Abstract
1. The actions of the n-alcohols from pentanol to dodecanol on nicotinic acetylcholine receptor (nAChR) channels were investigated by recording single ACh-activated channel activity from inside-out membrane patches isolated from cultured rat myotubes. Alcohols were applied to the cytoplasmic side of the membrane; aqueous concentrations ranged from 11.7 mM-pentanol to 0.02 mM-dodecanol. 2. The intermediate-chain alcohols (pentanol to octanol) caused channel currents to fluctuate between the fully open and closed state level so that openings occurred in bursts interrupted by brief gaps. Closed time distributions were fitted well with two exponential components, the fast component representing the closures within a burst. The number of gaps within a burst was dependent on alcohol concentration whereas gap duration was independent of concentration but increased with increasing chain length of the alcohol up to octanol. 3. Nonanol and decanol reduced the mean duration of bursts of openings but did not cause an increase in the number of short closed intervals within a burst. Beyond decanol there was a decline in the ability of the n-alcohols to affect channel function. A saturated solution of undecanol (0.07 mM) reduced the mean open time by 33 +/- 17%, whereas a saturated solution of dodecanol had no significant effect. 4. The current integral per burst was reduced by all the n-alcohols between pentanol and undecanol. The IC50S were as follows: hexanol, 0.53 +/- 0.14 mM; heptanol, 0.097 +/- 0.02 mM; octanol, 0.04 mM and nonanol, 0.16 +/- 0.035 mM. 5. The results were analysed in terms of an open channel block model with a long-lived closed-blocked state beyond the blocked state. Over the range of concentrations tested this describes the effects