Abstract
We studied the silencing of the cryptic mating-type loci HMLα and HMRa in the budding yeast Kluyveromyces lactis. A 102-bp minimal silencer fragment was defined that was both necessary and sufficient for silencing of HMLα. Mutagenesis of the silencer revealed three distinct regions (A, B, and C) that were important for silencing. Recombinant K. lactis ribosomal DNA enhancer binding protein 1 (Reb1p) could bind the silencer in vitro, and point mutations in the B box abolished both Reb1p binding and silencer function. Furthermore, strains carrying temperature-sensitive alleles of the REB1 gene derepressed the transcription of the HMLα1 gene at the nonpermissive temperature. A functional silencer element from the K. lactis cryptic HMRa locus was also identified, which contained both Reb1p binding sites and A boxes, strongly suggesting a general role for these sequences in K. lactis silencing. Our data indicate that different proteins bind to Kluyveromyces silencers than to Saccharomyces silencers. We suggest that the evolution of silencers is rapid in budding yeasts and discuss the similarities and differences between silencers in Saccharomyces and Kluyveromyces.
Two copies of the same gene can display different expression levels, even though the two genes are present in the same nucleus. As a consequence of the nuclear environment surrounding a gene, specialized chromatin structures can influence how accessible the promoter of the gene is to the transcriptional machinery. This phenomenon, called “position effect,” has been observed in many organisms, and intensively studied examples include X-chromosome inactivation in mammals (49), position effect variegation in Drosophila (23), and silencing of the cryptic mating-type loci in yeasts (40). In yeasts, loci that exhibit position effects are characterized by chromatin structures that are inaccessible to DNA-modifying enzymes (39, 58), and the chromatin of these loci contains hypoacetylated histones (11).
In the budding yeast Saccharomyces cerevisiae, position effects have been observed at the cryptic mating-type loci HMLα and HMRa (29, 45, 51), at telomeres (22), and at the ribosomal DNA (rDNA) locus (12, 19, 59). At the cryptic mating-type loci, this phenomenon is called “silencing” and requires DNA elements close to the inactivated loci and dedicated proteins. Because these DNA elements mediate transcriptional repression, the opposite effect of enhancers, they are called “silencers” (9). The polycomb response element in Drosophila (14, 57) and sequences near the cryptic mating-type cassettes in Schizosaccharomyces pombe (64) are other examples of silencers, demonstrating that silencers are not unique to budding yeast. Silencers can be required for establishing and/or maintaining inactive chromatin, but exactly how silencers accomplish this is not known.
The best-studied silencer is the Saccharomyces HMR-E silencer. HMR-E consists of a combination of binding sites for Rap1p, the origin recognition complex (ORC), and Abf1p (10, 55). Rap1p and Abf1p are transcription factors that bind to many promoters in yeast (13, 55). ORC is required for the initiation of replication (8), and HMR-E is in fact a chromosomal replication origin (52). It is believed that the combination of these binding sites presents a surface that nucleates the formation of a higher-order chromatin structure that spreads, thus repressing nearby promoters. Sir proteins (Sir2 to -4) are essential for silencing (26, 50) and appear to be part of the protein-DNA complex that defines silent chromatin (62). The Sir3 and Sir4 proteins are recruited to the silent domains by direct interaction with Rap1p (43). Euchromatic genes juxtaposed to telomeric sequences are also silenced in a Sir-dependent manner (3). Telomeres contain multiple Rap1p binding sites, and the Rap1p molecules bound to these sites are probably sufficient to recruit Sir proteins to telomeres. The single Rap1p binding site at HMR-E, however, is probably not sufficient for Sir recruitment, but also other interactions occur, such as the interaction between Sir proteins and ORC (18, 65). Sir3p and Sir4p also interact with each other (42) and with histone tails (24), providing additional evidence that multiple protein-protein interactions are required for silencing. HMR-E shows signs of redundancy, since deletion of the Rap1p, Abf1p, or ORC binding sites individually does not abolish silencing, but deletion of two of these sites simultaneously does (10). The Sir2 protein is a histone decacetylase (25, 34, 60) and probably contributes to the deacetylation of histone tails in silent chromatin (11).
Much less is known about silencing in Kluyveromyces. Kluyveromyces lactis also contains cryptic mating-type loci, and the cryptic α-locus (HMLα) requires Sir2p and Sir4p to remain silent (5). Both Sir2p and Sir4p are also required for maintaining a specialized chromatin structure at HMLα, as would be expected (4). Moreover, both Sir2p and Sir4p are functionally interchangeable with their Saccharomyces counterparts (5, 16), but K. lactis and S. cerevisiae Sir4p have remarkably divergent amino acid sequences. We previously defined silencers close to both HMLα and HMRa (4), but the features and possible protein binding sites of these silencers remained unknown.
To better understand the architecture of a silencer, we have analyzed the K. lactis HMLα silencer. We found three discrete sequences within the silencer that were essential for silencing. One of these sequences was a Reb1p DNA binding site, and we could show that Reb1p itself was important for silencing. Similarly to Rap1p, Reb1p is a Myb domain transcription factor (27), suggesting that evolution has used Myb domain-containing proteins repeatedly to generate silencers.
MATERIALS AND METHODS
Plasmid construction.
