Nikparast F, Akbari-Lalimi H, Zare H. Impaired white matter integrity in infants and young children with autism spectrum disorder: what evidence does diffusion tensor imaging provide? Psychiatry Res Neuroimag. 2023;335:111711. [DOI] [PubMed] [Google Scholar]
- 46.Feng T, Yamashita T, Sasaki R, et al. Protective effects of edaravone on white matter pathology in a novel mouse model of Alzheimer’s disease with chronic cerebral hypoperfusion. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2021;41(6):1437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruchoux MM, Kalaria RN, Román GC. The pericyte: a critical cell in the pathogenesis of CADASIL. Cereb Circ Cognit Behav. 2021;2:100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Z, Gao C, Gao D, et al. Reduction in pericyte coverage leads to blood-brain barrier dysfunction via endothelial transcytosis following chronic cerebral hypoperfusion. Fluids Barriers CNS. 2021;18(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dupré N, Drieu A, Joutel A. Pathophysiology of cerebral small vessel disease: a journey through recent discoveries. J Clin Investig. 2024. 10.1172/JCI172841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erdener Ş, Küreli E, Dalkara G. Contractile apparatus in CNS capillary pericytes. Neurophotonics. 2022;9(2):021904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahn W, Chi G, Kim S, Son Y, Zhang M, Substance P. Reduces infarct size and mortality after ischemic stroke, possibly through the M2 polarization of Microglia/Macrophages and Neuroprotection in the ischemic rat brain. Cell Mol Neurobiol. 2023;43(5):2035–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu KK. Control of tissue fibrosis by 5-Methoxytryptophan, an innate anti-inflammatory metabolite. Front Pharmacol. 2021;12:759199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding R, Hase Y, Burke M, et al. Loss with ageing but preservation of frontal cortical capillary pericytes in post-stroke dementia, vascular dementia and Alzheimer’s disease. Acta Neuropathol Commun. 2021;9(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shibahara T, Nakamura K, Wakisaka Y, et al. PDGFRβ-positive cell-mediated post-stroke remodeling of fibronectin and laminin α2 for tissue repair and functional recovery. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2023;43(4):518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Z, Zhao H, Yang S, et al. Edaravone Dexborneol protects against blood-brain barrier disruption following cerebral ischemia/reperfusion by upregulating pericyte coverage via vitronectin-integrin and PDGFB/PDGFR-β signaling. Free Radic Biol Med. 2024;225:758–66. [DOI] [PubMed] [Google Scholar]
- 56.Sanicola HW, Stewart CE, Luther P, et al. Pathophysiology, management, and therapeutics in subarachnoid hemorrhage and delayed cerebral ischemia: an overview. Pathophysiol Off J Int Soc Pathophysiol. 2023;30(3):420–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim JH, Yook JM, Oh SH, et al. Paricalcitol improves Hypoxia-Induced and TGF-β1-Induced Injury in kidney pericytes. Int J Mol Sci. 2021;22:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng B, Hu Q, He R, et al. Baicalein alleviates fibrosis and inflammation in systemic sclerosis by regulating B-cell abnormalities. BMC Complement Med Ther. 2023;23(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumari S, Dhapola R, Sharma P, Nagar P, Medhi B, HariKrishnaReddy D. The impact of cytokines in neuroinflammation-mediated stroke. Cytokine Growth Factor Rev. 2024;78:105–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing data has been deposited in the NCBI Sequence Read Archive (SRA) repository under project PRJNA1178705.