of all the n-alcohols (C5 to C12) on channel gating reasonably well. 6. Blocking rate constants (k+B) for pentanol through to nonanol were calculated to be between 2.8 and 5.7 X 10(6) M-1 S-1. These values are based on the assumption that the concentration of the alcohols at their site(s) of action was equal to the aqueous concentration applied to the membrane. 7. Equilibrium dissociation constants (KD), calculated from the blocking and unblocking rate constants (KD = k-B/k+B), decreased with increasing chain length from 8 mM for pentanol to 0.15 mM for octanol. The standard free energy per methylene group for adsorption to the site of action was calculated to be about -3.3 kJ mol-1.(ABSTRACT TRUNCATED AT 400 WORDS)
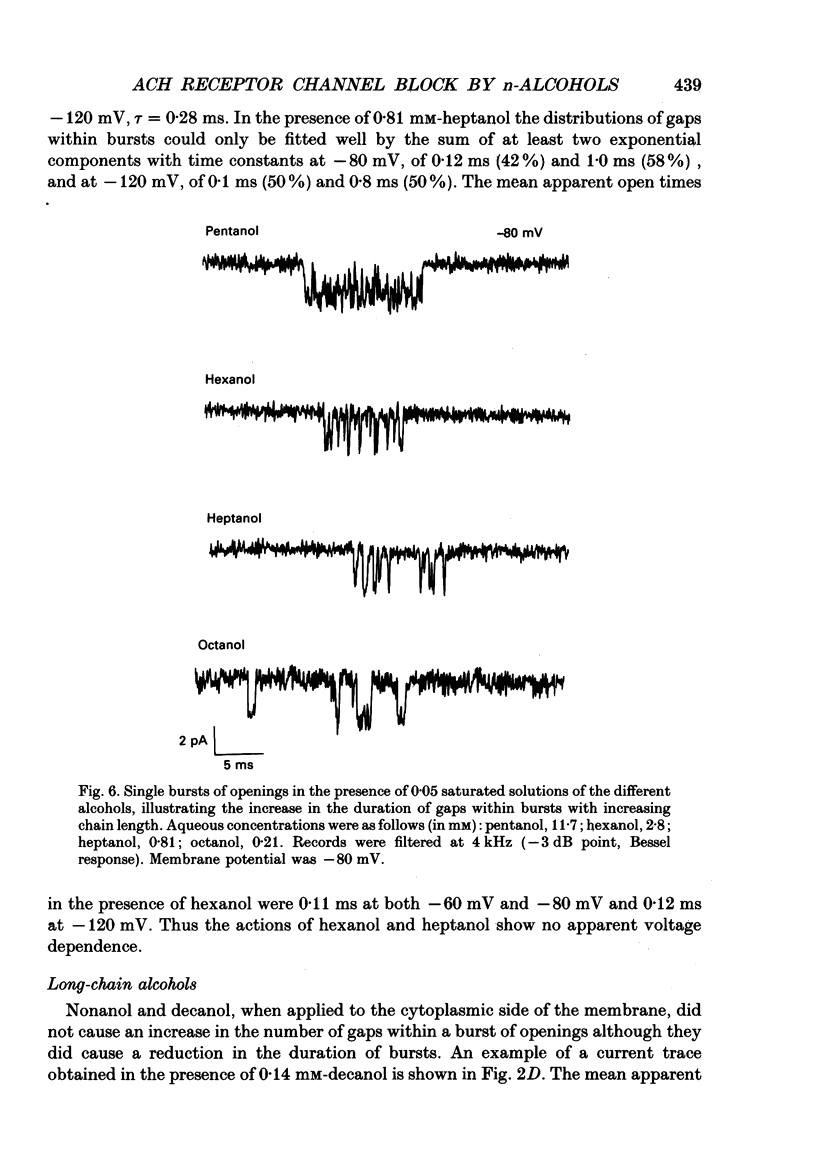
Full text
PDF



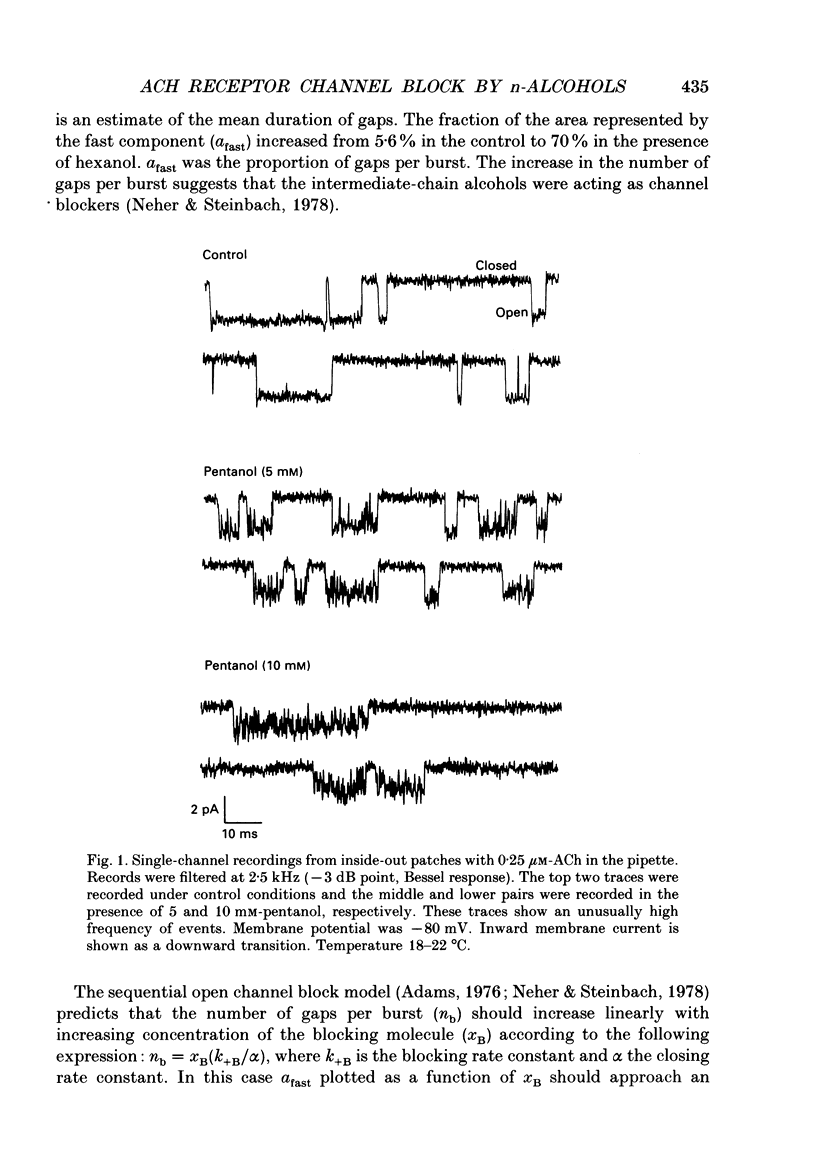
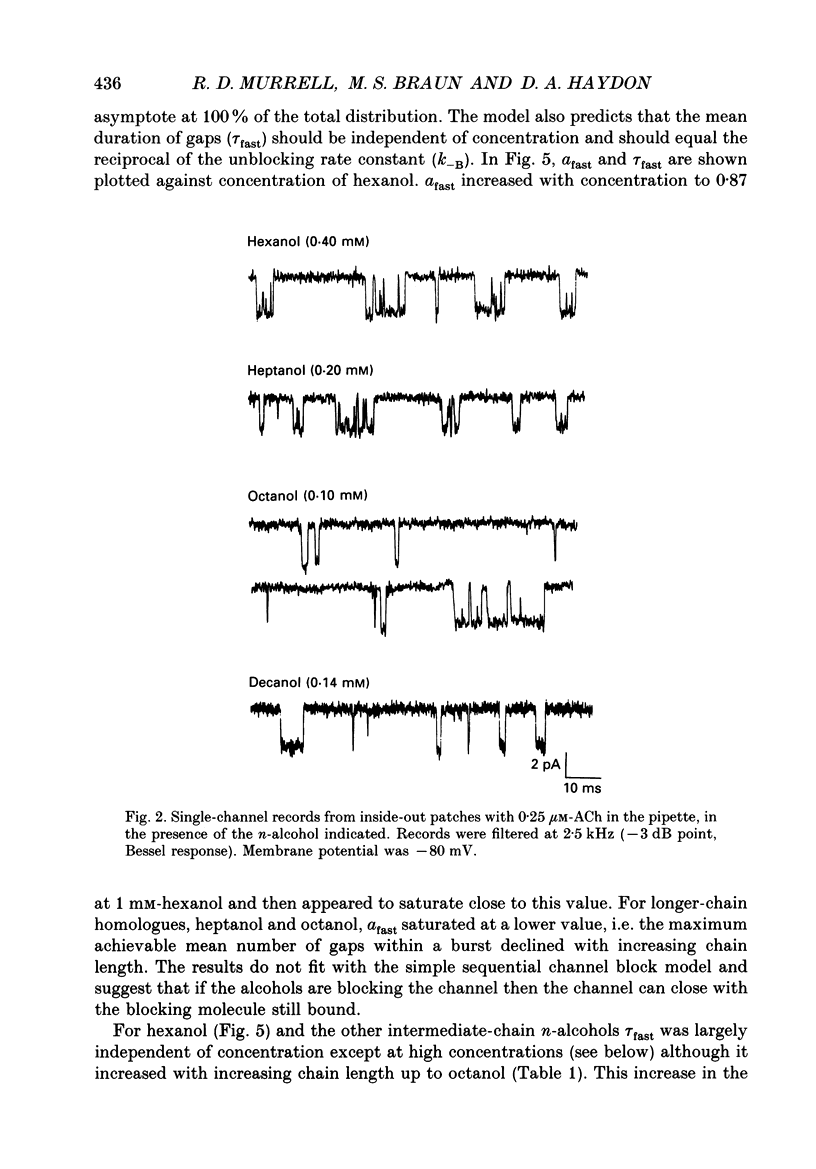