Plasmids p291 (pCXJ18-HMLα without silencer) p300 (pCXJ18-HMLα plus 1.6-kb silencer), p326 (pCXJ18-HMLα plus 719-bp silencer), and p413 (pCXJ18-HMLα plus 102-bp minimal silencer) were described previously (4). Plasmid p423 was p291 with a 301-bp BamHI-KpnI PCR fragment originating from the HMRa flanking region, corresponding to nucleotides 425 to 726 (the first base after the stop codon of the a1 gene was defined as nucleotide 1). A BamHI-KpnI silencer fragment from p413 and p326 was cloned into the corresponding sites of pRS306 (56), generating plasmids pp1.6 and p318, respectively. Phagemids were produced from pp1.6 and p318, and then the mutations indicated in Fig. 1 were introduced with the appropriate oligonucleotides by in vitro mutagenesis (32). The mutant silencers were cloned back into the p291 vector (BamHI-KpnI) after the mutations had been confirmed by DNA sequencing. Plasmid pAB24-REB1 (from J. Warner, Albert Einstein College of Medicine, New York, N.Y.) was used as a template to generate a 2.2-kb REB1 PCR fragment containing SacI-NsiI sites at the ends, and this fragment was cloned into the SacI-PstI sites of pCXJ20 (LEU2 CEN) (15). A PCR fragment including the Myb domain of REB1 was cloned into a T-vector (pGEM-T; Promega). Phagemids were produced, and the mutations indicated in Fig. 4 were generated with the appropriate oligonucleotides by in vitro mutagenesis (32). The mutations were confirmed by DNA sequencing, and from the resulting plasmids, a 415-bp XbaI-BsiWI fragment was exchanged for the corresponding fragment of pCXJ20-REB1. The resulting plasmids were p500 (reb1 RRK329 to AAA329), p502 (reb1 EED346 to AAA346, reb1-1), and p504 (reb1 EEE398 to AAA398 reb1-2). A pCXJ20-ScREB1 plasmid was generated by cloning a 2.8-kb SacI-BamHI PCR fragment corresponding to the entire Saccharomyces REB1 gene, including the promoter region, into the corresponding sites of pCXJ20. A maltose binding protein (MBP)-Reb1 fusion protein was generated by cloning an EcoRI-SalI PCR fragment corresponding to amino acids 1 to 595 of Reb1p into the corresponding sites of pMALc2 (New England Biolabs).
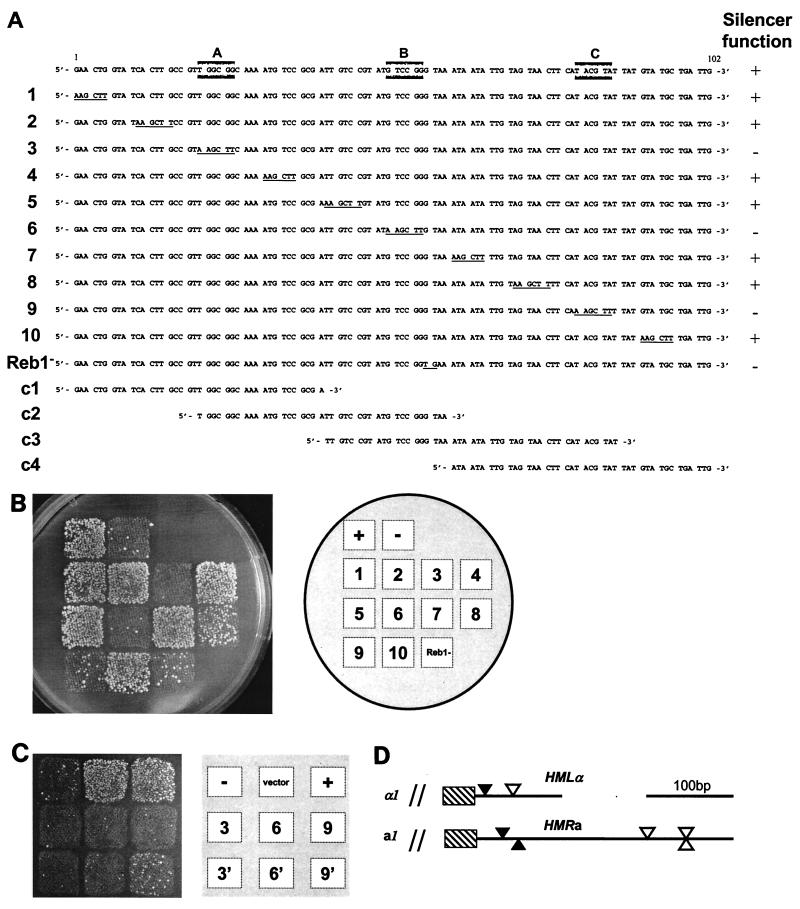
FIG. 1.
