Abstract
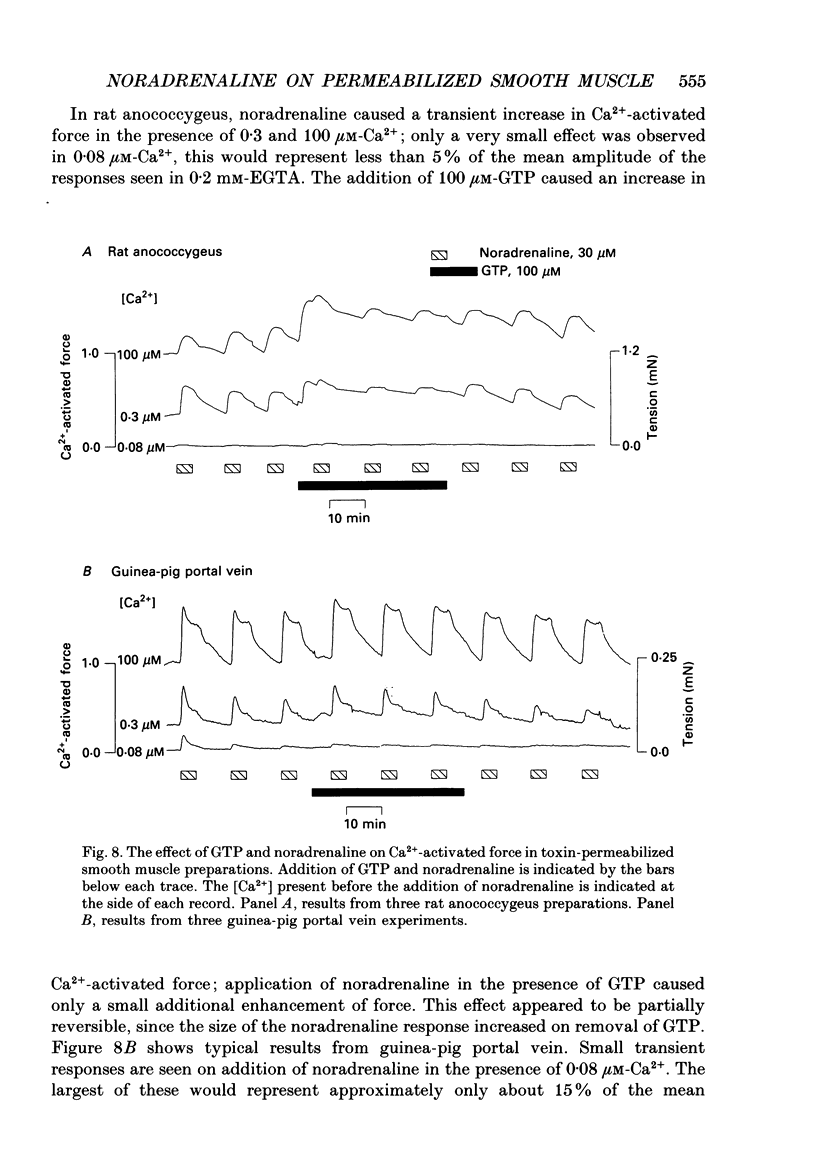
1. Strips of smooth muscle from rat anococcygeus and guinea-pig portal vein were treated with solutions containing crude alpha-toxin from the bacterium Staphylococcus aureus. This rendered the surface membrane permeable to small molecular weight substances, but left functional sarcolemmal adrenoceptors. Tension measurements from these preparations were used to investigate the effects of guanosine-5'-triphosphate (GTP) on the noradrenaline-induced Ca2+ release from the sarcoplasmic reticulum (SR) of the smooth muscle of rat anococcygeus and guinea-pig portal vein. 2. Under conditions of low Ca2+ buffering (0.2 mM-EGTA), applying a maximal dose of noradrenaline (30 microM) to a toxin-permeabilized strip of anococcygeus muscle and longitudinal muscle of guinea-pig portal vein caused a transient contracture. Subsequent exposures to noradrenaline resulted in progressively smaller contractures. However, the rate of decline in the size of the noradrenaline-induced contracture was greater in rat anococcygeus muscle than in guinea-pig portal vein preparations. The decline in the size of the contracture in toxin-permeabilized anococcygeus muscle was not due to a fall in the Ca2+ content of the SR or a reduced Ca2+ release from the SR in response to myo-inositol 1,4,5-trisphosphate (IP3). 