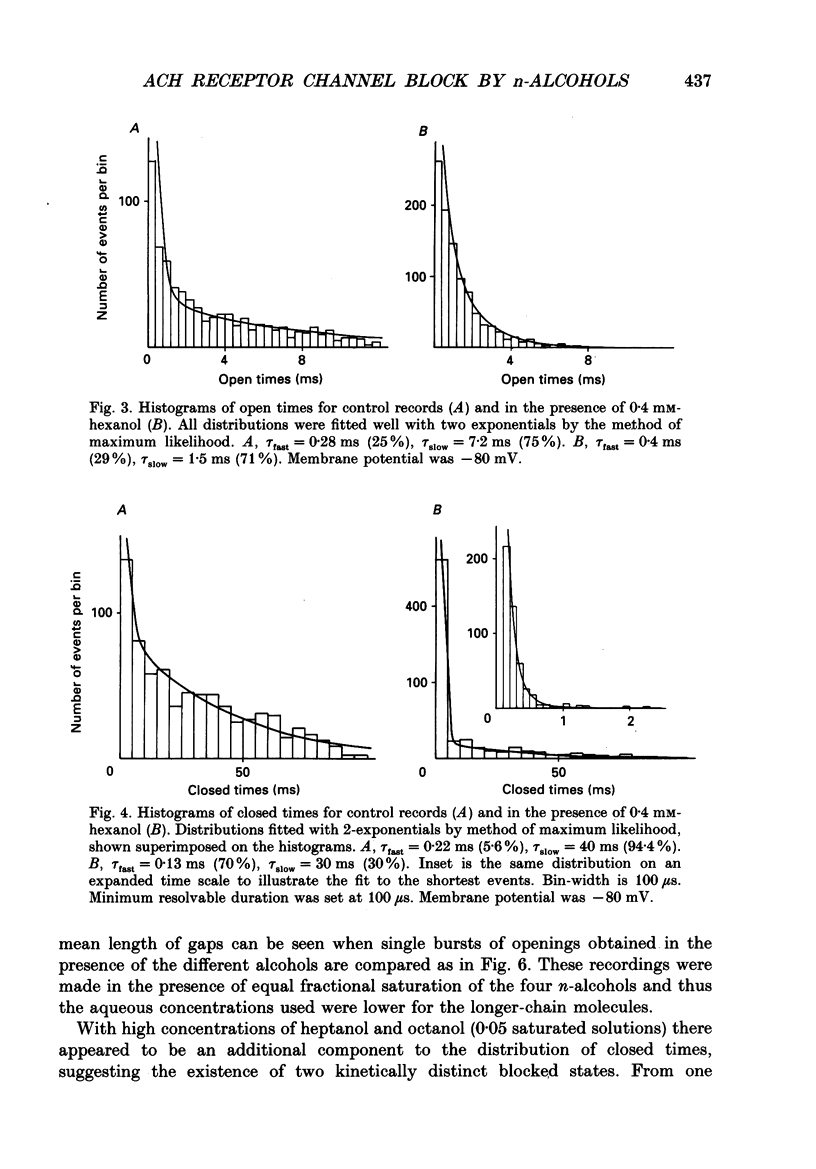
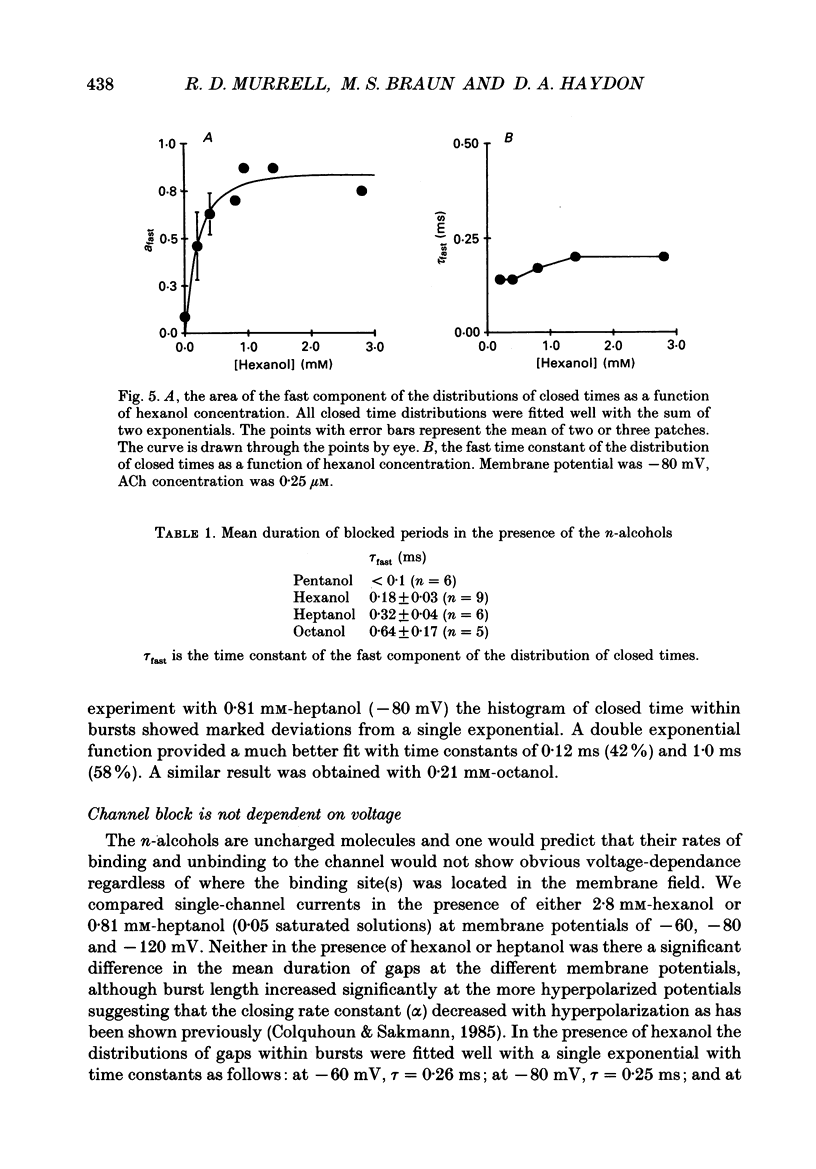
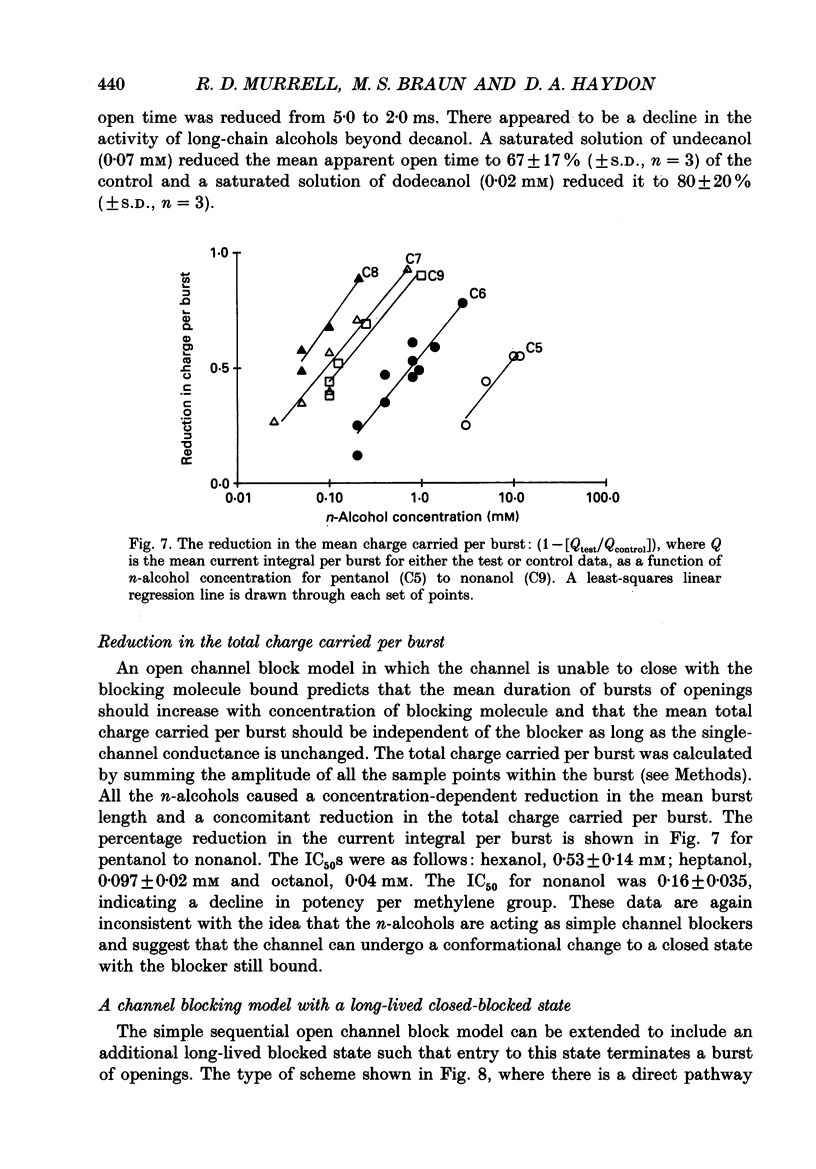

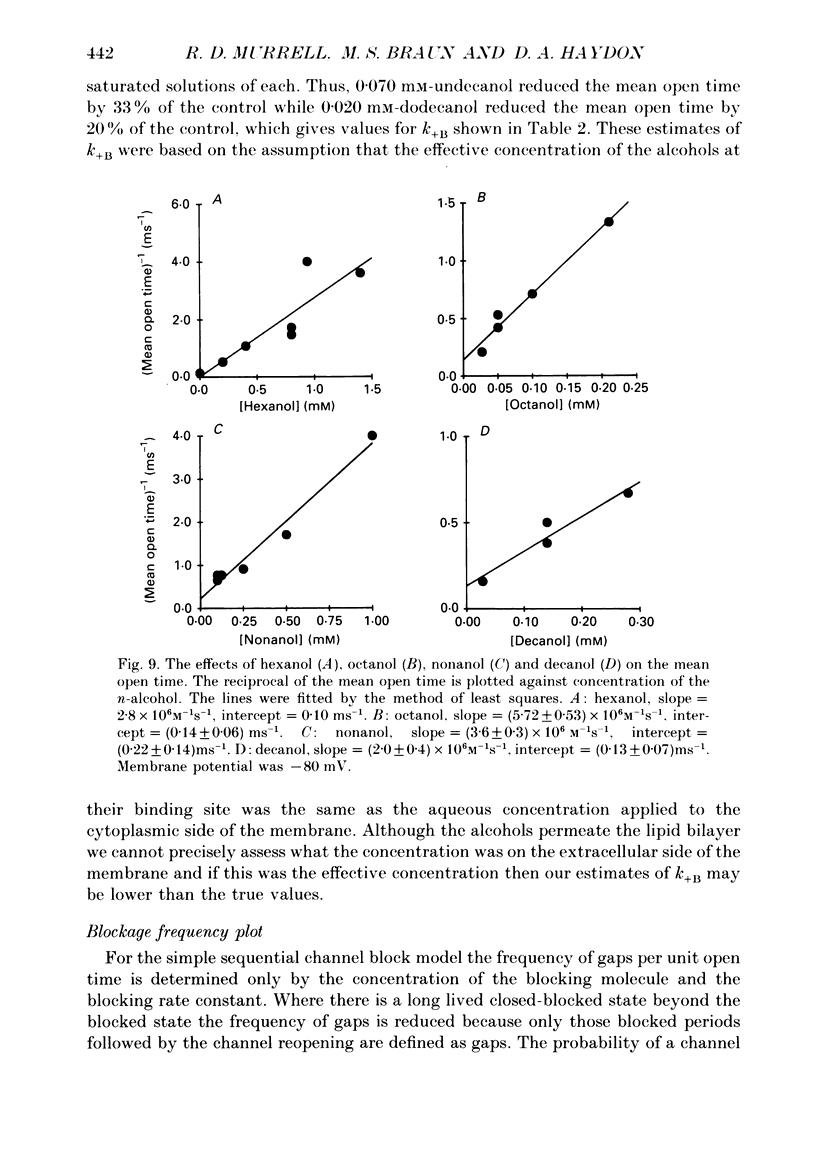
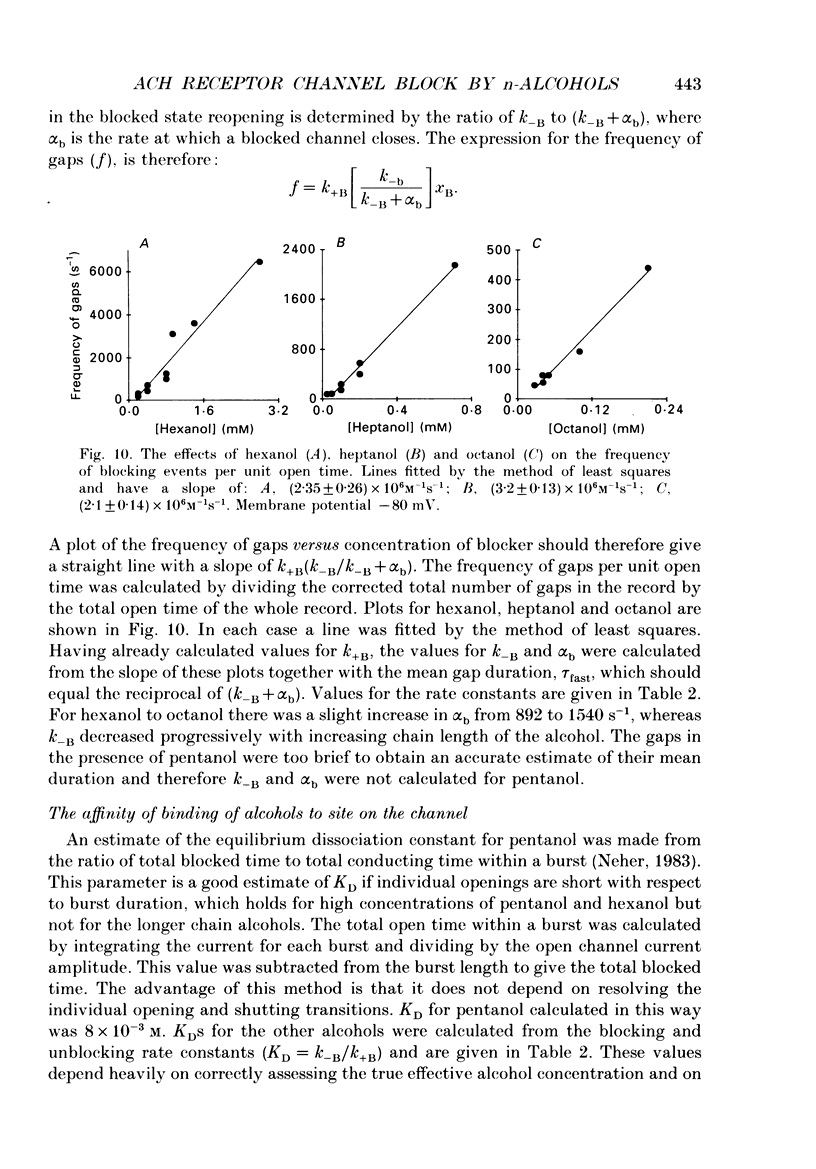




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
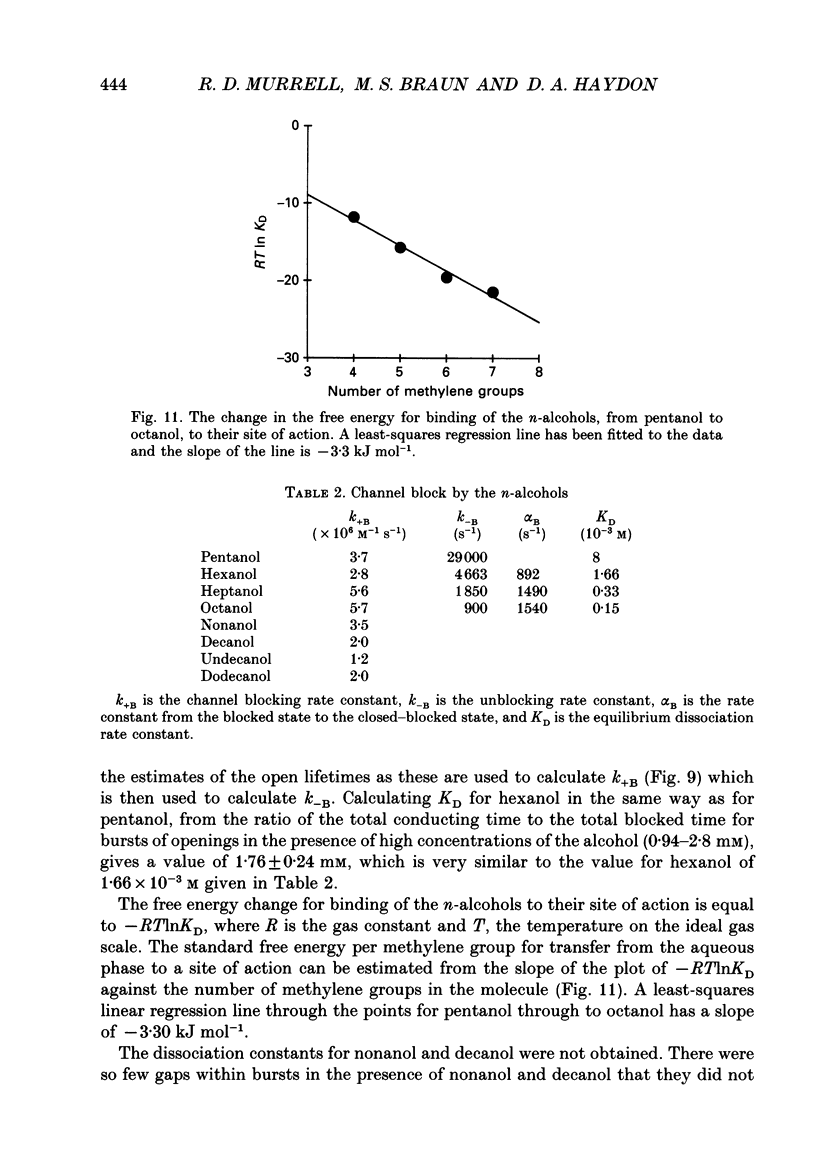
- Alifimoff J. K., Firestone L. L., Miller K. W. Anaesthetic potencies of primary alkanols: implications for the molecular dimensions of the anaesthetic site. Br J Pharmacol. 1989 Jan;96(1):9–16. doi: 10.1111/j.1476-5381.1989.tb11777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahm J. Permeability of human red cells to a homologous series of aliphatic alcohols. Limitations of the continuous flow-tube method. J Gen Physiol. 1983 Feb;81(2):283–304. doi: 10.1085/jgp.81.