Abstract
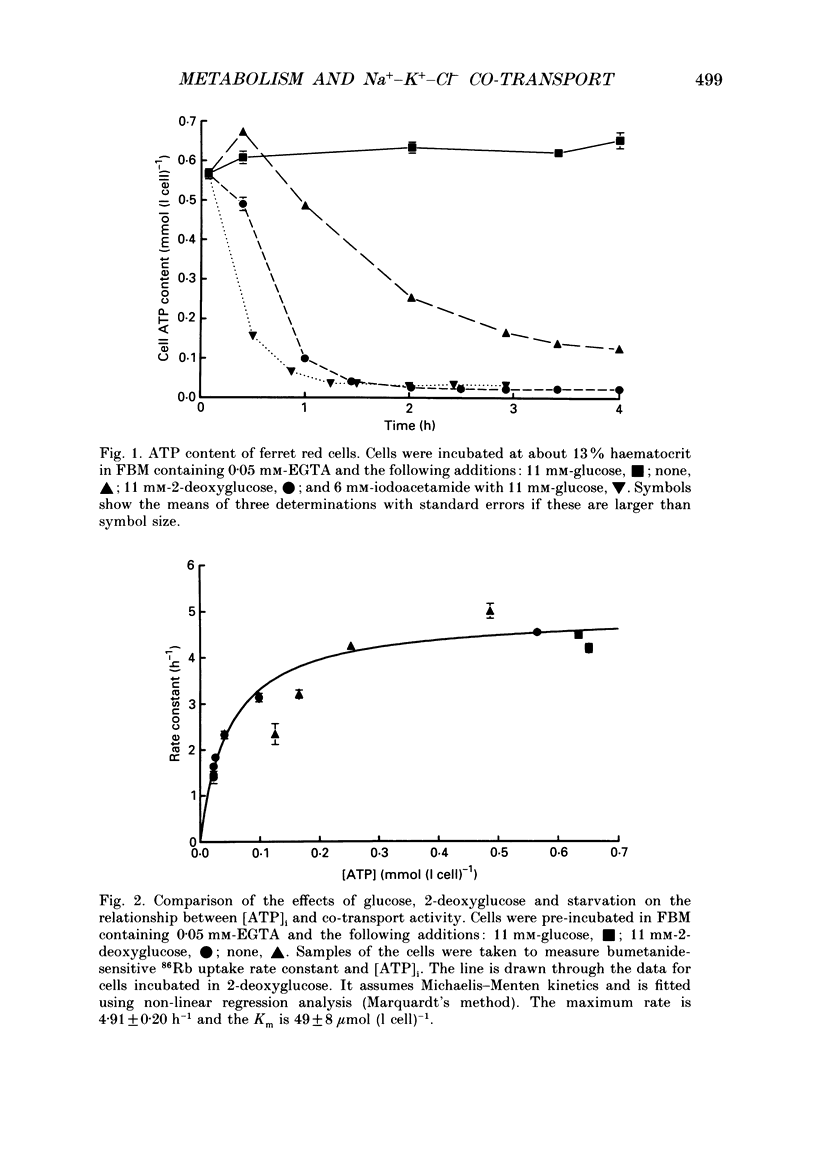
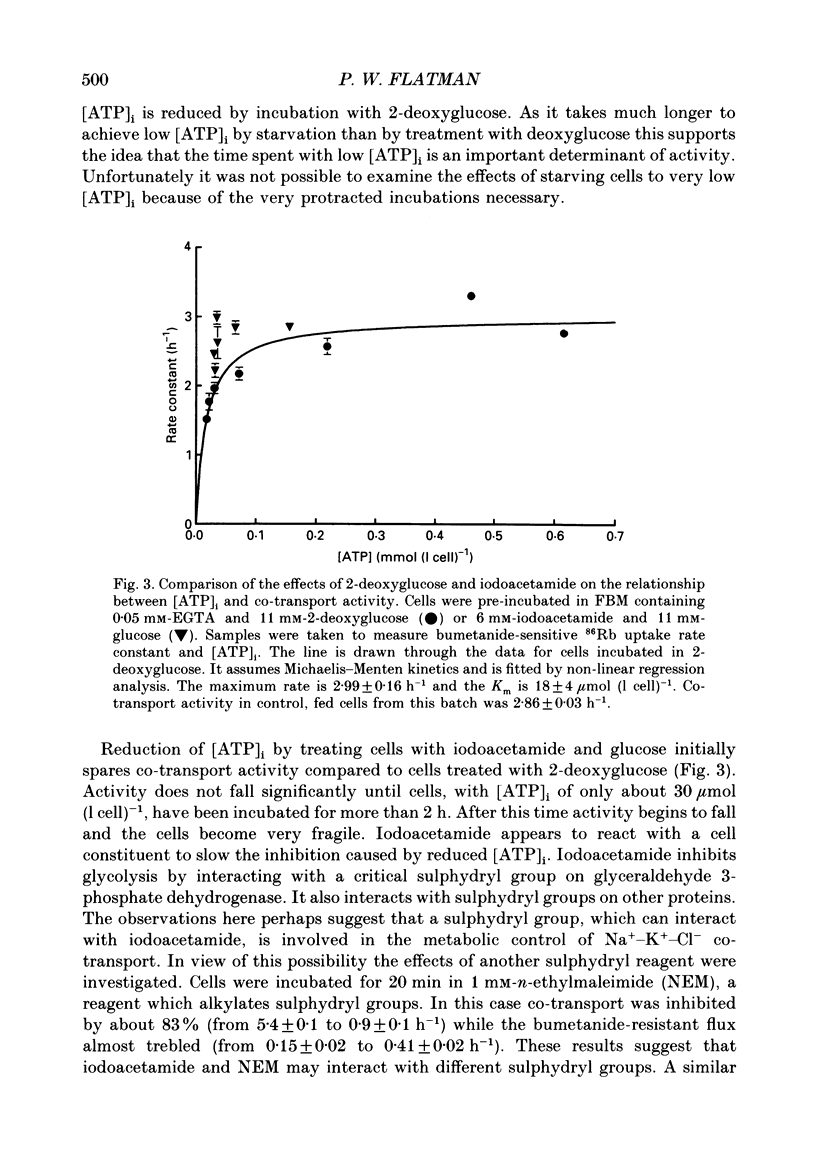
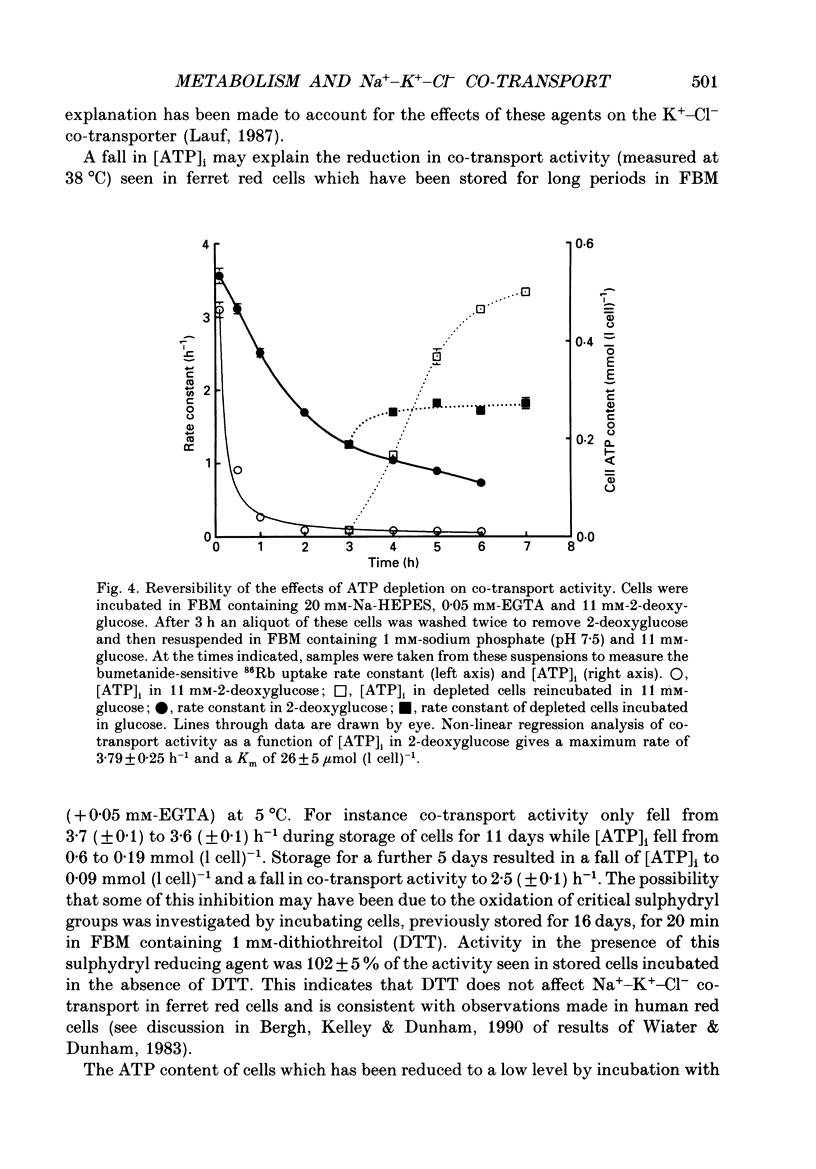
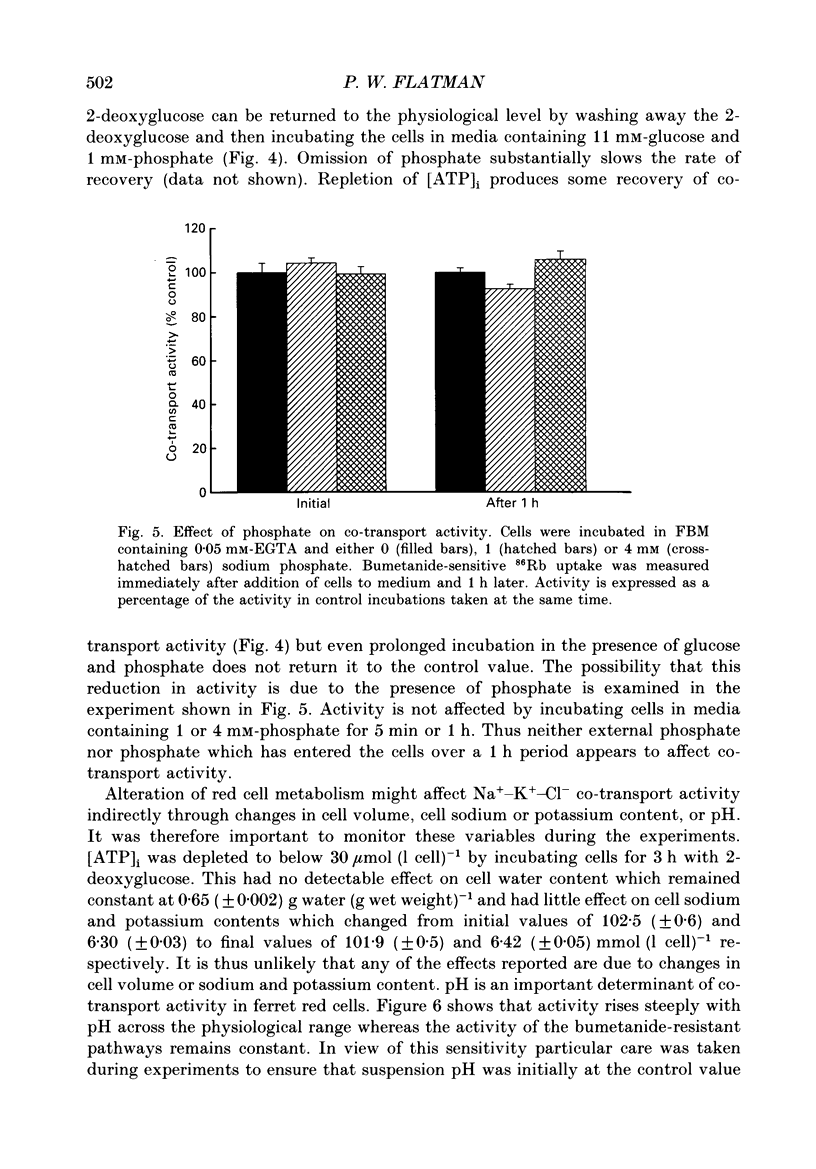
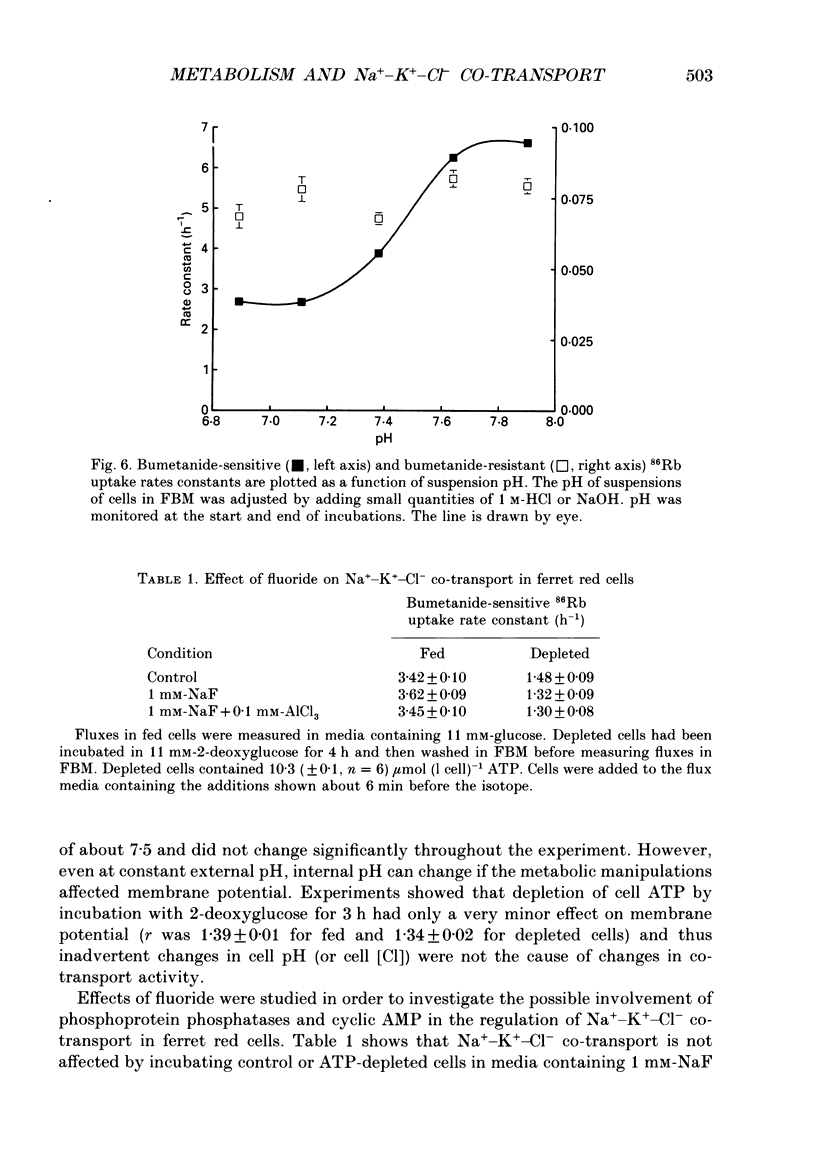
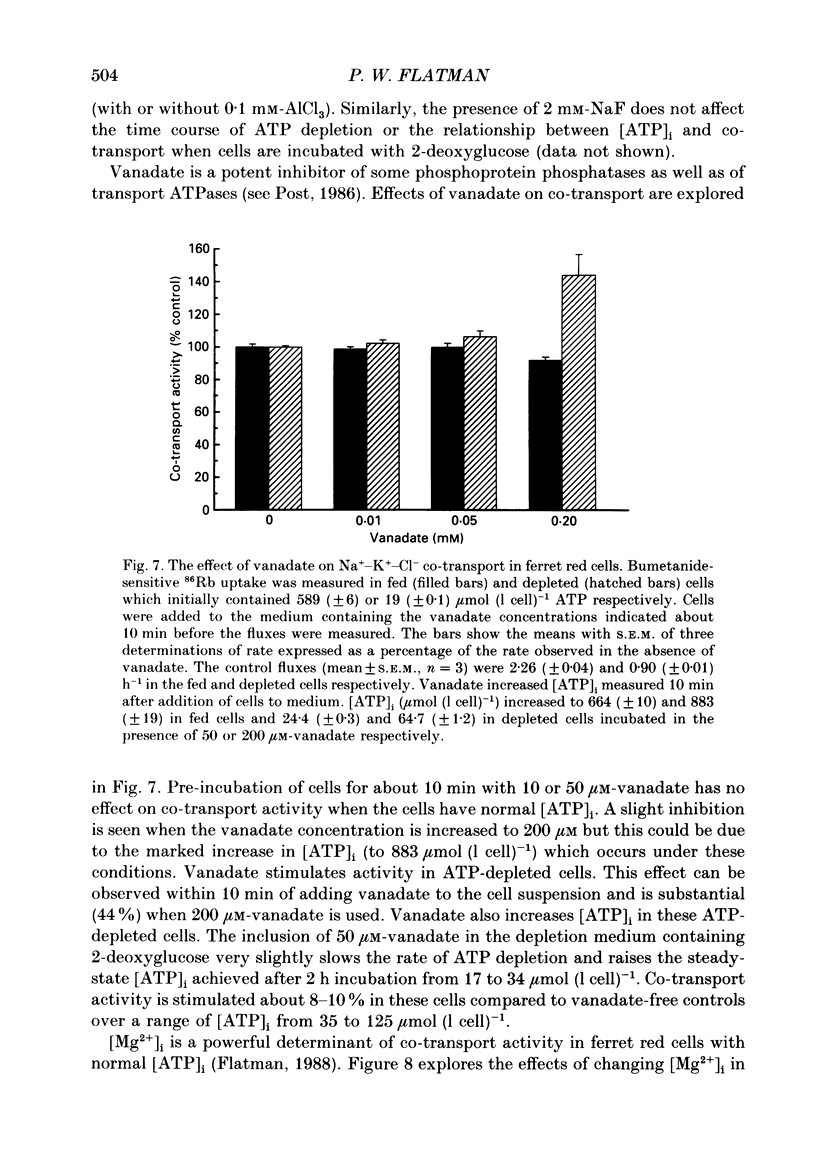
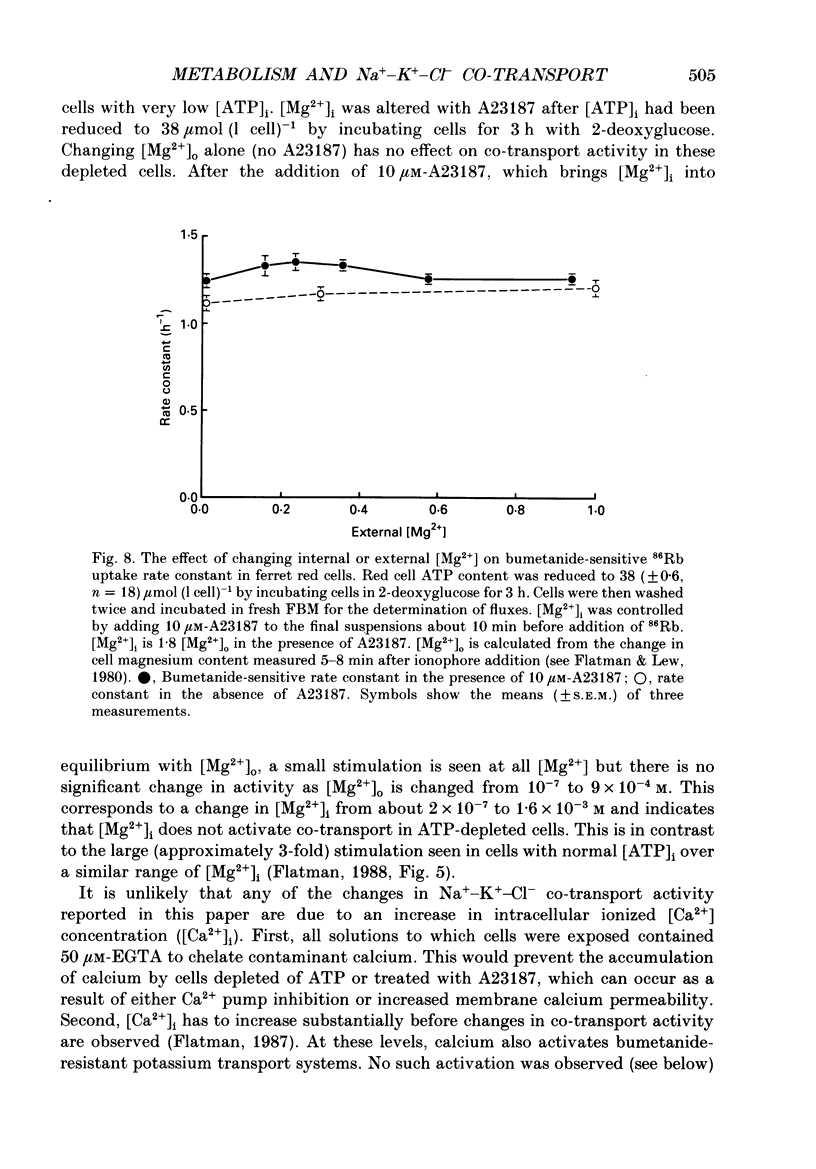
1. The effects of altering metabolism on Na(+)-K(+)-Cl- co-transport were studied in ferret red cells. Na(+)-K(+)-Cl- co-transport was measured as the bumetanide-sensitive uptake of 86Rb. 2. Glucose, but not inosine or adenosine, sustained metabolism and maintained cell ATP content ([ATP]i) at the physiological level. [ATP]i could be reduced by prolonged incubation of cells in a substrate-free medium or more quickly by incubating cells with 2-deoxyglucose or with a mixture of iodoacetamide and glucose. 3. Na(+)-K(+)-Cl- co-transport activity was inhibited when [ATP]i was reduced to below 100 mumol (1 cell)-1 by starvation or by treatment with 2-deoxyglucose. However, a unique relationship between [ATP]i and activity could not be found. [ATP]i and the method and time course of ATP depletion all influenced activity. The inhibition of Na(+)-K(+)-Cl- co-transport, caused by reducing [ATP]i could be partially reversed by restoring [ATP]i to normal. 