Three discrete sites were required for silencer function. (A) DNA sequence of the HMLα silencer and mutations generated. The top line represents the wild-type sequence, and lines 1 to 10 represent mutations generated in the silencer. Changed bases are underlined. Reb1− shows the 2-bp substitution in the Reb1p recognition sequence. Lines c1 to c4 represent the DNA fragments used for competition in the gel mobility shift experiment. A, B, and C (shaded boxes) indicate the sequences defined to be essential for silencer function. Silencer function in the mating assay is indicated on the right. (B) Mating assay for silencer function. A tester mater strain (MATα WM52V4) was mated to a MATa strain (CK213-4C) containing different plasmids. These plasmids contained the HMLα locus either lacking a silencer (p291; −) or containing a wild-type silencer (p413; +). Mutant silencers were as indicated in panel A. Diploids were selected on synthetic dextrose plates. (C) Introduction of ORC binding sites did not improve the silencer function of A and B box mutations. A mating assay was performed as described in panel B. Squares 3, 6, and 9 are the same silencer mutations indicated in panel A, and 3′, 6′, and 9′ indicate that the adjacent ORC binding site was included on the plasmid. A plasmid without an insert is denoted as the vector. (D) Schematic drawing of the HMLα and HMRa silencers. Sequence analysis with MatInspector software revealed the presence of both A boxes (solid arrowhead) and B boxes (Reb1 binding-sites) (open arrowhead) at both the HMLα and HMRa silencers.
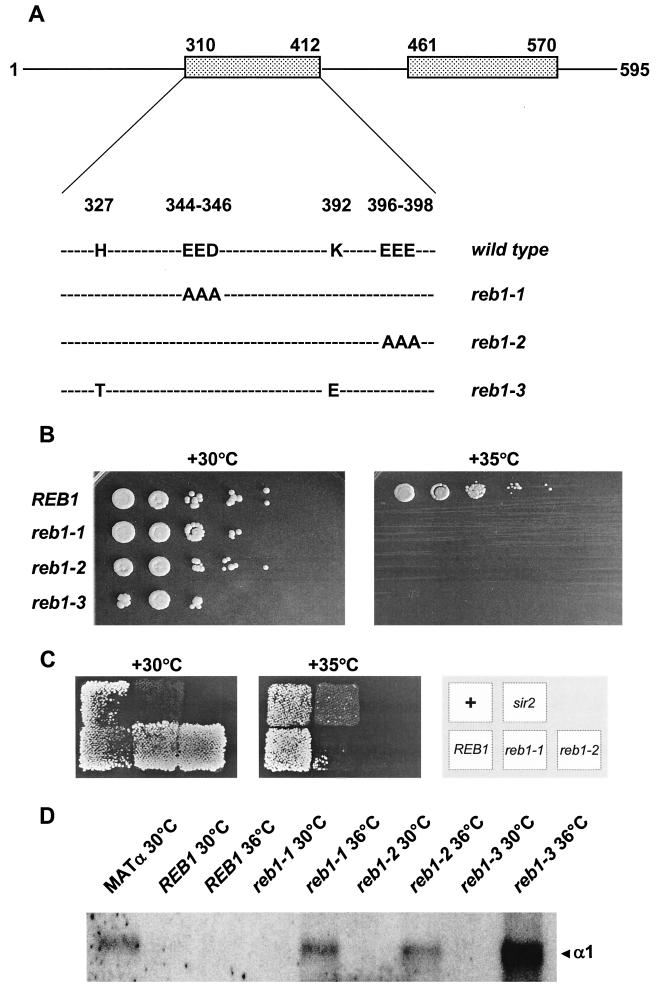
FIG. 4.
Reb1p was essential for silencing in K. lactis. (A) Schematic drawing of three reb1(Ts) alleles. K. lactis Reb1p (595 amino acids long) contains two Myb domains (shaded boxes, top row), and the amino acid substitutions generated in the reb1(Ts) mutants are indicated below. The reb1-1 and reb1-2 alleles were generated by site-directed mutagenesis, and the reb1-3 allele was generated by hydroxylamine mutagenesis. Amino acid numbers in which the mutations were generated are also indicated. (B) The reb1 alleles generated were temperature sensitive. Serial dilutions (10-fold) of a K. lactis reb1::hisG strain (SAY206) dependent on plasmid-encoded Reb1p were spotted onto rich medium (yeast extract-peptone-dextrose) at 30 and 35°C. The plasmid contained either the wild-type REB1 gene or the reb1-1, reb1-2, and reb1-3 temperature-sensitive alleles,as indicated on the left. (C) Strains dependent on the reb1-1 and reb1-2 alleles were sterile at the restrictive temperature. A mating assay was performed, crossing strain SAY206 (MATa reb1::hisG) containing plasmids with the indicated reb1 allele with strain WM52V4 (MATα). Matings were allowed to proceed for 24 h at the indicated temperature and replica plated onto selective medium (synthetic dextrose plus uracil), and diploids were allowed to grow for an additional 72 h. As a control, strains CK213 (+ [wild type]) and SAY156 (sir2) were included. (D) The cryptic HMLα1 gene was derepressed at the nonpermissive temperature in the reb1(Ts) strains. RNA-blot analysis was performed with total RNA prepared from a MATa reb1::hisG strain (SAY206) dependent on plasmid-encoded Reb1p grown at either the permissive temperature (30°C) or the nonpermissive temperature (36°C). The REB1 allele present in the strain, as well as the temperature used, is indicated above the lanes. The blot was probed with a 32P-labeled PCR fragment corresponding to the HMLα1 gene, and RNA prepared from a MATα strain (left) was used as a control. The last lane (reb1-3 [36°C]) was overloaded.