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnet P., Labarca C., Leonard R. J., Vogelaar N. J., Czyzyk L., Gouin A., Davidson N., Lester H. A. An open-channel blocker interacts with adjacent turns of alpha-helices in the nicotinic acetylcholine receptor. Neuron. 1990 Jan;4(1):87–95. doi: 10.1016/0896-6273(90)90445-l. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A. A., Elliott J. R. The role of inactivation in the effects of n-alkanols on the sodium current of cultured rat sensory neurones. J Physiol. 1989 Aug;415:19–33. doi: 10.1113/jphysiol.1989.sp017709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Haydon D. A. The actions of neutral anaesthetics on ion conductances of nerve membranes. Biochim Biophys Acta. 1989 May 9;988(2):257–286. doi: 10.1016/0304-4157(89)90021-x. [DOI] [PubMed] [Google Scholar]
- Firestone L. L., Sauter J. F., Braswell L. M., Miller K. W. Actions of general anesthetics on acetylcholine receptor-rich membranes from Torpedo californica. Anesthesiology. 1986 Jun;64(6):694–702. doi: 10.1097/00000542-198606000-00004. [DOI] [PubMed] [Google Scholar]
- Forman S. A., Miller K. W. Molecular sites of anesthetic action in postsynaptic nicotinic membranes. Trends Pharmacol Sci. 1989 Nov;10(11):447–452. doi: 10.1016/S0165-6147(89)80009-4. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N., Schneider G. T. Effects of some aliphatic alcohols on the conductance change caused by a quantum of acetylcholine at the toad end-plate. J Physiol. 1975 Jan;244(2):409–429. doi: 10.1113/jphysiol.1975.sp010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Chang J. Y., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: serine-262 of the delta subunit is labeled by [3H]chlorpromazine. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2719–2723. doi: 10.1073/pnas.83.8.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Brodwick M. S. Acetylcholine-induced current in perfused rat myoballs. J Gen Physiol. 