4. Increasing the concentration of intracellular ionized magnesium [( Mg2+]i) did not stimulate co-transport activity in ATP-depleted cells. This contrasts with the substantial stimulation seen in cells with normal [ATP]i. 5. Vanadate stimulated Na(+)-K(+)-Cl- co-transport activity in ATP-depleted cells but not in cells with normal [ATP]i. Fluoride did not affect activity at any [ATP]i. 6. The effects of some sulphydryl reagents on Na(+)-K(+)-Cl- co-transport were also examined. n-Ethylmaleimide (1 mM) inhibited Na(+)-K(+)-Cl- co-transport while it stimulated bumetanide-resistant potassium transport. Dithiothreitol (1 mM) did not affect activity. Iodoacetamide (6 mM) appeared to reduce the inhibition of cotransport activity seen at low [ATP]i but also greatly increased cell fragility. 7. The data suggest that activity of the Na(+)-K(+)-Cl- co-transport system is controlled by a cycle of phosphorylation and dephosphorylation with the phosphorylated form being active. Phosphorylation and transport appear to be almost maximal in ferret red cells with normal [ATP]i. Reduction of [ATP]i may allow changes in phosphatase activity to manifest as changes in transport rate. Differences in the balance between phosphorylation and dephosphorylation may explain tissue-dependent variations in the response of the system to various stimuli.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adragna N. C., Perkins C. M., Lauf P. K. Furosemide-sensitive Na+-K+ cotransport and cellular metabolism in human erythrocytes. Biochim Biophys Acta. 1985 Jan 10;812(1):293–296. doi: 10.1016/0005-2736(85)90551-6. [DOI] [PubMed] [Google Scholar]
- Alper S. L., Beam K. G., Greengard P. Hormonal control of Na+-K+ co-transport in turkey erythrocytes. Multiple site phosphorylation of goblin, a high molecular weight protein of the plasma membrane. J Biol Chem. 1980 May 25;255(10):4864–4871. [PubMed] [Google Scholar]
- Altamirano A. A., Breitwieser G. E., Russell J. M. Vanadate and fluoride effects on Na+-K+-Cl- cotransport in squid giant axon. Am J Physiol. 1988 Apr;254(4 Pt 1):C582–C586. doi: 10.1152/ajpcell.1988.254.4.C582. [DOI] [PubMed] [Google Scholar]
- Bergh C., Kelley S. J., Dunham P. B. K-Cl cotransport in LK sheep erythrocytes: kinetics of stimulation by cell swelling. J Membr Biol. 1990 Aug;117(2):177–188. doi: 10.1007/BF01868684. [DOI] [PubMed] [Google Scholar]