Strain construction.
The genotypes of all strains used in this study are indicated in Table 1. All disruptions were confirmed on DNA blots. A diploid strain generated by crossing strains CK213 and SAY119 was transformed with HindIII-linearized pRS306-reb1::LEU2 to generate a REB1/reb1::LEU2 heterozygous diploid by a two-step gene disruption procedure (53). Tetrad analysis of this diploid revealed that viability segregated 2:2, and all survivors were leucine auxotrophs, indicating that the REB1 gene was essential. The reb1::LEU2 allele was converted into a reb1::hisG allele by using plasmid pNKY85 (2). Plasmid pAB24-REB1 (URA3) (44) was introduced into the resulting REB1/reb1::hisG diploid strain, and tetrad analysis was performed. 5-Fluoro-orotic acid (5-FOA)-sensitive progeny were identified, resulting in strains SAY205 and SAY206. Strain SAY208 was generated by crossing SAY205 to SAY130 followed by tetrad analysis. Strains dependent on either S. cerevisiae REB1 or mutant derivatives of K. lactis REB1 were generated by introducing LEU2 plasmids with the tested gene into SAY206, and the transformants were plated on synthetic complete medium lacking leucine but containing 5-FOA (5-FOA-LEU). The result of this plasmid shuffling procedure was that the pAB24-REB1 plasmid was exchanged for a LEU2 plasmid containing a mutant REB1 gene.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| K. lactis | ||
| WM52V4 | MATα ade1 adeX his7 uraA1 | 16 |
| CK213-4C | MATalysA1 trp1 leu2 metA1 uraA1 | 16 |
| SAY119 | MATα uraA1 ade1 leu2 trp1 metA1 | 4 |
| SAY130 | CK213 hmlα1::KanMX | 4 |
| SAY156 | SAY130 sir2 | 4 |
| SAY205 | MATα uraA1 ade1 leu2 trp1 metA1 reb1::hisG + pAB24-REB1 | This study |
| SAY206 | MATauraA1 leu2 trp1 metA1 reb1::hisG + pAB24-REB1 | This study |
| SAY208 | MATauraA1 leu2 lysA1 trp1 metA1 reb1::hisG hmlα1::KanMX + pAB24-REB1 | This study |
| S. cerevisiae J343 | MAT? ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 reb1::LEU2 + pAB24-KlREB1 | J. Warner, Albert Einstein College of Medicine, New York, N.Y. |
Yeast media and methods.
DNA manipulations, RNA-DNA blots, DNA sequencing, and media for growth of yeast and bacteria followed standard protocols (1, 6). Mating assays were also performed as described previously (5). For the gel mobility shift assay, the binding reaction was performed in a mixture of 20 mM HEPES-KOH (pH 7.6), 10% glycerol, 100 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 0.05% NP-40, 70 ng of poly(dI-dC) per μl, 300 μg of bovine serum albumin per ml, 20,000 cpm of 32P-labeled DNA probe (0.1 to 0.5 ng of 102-bp minimal silencer), and approximately 15 μg of crude extract or 10 ng of purified MBP-Reb1 protein. For competition assays, unlabeled competitor DNA was added at approximately 30-fold molar excess over the probe. The reaction mixture (15 μl) was incubated at 30°C for 15 min. The samples were then analyzed by nondenaturing polyacrylamide gel electrophoresis (40:1 acrylamide/bisacrylamide ratio) with Tris-acetate (TAE) (6) as gel and electrophoresis buffer. The gel was dried and autoradiographed. Total RNA was prepared from late-logarithmic-phase-growing cells (SAY206 plus plasmids) grown at 30 or at 36°C for approximately 14 h. Strains dependent on the temperature-sensitive reb1 alleles [reb1(Ts)] were viable after 14 h at 36°C, but had ceased to divide.
RESULTS
Three discrete sequence elements were important for silencer function.
Previously, we identified a minimal silencer element, 102 bp long, that was both necessary and sufficient for silencing of HMLα in a silencing assay. This assay measured the mating proficiency of a MATa strain containing plasmids with HMLα sequences (4). Since the simultaneous expression of a and α information inhibits mating in K. lactis, mutant plasmids that inhibited mating of the MATa strain contained expressed α1 and α2 genes. The HMLα DNA fragments tested contained the entire α1 and α2 genes, but only included half of the α3 gene. If a silencer fragment was included on this plasmid, the α genes were not expressed, and the strain mated efficiently (4). To pinpoint short sequences within the minimal silencer that were important for silencing and thus might define protein-binding sites, we performed an extensive mutational analysis of the minimal silencer. Ten mutant derivatives of the silencer were generated by oligonucleotide-directed mutagenesis. For convenience, the altered base pairs were changed into a HindIII site (Fig. 1A). By using the plasmid-based assay for silencing, we determined that three of these mutant silencers could no longer support silencing in the mating assay. The remaining seven mutant silencers had mating efficiencies similar to that of the wild-type silencer (Fig. 1B). We call the sequences that were defined as important for silencing by this assay the “A, B, and C boxes.”