3. The tension transients due to noradrenaline were enhanced and maintained in the presence of 100 microM-GTP in toxin-permeabilized guinea-pig portal vein. Addition of 100 microM-GTP caused a transient contracture in permeabilized rat anococcygeus muscle and only promoted the next noradrenaline response, thereafter the amplitude of the contractures decayed to zero. 4. Addition of guanosine-5'-O-(2 thiodiphosphate) (GDP-beta-S, 100 microM) would be expected to cause a reversible reduction of the noradrenaline response by binding to the intermediary G-protein. This was observed in toxin-permeabilized portal vein, but in rat anococcygeus muscle, GDP-beta-S caused slowing of the response to noradrenaline, thereafter the response to noradrenaline was absent. The noradrenaline response did not recover when GDP-beta-S was removed. 5. The non-metabolizable form of GTP, guanosine-5'-O-(3-thiotriphosphate) (GTP-gamma-S, 100 microM), caused a transient contracture in both toxin-permeabilized rat anococcygeus muscle and guinea-pig portal vein. In both these tissues, the addition of GTP-gamma-S resulted in the irreversible inhibition of the response to noradrenaline. 6. In the presence of a high concentration (10 mM) of the Ca2+ buffer EGTA, GTP (100 microM) and noradrenaline (30 microM) increased Ca(2+)-activated force in both tissues.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