- Chipperfield A. R. The (Na+-K+-Cl-) co-transport system. Clin Sci (Lond) 1986 Nov;71(5):465–476. doi: 10.1042/cs0710465. [DOI] [PubMed] [Google Scholar]
- Dagher G., Brugnara C., Canessa M. Effect of metabolic depletion on the furosemide-sensitive Na and K fluxes in human red cells. J Membr Biol. 1985;86(2):145–155. doi: 10.1007/BF01870781. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. In squid axons, ATP modulates Na+-Ca2+ exchange by a Ca2+i-dependent phosphorylation. Biochim Biophys Acta. 1987 Mar 12;897(3):347–354. doi: 10.1016/0005-2736(87)90432-9. [DOI] [PubMed] [Google Scholar]
- Duhm J., Göbel B. O. Role of the furosemide-sensitive Na+/K+ transport system in determining the steady-state Na+ and K+ content and volume of human erythrocytes in vitro and in vivo. J Membr Biol. 1984;77(3):243–254. doi: 10.1007/BF01870572. [DOI] [PubMed] [Google Scholar]
- Flatman P. W., Lew V. L. Magnesium buffering in intact human red blood cells measured using the ionophore A23187. J Physiol. 1980 Aug;305:13–30. doi: 10.1113/jphysiol.1980.sp013346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. Sodium and potassium transport in ferret red cells. J Physiol. 