In Saccharomyces, the HMR-E silencer is partly redundant for the Abf1p, Rap1p, and ORC binding sites. Thus, a silencer lacking only one of these elements is still functional, but if two of the sites are mutated simultaneously, then silencing is abolished (10). Since we previously found an ARS sequence (ORC binding site) close to the minimal silencer at HMLα, we wanted to determine whether the A, B, and C boxes were equally important for silencing when the ARS sequence was included on the silencer fragment. We thus generated the A, B, and C box mutations in a silencer fragment that was 712 bp long and thus included the ARS sequence (4). In the silencing assay, none of these mutant silencers were completely functional, thus indicating that the ARS sequence was not redundant with the A, B, or C boxes for silencer function (Fig. 1C). In the case of the C box mutation, a slight improvement of silencing was observed when the longer silencer fragment was used, but we assume that this improvement may be due to cryptic C boxes in the longer fragment (see Discussion). Thus, we defined three short sequence elements that were important for silencing of HMLα and found no evidence for involvement of ORC.
Previously, we defined a 301-bp fragment close to the K. lactis HMRa locus that could act as a silencer in our mating assay. In Saccharomyces, the HMRa and HMLα silencers are similar and consist of similar protein binding sites. We wanted to investigate if sequences similar to the A, B, and C boxes could be found in the K. lactis HMRa silencer. The HMRa silencer was thus analyzed with the MatInspector software, and we found two good matches to both the A box sequence and three good matches to the B box sequence (Fig. 1D). The HMRa silencer did not contain sequences similar to those of the C box. Since A and B box sequences were found in both silencers, they were excellent candidates for protein binding sites that mediated silencing in Kluyveromyces.
K. lactis extracts contained a protein that bound to the B box.
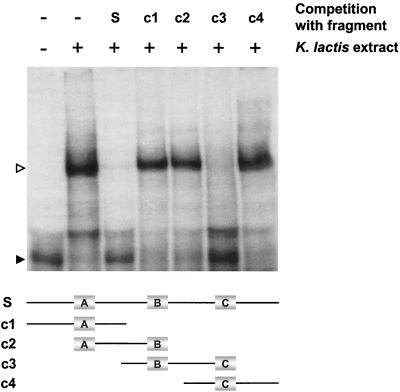
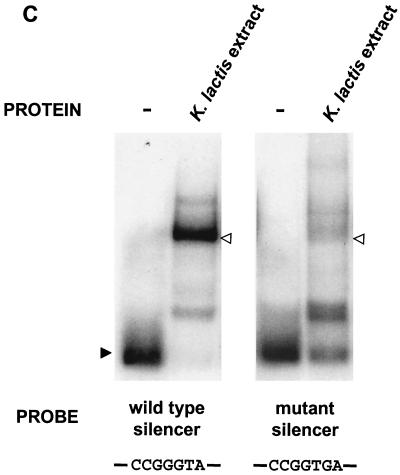
Since we suspected that the silencer contained protein binding sites, we performed a DNA mobility shift assay, using the minimal silencer and protein extracts from K. lactis. One major, slower-migrating mobility shift and one minor, faster-migrating mobility shift were observed (Fig. 2). To determine whether the binding was sequence specific and what part of the silencer these activities recognized, we added competing DNA to the band shift reaction. A 30-fold excess of unlabeled wild-type silencer abolished the slower-migrating mobility shift, but had less impact on the faster-migrating mobility shift. The faster-migrating mobility shift was thus probably the result of a nonspecific interaction and was not considered further. The use of competing DNA from different parts of the silencer revealed that a 40-bp fragment from the central part of the 102-bp fragment (fragment c3, Fig. 2) could efficiently compete for binding, but other parts of the silencer could not. Two of the fragments that failed to compete for binding (fragments c2 and c4) actually overlapped with the competing fragment, thus indicating that the binding site of this protein was at the junction between fragments c2 and c4. We confirmed this notion by adding both fragments c2 and c4 simultaneously to the band shift reaction, which did not result in efficient competition (data not shown). Thus, for the competition to work, it was important that the sequence at the junction of fragments c2 and c4 was covalently attached, such as in fragment c3. Interestingly, the B box sequence defined above as important for silencing was found at the junction between fragments c2 and c4.
FIG. 2.
A fragment containing a consensus Reb1p binding site efficiently competed for binding to the silencer by a K. lactis protein extract. An electrophoretic mobility shift assay showing the ability of unlabeled parts of the silencer to compete with a labeled 102-bp minimal silencer for binding to a K. lactis protein extract was performed. The competing DNA fragments (30-fold molar excess; Fig. 1A) are schematically depicted below. A Reb1p binding site was present in competing fragment c3, but not present or only partially present in the overlapping fragments c2 and c4. The free silencer fragment (solid arrowhead) and shifted silencer fragment (open arrowhead) are indicated.
The B box corresponded to a Reb1p binding site.