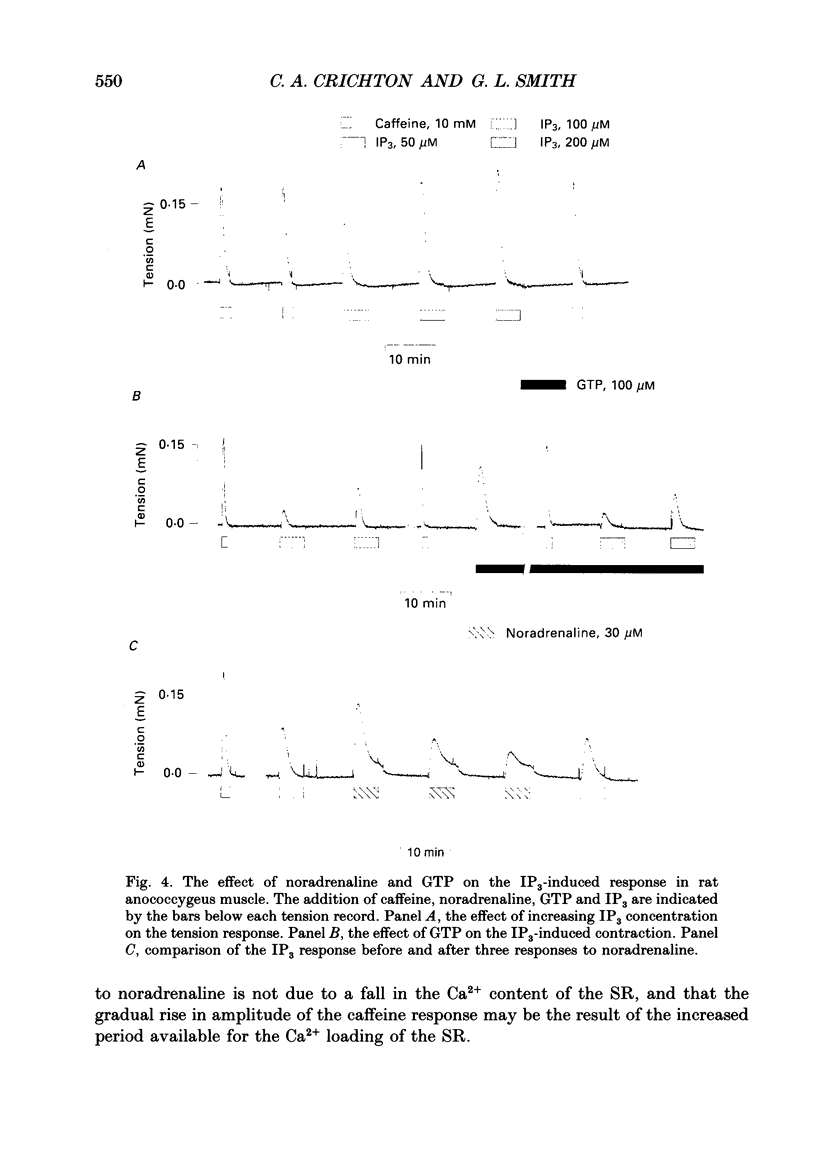

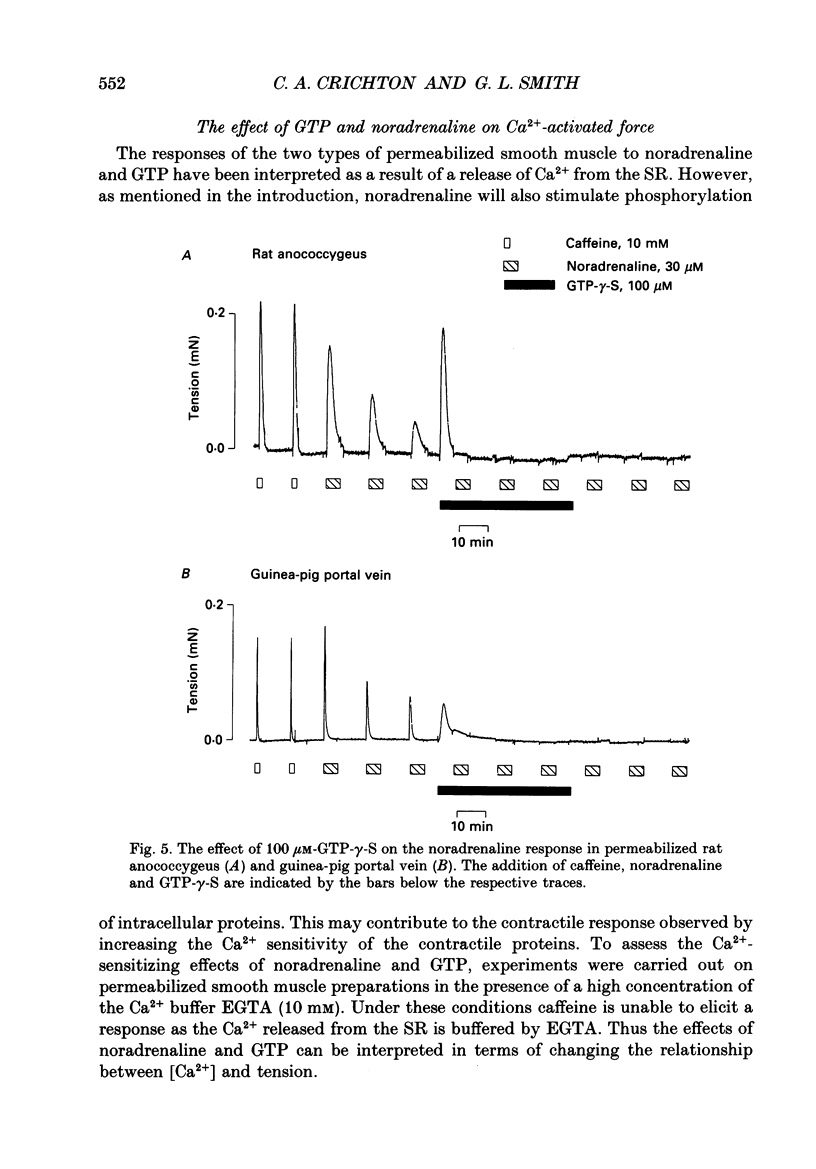
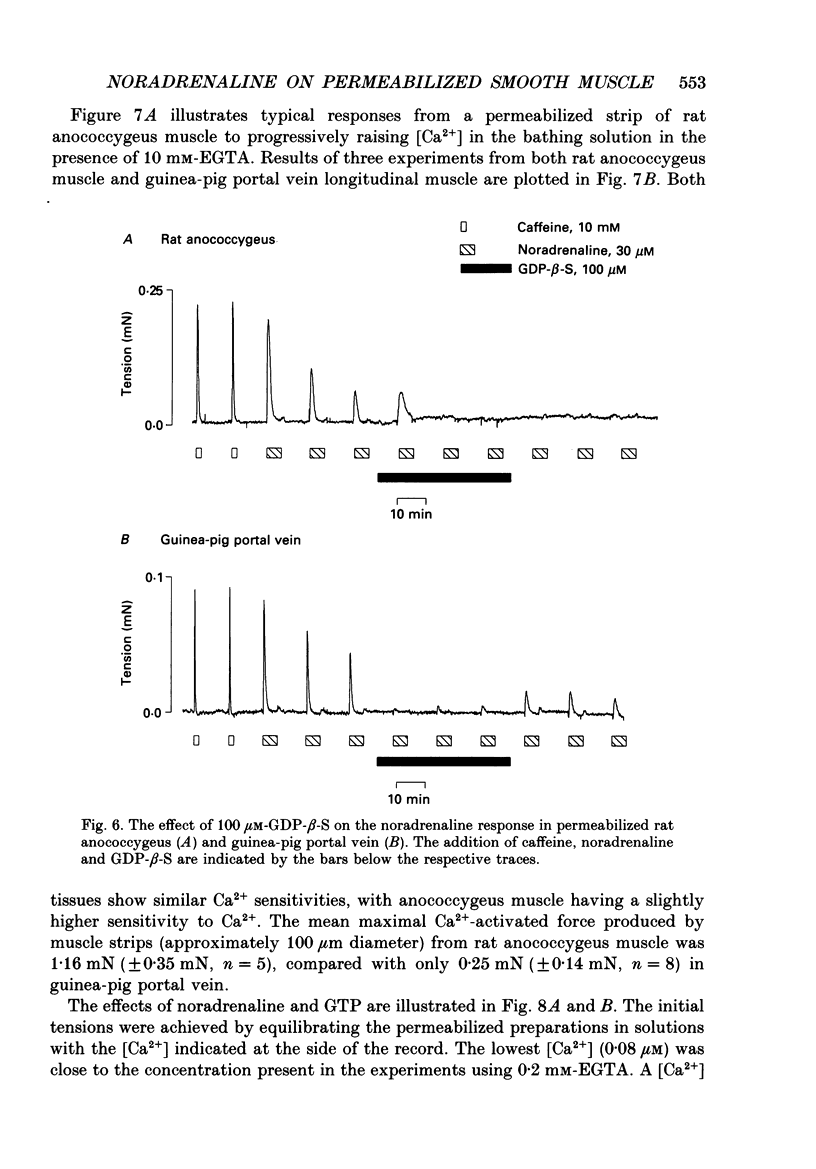
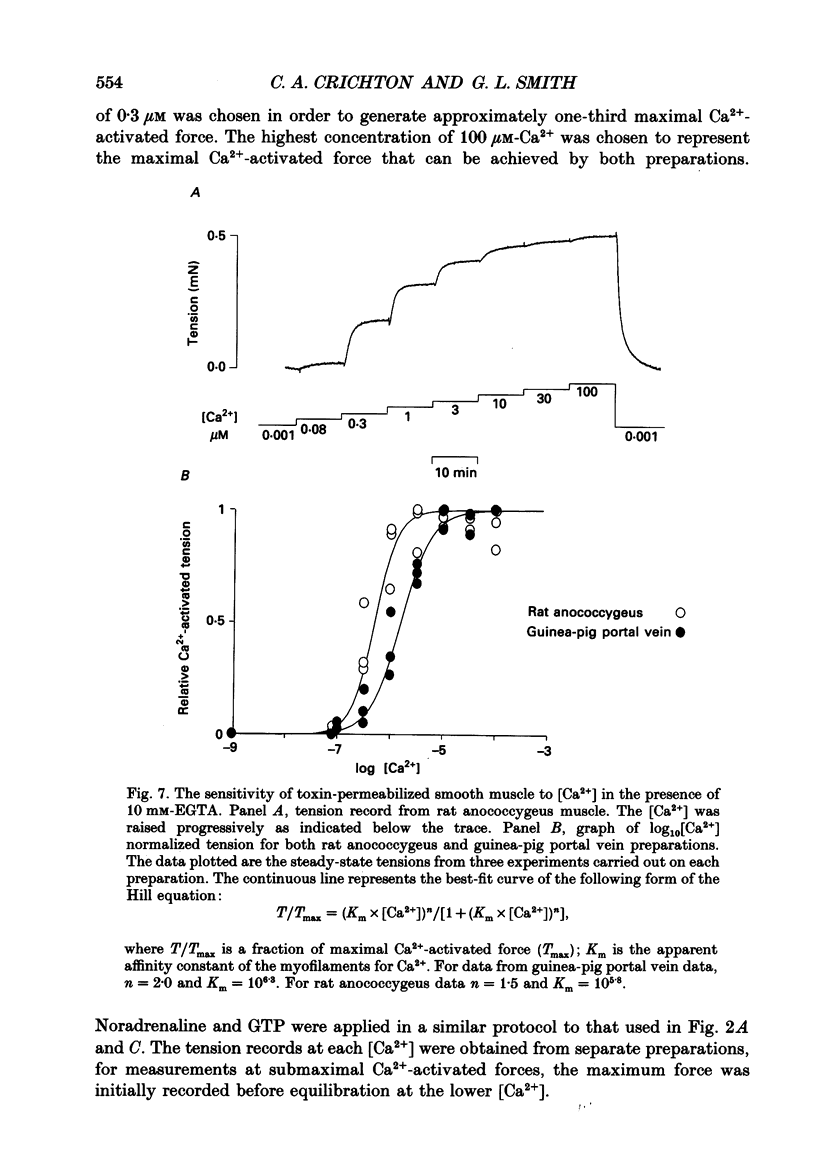






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chueh S. H., Mullaney J. M., Ghosh T. K., Zachary A. L., Gill D. L. GTP- and inositol 1,4,5-trisphosphate-activated intracellular calcium movements in neuronal and smooth muscle cell lines. J Biol Chem. 1987 Oct 5;262(28):13857–13864. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fujiwara T., Itoh T., Kubota Y., Kuriyama H. Effects of guanosine nucleotides on skinned smooth muscle tissue of the rabbit mesenteric artery. J Physiol. 1989 Jan;408:535–547. doi: 10.1113/jphysiol.1989.sp017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. L., Mullaney J. M., Ghosh T. K. Intracellular calcium translocation: mechanism of activation by guanine nucleotides and inositol phosphates. J Exp Biol. 1988 Sep;139:105–133. doi: 10.1242/jeb.139.1.105. [DOI] [PubMed] [Google Scholar]
- Gill D. L., Ueda T., Chueh S. H., Noel M. W. Ca2+ release from endoplasmic reticulum is mediated by a guanine nucleotide regulatory mechanism. Nature. 1986 Apr 3;320(6061):461–464. doi: 10.1038/320461a0. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Calcium dependent inositol trisphosphate-induced calcium release in the guinea-pig taenia caeci. Biochem Biophys Res Commun. 