1983 Aug;341:545–557. doi: 10.1113/jphysiol.1983.sp014823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. The effects of calcium on potassium transport in ferret red cells. J Physiol. 1987 May;386:407–423. doi: 10.1113/jphysiol.1987.sp016541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. The effects of magnesium on potassium transport in ferret red cells. J Physiol. 1988 Mar;397:471–487. doi: 10.1113/jphysiol.1988.sp017013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geck P., Pietrzyk C., Burckhardt B. C., Pfeiffer B., Heinz E. Electrically silent cotransport on Na+, K+ and Cl- in Ehrlich cells. Biochim Biophys Acta. 1980 Aug 4;600(2):432–447. doi: 10.1016/0005-2736(80)90446-0. [DOI] [PubMed] [Google Scholar]
- Haas M. Properties and diversity of (Na-K-Cl) cotransporters. Annu Rev Physiol. 1989;51:443–457. doi: 10.1146/annurev.ph.51.030189.002303. [DOI] [PubMed] [Google Scholar]
- Haas M., Schmidt W. F., 3rd, McManus T. J. Catecholamine-stimulated ion transport in duck red cells. Gradient effects in electrically neutral [Na + K + 2Cl] Co-transport. J Gen Physiol. 1982 Jul;80(1):125–147. doi: 10.1085/jgp.80.