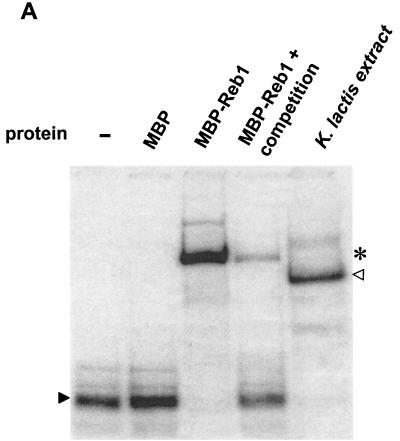
Inspection of the sequence at the junction between fragments c2 and c4 revealed a consensus binding site for the rDNA enhancer binding protein 1 (Reb1p), CCGGGTA (37). The REB1 gene was previously identified in K. lactis (44), facilitating the cloning of REB1 into an Escherichia coli expression vector. We purified recombinant MBP-Reb1p fusion protein and tested whether this protein could bind to the minimal silencer in the DNA mobility shift assay. Recombinant MBP-Reb1p efficiently bound to the silencer element, and the position of the shifted complex was consistent with that of the shift of the silencer fragment with K. lactis protein extracts, considering that the recombinant protein contained an MBP moiety (Fig. 3A). Also the binding of the recombinant MBP-Reb1p protein to the silencer was specific, since a 30-fold excess of unlabeled silencer DNA almost abolished binding. We thus conclude that the HMLα silencer contained a Reb1p binding site.
FIG. 3.
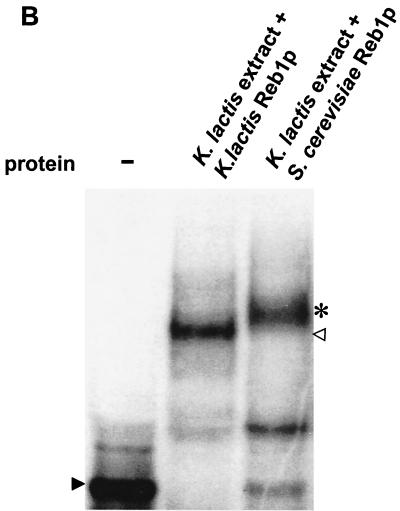
Reb1p binds to the minimal silencer. (A) Recombinant MBP-Reb1p bound to the minimal silencer. An electrophoretic mobility shift assay (EMSA) of labeled 102-bp minimal silencer incubated without protein (−), with MBP alone, MBP-Reb1p, and a K. lactis protein extract was performed. Additionally, wild-type unlabeled silencer at a 30-fold molar excess was incubated together with MBP-Reb1p to compete for labeled silencer binding. A solid arrowhead indicates unbound minimal silencer, an open arrowhead indicates the Reb1p mobility shift, and an asterisk indicates the MBP-Reb1p shift. (B) Reb1p was the major protein that bound to the silencer in K. lactis protein extracts. Protein extracts prepared from reb1::hisG strain SAY206, containing plasmids carrying either the K. lactis REB1 gene or the S. cerevisiae REB1 gene, were tested in an EMSA for the ability to bind the minimal silencer. Since the K. lactis and S. cerevisiae Reb1p proteins are of different sizes, the S. cerevisiae Reb1p mobility shift (asterisk) migrated slower than the K. lactis Reb1p mobility shift (open triangle). The unbound silencer is indicated by the solid arrowhead. (C) Inefficient binding of a silencer containing point mutations in the Reb1p binding site. EMSA with either a labeled wild-type silencer fragment or a labeled silencer containing two point mutations in the Reb1p binding site (Reb1− in Fig. 1A) was performed. A K. lactis protein extract was incubated either with wild-type silencer or the Reb1− silencer. −, no protein added to the reaction mixture. The free probe is indicated by a solid arrowhead, and the shifted probe is indicated by an open arrowhead.
Next we wanted to determine if the silencer DNA binding activity from K. lactis extracts really corresponded to Reb1p. An alternative hypothesis was that K. lactis contained another DNA binding protein, which binds to a sequence similar to the Reb1p DNA binding site. To determine this, we generated a strain containing a deletion of the genomic K. lactis REB1 gene (see Materials and Methods). Tetrad analysis of a heterozygous REB1/reb1::hisG diploid indicated that REB1 was an essential gene, since the viability of this meiosis segregated 2:2. This lethality could be complemented, however, if the heterozygous REB1/reb1::hisG diploid was transformed with a plasmid containing the REB1 gene. We thus generated a haploid strain containing a reb1::hisG-null allele that was rescued by a URA3 plasmid containing the REB1 gene. The possibility that Reb1p was only required for spore germination and not vegetative growth was tested by plating this haploid strain on medium containing 5-FOA. Since 5-FOA selects against the URA3 gene, cells that contain the URA3 plasmid cannot grow on this medium. The REB1 gene was required for vegetative growth of K. lactis, since the haploid reb1::hisG strain was unable to cut loose the URA3-REB1 plasmid and thus grow on 5-FOA medium. Others previously showed that K. lactis REB1 complemented S. cerevisiae reb1 strains (44). We wanted to test if the reverse was true and thus made the K. lactis reb1::hisG strain dependent on Saccharomyces REB1 with plasmid shuffling (see Materials and Methods). The resulting strain was viable and displayed no growth rate defect. K. lactis and S. cerevisiae REB1 thus cross-complemented each other in both organisms. Next we took advantage of the fact that the K. lactis and S. cerevisiae REB1 genes encode proteins of different sizes. Kluyveromyces Reb1p is only 595 amino acids long, whereas Saccharomyces Reb1p is 810 amino acids long. If the silencer DNA binding activity that was present in K. lactis protein extracts corresponded to Reb1p, then we would expect to se a slower-migrating gel mobility shift with protein extract from the reb1::hisG strain that exclusively expressed Saccharomyces Reb1p. This notion was confirmed (Fig. 3B), and we thus concluded that the HMLα silencer contained a Reb1p binding site, which was recognized both by endogenous Reb1p and Saccharomyces Reb1p.