1980 Mar;75(3):297–321. doi: 10.1085/jgp.75.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Imoto K., Methfessel C., Sakmann B., Mishina M., Mori Y., Konno T., Fukuda K., Kurasaki M., Bujo H., Fujita Y. Location of a delta-subunit region determining ion transport through the acetylcholine receptor channel. Nature. 1986 Dec 18;324(6098):670–674. doi: 10.1038/324670a0. [DOI] [PubMed] [Google Scholar]
- Jaramillo F., Schuetze S. M. Kinetic differences between embryonic- and adult-type acetylcholine receptors in rat myotubes. J Physiol. 1988 Feb;396:267–296. doi: 10.1113/jphysiol.1988.sp016962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J., Gruener R. Halothane shortens acetylcholine receptor channel kinetics without affecting conductance. Proc Natl Acad Sci U S A. 1984 May;81(9):2929–2933. doi: 10.1073/pnas.81.9.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. J., Labarca C. G., Charnet P., Davidson N., Lester H. A. Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science. 1988 Dec 16;242(4885):1578–1581. doi: 10.1126/science.2462281. [DOI] [PubMed] [Google Scholar]
- Miller K. W. The nature of the site of general anesthesia. Int Rev Neurobiol. 1985;27:1–61. doi: 10.1016/s0074-7742(08)60555-3. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Siegelbaum S. A., Colquhoun D. Block of acetylcholine-activated ion channels by an uncharged local anaesthetic. Nature. 1981 Feb 12;289(5798):596–598. doi: 10.1038/289596a0. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Trautmann A., Koenig J. Single acetylcholine-activated channel currents in developing muscle cells. Dev Biol. 1984 Aug;104(2):366–379. doi: 10.1016/0012-1606(84)90092-7. [DOI] [PubMed] [Google Scholar]