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. C., Ellory J. C. Measurement and stoichiometry of bumetanide-sensitive (2Na:1K:3Cl) cotransport in ferret red cells. J Membr Biol. 1985;85(3):205–213. doi: 10.1007/BF01871515. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., al-Rohil N. Kinetics of activation and inactivation of swelling-stimulated K+/Cl- transport. The volume-sensitive parameter is the rate constant for inactivation. J Gen Physiol. 1990 Jun;95(6):1021–1040. doi: 10.1085/jgp.95.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. D., Tsai Y. S., Franklin C. C., Turner J. T. Characterization of Na+/K+/Cl- cotransport in cultured HT29 human colonic adenocarcinoma cells. Biochim Biophys Acta. 1988 Dec 22;946(2):397–404. doi: 10.1016/0005-2736(88)90415-4. [DOI] [PubMed] [Google Scholar]
- Lauf P. K. K+:Cl- cotransport: sulfhydryls, divalent cations, and the mechanism of volume activation in a red cell. J Membr Biol. 1985;88(1):1–13. doi: 10.1007/BF01871208. [DOI] [PubMed] [Google Scholar]
- Lauf P. K. Thiol-dependent passive K/Cl transport in sheep red cells: VII. Volume-independent freezing by iodoacetamide, and sulfhydryl group heterogeneity. J Membr Biol. 1987;98(3):237–246. doi: 10.1007/BF01871186. [DOI] [PubMed] [Google Scholar]