Reb1p was required for silencing.
We noted that the Reb1p binding site overlapped with the previously defined B box sequence and wanted to learn if Reb1p binding was important for silencing. Since the REB1 gene is essential in K. lactis, we could not assay silencing in a reb1-deficient strain. Instead, we generated a silencer that contained two point mutations (in boldface) in the Reb1p binding site (Fig. 1) by changing CCGGGTA into CCGGTGA. The identical mutations were previously shown to abolish the binding of Saccharomyces Reb1p (36), which has the same recognition sequence as K. lactis Reb1p (44). This mutant silencer did not silence HMLα in the plasmid assay (Fig. 1B), and mobility shift experiments with a DNA fragment with the same point mutations showed that Reb1p binding was severely impaired (Fig. 3C). These correlative data strongly suggested that Reb1p binding to the silencer was important for silencing in K. lactis.
To confirm that Reb1p indeed was required for silencing, we wanted to generate conditional alleles of the REB1 gene, so that we could inactivate Reb1p in living cells and investigate if this would lead to the derepression of the cryptic HMLα locus. An interesting observation was that K. lactis strains exclusively expressing Saccharomyces Reb1p displayed normal mating and did not derepress a KanMX marker gene at HMLα (data not shown), demonstrating that the Saccharomyces protein was proficient for silencing. The K. lactis and S. cerevisiae Reb1p proteins share only 40% sequence identity, and we expected that the K. lactis REB1 gene had to be mutationally compromised in positions conserved between Saccharomyces and Kluyveromyces in order to generate conditional alleles. We thus performed a limited alanine-scanning mutagenesis of charge clusters (67) (at least three charged amino acids in a row) that were conserved between the two yeasts. All of the conserved charge clusters were found in the two Myb domains of Reb1p, which are responsible for DNA binding. To avoid generating mutations that would abolish DNA binding and thus generate a completely inactive Reb1p molecule, we took advantage of a previous study that solved the structure of the Myb domain of a mouse protein called “c-myb” (46, 47). Utilization of this structure to predict the structure of the REB1 Myb domains revealed that several of the conserved charge clusters probably were present in the potential DNA binding helices. We chose to generate mutations predicted to be on the surface of the first Myb domain (Fig. 4A ). Three mutants bearing targeted mutations in this domain were generated, two of which had a phenotype (Fig. 4A). A K. lactis reb1::hisG strain was made dependent on these alleles by plasmid shuffling, and the resulting strains were tested for growth at high temperature. Compared to the strain containing wild-type REB1, strains expressing the reb1 EED346-to-AAA346 allele (reb1-1) and the reb1 EEE398-to-AAA398 allele (reb1-2) were unable to grow at 35°C, but grew normally at 30°C (Fig. 4B). We had thus generated two temperature-sensitive alleles in the REB1 gene. We also attempted to isolate reb1(Ts) alleles by a random mutagenesis approach. A plasmid containing the REB1 open reading frame was mutagenized with hydroxylamine and tested for a conditional phenotype in the reb1::hisG strain. By this procedure, we isolated one additional reb1(Ts) allele, which we call “reb1-3.” DNA sequencing of this allele revealed that the REB1 open reading frame contained five missense mutations. By subcloning different fragments of the reb1-3 allele into a wild-type REB1 gene, we determined that the mutations responsible for the conditional growth phenotype mapped to the first Myb domain (H327 to T327 and K392 to E392; Fig. 4). We did not investigate which one of these two mutations (or both) generated the temperature sensitivity.
At temperatures below the restrictive temperature, strains carrying the reb1-1, reb1-2, and reb1-3 alleles were not silencing deficient, since these strains did not derepress an hmlα1::KanMX marker gene (assayed on plates containing Geneticin). Then, we compared the mating efficiency of a strain containing the wild-type REB1 gene to strains containing reb1(Ts) alleles (Fig. 4C). The reb1-1 and reb1-2 strains remained viable for at least 24 h at the restrictive temperature (data not shown), making it possible to test the mating efficiency at both the permissive and restrictive temperatures. At the permissive temperature, the reb1(Ts) strains did not have a mating defect, but at the restrictive temperature, neither the reb1-1 strain nor the reb1-2 strain could mate (Fig. 4C). This result was consistent with the finding that Reb1p was essential for silencing, but it was also possible that this was a pleiotrophic phenotype caused by another function of Reb1p, distinct from silencing. Therefore, we wanted to test whether silencing of HMLα was lost when strains carrying the reb1(Ts) alleles were shifted to the nonpermissive temperature. RNA was prepared from wild-type, reb1-1, reb1-2, and reb1-3 strains grown at 30°C and then shifted to the nonpermissive temperature (36°C, in liquid) thus inactivating Reb1p in the temperature-sensitive strains. An RNA-blot analysis was then performed with the HMLα1 gene as a probe (Fig. 4D). This analysis revealed that the reb1(Ts) strains grown at 36°C derepressed the cryptic HMLα1 gene. The wild-type strain did not derepress HMLα1 transcription at 36°C. These data thus confirmed the notion that Reb1p was essential for silencing in K. lactis.