1987 Jan 15;142(1):47–52. doi: 10.1016/0006-291x(87)90449-9. [DOI] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Kitazawa T., Kobayashi S., Horiuti K., Somlyo A. V., Somlyo A. P. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem. 1989 Apr 5;264(10):5339–5342. [PubMed] [Google Scholar]
- Kobayashi S., Somlyo A. V., Somlyo A. P. Heparin inhibits the inositol 1,4,5-trisphosphate-dependent, but not the independent, calcium release induced by guanine nucleotide in vascular smooth muscle. Biochem Biophys Res Commun. 1988 Jun 16;153(2):625–631. doi: 10.1016/s0006-291x(88)81141-0. [DOI] [PubMed] [Google Scholar]
- McGrath J. C., Brown C. M., Wilson V. G. Alpha-adrenoceptors: a critical review. Med Res Rev. 1989 Oct-Dec;9(4):407–533. doi: 10.1002/med.2610090403. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Ruegg J. C. Dependence of cyclic-AMP induced relaxation on Ca2+ and calmodulin in skinned smooth muscle of guinea pig Taenia coli. Pflugers Arch. 1983 Dec;399(4):315–320. doi: 10.1007/BF00652759. [DOI] [PubMed] [Google Scholar]
- Minneman K. P. Alpha 1-adrenergic receptor subtypes, inositol phosphates, and sources of cell Ca2+. Pharmacol Rev. 1988 Jun;40(2):87–119. [PubMed] [Google Scholar]