- Lew V. L. On the ATP dependence of the Ca 2+ -induced increase in K + permeability observed in human red cells. Biochim Biophys Acta. 1971 Jun 1;233(3):827–830. doi: 10.1016/0005-2736(71)90185-4. [DOI] [PubMed] [Google Scholar]
- Li H. C. Phosphoprotein phosphatases. Curr Top Cell Regul. 1982;21:129–174. [PubMed] [Google Scholar]
- Macdonald T. L., Martin R. B. Aluminum ion in biological systems. Trends Biochem Sci. 1988 Jan;13(1):15–19. doi: 10.1016/0968-0004(88)90012-6. [DOI] [PubMed] [Google Scholar]
- Palfrey H. C., Rao M. C. Na/K/Cl co-transport and its regulation. J Exp Biol. 1983 Sep;106:43–54. doi: 10.1242/jeb.106.1.43. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., McRoberts J. A., Saier M. H., Jr (Na+,K+)-cotransport in the Madin-Darby canine kidney cell line. Kinetic characterization of the interaction between Na+ and K+. J Biol Chem. 1982 Mar 10;257(5):2254–2259. [PubMed] [Google Scholar]
- Russell J. M. Anion transport mechanisms in neurons. Ann N Y Acad Sci. 1980;341:510–523. doi: 10.1111/j.1749-6632.1980.tb47195.x. [DOI] [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. Substrate specificity of brain hexokinase. J Biol Chem. 1954 Oct;210(2):581–595. [PubMed] [Google Scholar]
- Saier M. H., Jr, Boyden D. A. Mechanism, regulation and physiological significance of the loop diuretic-sensitive NaCl/KCl symport system in animal cells. Mol Cell Biochem. 1984;59(1-2):11–32. doi: 10.1007/BF00231303. [DOI] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982 Aug;107(3):1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Ueberschär S., Bakker-Grunwald T. Effects of ATP and cyclic AMP on the (Na+ + K+ + 2Cl-)-cotransport system in turkey erythrocytes. Biochim Biophys Acta. 1985 Aug 27;818(2):260–266. doi: 10.1016/0005-2736(85)90566-8. [DOI] [PubMed] [Google Scholar]
- Whittam R., Wiley J. S. Some aspects of adenosine triphosphate synthesis from adenine and adenosine in human red blood cells. J Physiol. 1968 Dec;199(2):485–494. doi: 10.1113/jphysiol.1968.sp008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiater L. A., Dunham P. B. Passive transport of K+ and Na+ in human red blood cells: sulfhydryl binding agents and furosemide. Am J Physiol. 1983 Nov;245(5 Pt 1):C348–C356. doi: 10.1152/ajpcell.1983.245.5.C348. [DOI] [PubMed] [Google Scholar]