- Nishimura J., Kolber M., van Breemen C. Norepinephrine and GTP-gamma-S increase myofilament Ca2+ sensitivity in alpha-toxin permeabilized arterial smooth muscle. Biochem Biophys Res Commun. 1988 Dec 15;157(2):677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Patel P., Bose D., Greenway C. Effects of prazosin and phenoxybenzamine on alpha- and beta-receptor-mediated responses in intestinal resistance and capacitance vessels. J Cardiovasc Pharmacol. 1981 Sep-Oct;3(5):1050–1059. doi: 10.1097/00005344-198109000-00015. [DOI] [PubMed] [Google Scholar]
- Sagi-Eisenberg R. GTP-binding proteins as possible targets for protein kinase C action. Trends Biochem Sci. 1989 Sep;14(9):355–357. doi: 10.1016/0968-0004(89)90001-7. [DOI] [PubMed] [Google Scholar]
- Saida K., Twort C., van Breemen C. The specific GTP requirement for inositol 1,4,5-trisphosphate-induced Ca2+ release from skinned vascular smooth muscle. J Cardiovasc Pharmacol. 1988;12 (Suppl 5):S47–S50. [PubMed] [Google Scholar]
- Somlyo A. P., Walker J. W., Goldman Y. E., Trentham D. R., Kobayashi S., Kitazawa T., Somlyo A. V. Inositol trisphosphate, calcium and muscle contraction. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):399–414. doi: 10.1098/rstb.1988.0084. [DOI] [PubMed] [Google Scholar]
- Walker J. W., Somlyo A. V., Goldman Y. E., Somlyo A. P., Trentham D. R. Kinetics of smooth and skeletal muscle activation by laser pulse photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1987 May 21;327(6119):249–252. doi: 10.1038/327249a0. [DOI] [PubMed] [Google Scholar]