DISCUSSION
In this study, we characterized the K. lactis HMLα silencer. We found three discrete sequences that were essential for silencing and identified one of them as a Reb1p binding site. We found no evidence for the involvement of Rap1p or ORC binding sites. Additionally, Reb1p binding sites are not found in silencer sequences in Saccharomyces. The S. cerevisiae and K. lactis species are both classified as hemiascomycetes and belong to the same order, Saccharomycetales (61). They are more closely related to each other than either is related to budding yeasts, such as Candida albicans and Yarrowia lipolytica (7). Given this close evolutionary relationship, it was surprising that the silencers appear so different in the two yeasts. A straightforward interpretation would be that the evolution of silencers is rapid. Others showed that the conservation of transcription factors among 13 different hemiascomycetous yeasts were lower than would be expected (20). They speculated that rapid evolution of transcription factors was somehow selected during speciation. The difference between Saccharomyces and Kluyveromyces silencers could thus be partly explained by rapid evolution of regulatory factors. Several similarities exist, however, and below we will discuss similarities and differences between the Saccharomyces and Kluyveromyces silencers.
Even though a functional ARS element was found close to the HMLα silencer in K. lactis, this ORC binding site did not contribute to silencing. The A and B box mutations completely abolished silencing, even in the presence of the ORC-binding site. The C box mutation, however, was partially suppressed by including the DNA fragment that contained the ARS activity. Not only was the ORC-binding site included on this fragment, but flanking DNA was included as well, and we prefer an explanation in which cryptic C boxes were present in these flanking sequences. The C box, defined by the mutagenesis of the silencer, bears a weak resemblance to an Abf1p binding site. In the DNA sequence between the minimal silencer and the ORC-binding site, there are in fact other sites that show a closer resemblance to an Abf1p binding site than the C box does. It should be noted that the resemblance of the C box to an Abf1p binding site is weak, and thus it would be premature to conclude that Abf1 binds to this site. Moreover, in the HMRa silencer, we could not find sequences that resembled Abf1p binding sites or the C box, indicating that the protein binding to the C box was only important for silencing of HMLα and not HMRa.
The A box, found both in the HMLα and HMRa silencers, was similar to a binding site for the Saccharomyces Ume6p protein (63). One of the two A boxes in the HMRa silencer was in fact a perfect match to the Ume6p consensus sequence. In some gel mobility shift experiments (see, for example, Fig. 3C) using K. lactis protein extracts, we observed protein-DNA complexes that were migrating slower than the Reb1p-silencer complex. This observation is consistent with the idea that other proteins bind to the silencer, but such proteins are of low abundance or require different conditions for optimal DNA binding. In Saccharomyces, Ume6p is required for repressing transcription from meiosis-specific promoters during vegetative growth (48, 63), but has not been implicated in silencing. Ume6p recruits the Sin3p-Rpd3p complex to meiotic promoters (28), and therefore we tested whether Rpd3p was important for silencing in Kluyveromyces. Strains containing an rpd3-null allele showed improved silencing, indicating that Rpd3p counteracted silencing in Kluyveromyces (data not shown). Thus, if the A box was a Ume6p binding site, then recruitment of Rpd3p to this binding site cannot be important for maintaining silencing. It is still possible that the A box binds Ume6p, but Ume6p in this context recruits a complex distinct from the Rpd3p-containing complex. In Saccharomyces, Ume6p can in fact recruit a chromatin-remodeling complex containing the ISWI2 gene product (21). We are currently investigating the role of the K. lactis UME6 and ISW2 genes in silencing.
In this study, we showed that the B box was a binding site for the Reb1 protein, and by using point mutations in the B box and conditional alleles of the REB1 gene, we concluded that Reb1p binding to the silencer was essential for silencing. The Reb1 protein contains two so-called “Myb domains.” The Myb domain is characterized by regularly spaced tryptophan residues, separated by 18 or 19 amino acids (46). This domain forms a structure related to, but distinct from, the canonical helix-loop-helix motif. The tryptophan repeat structure appears to be a general motif, utilized in the many members of the Myb gene family. It has already been noted that proteins that bind to eukaryotic telomeres often contain Myb-like domains (54). In humans (Trf1) (66), the budding yeasts S. cerevisiae and K. lactis (Rap1) (30, 38, 41), and the fission yeast (Taz1) (17), Myb domain proteins bind to telomeres. Moreover, in the case of S. pombe Taz1p and S. cerevisiae Rap1p, the interaction with telomeres contributes to telomeric position effect (17, 33, 43). In this study, we show that yet another Myb domain protein contributes to silencing in K. lactis. Thus, the Myb domain may have features that make it suited for silencers, since Myb domain proteins are required for silencing in such a diverse set of organisms.
It is tempting to speculate about the role, if any, of Reb1p in the phenomenon rDNA silencing. Reb1p binding to the rDNA locus to at least two different binding sites ensures correct transcriptional termination by RNA polymerase I (35, 36) and probably also affects the expression of rRNA (31). The rDNA locus can also impart position effects on RNA polymerase II-transcribed genes artificially placed in this locus (12, 19, 59). This phenomenon, rDNA silencing, requires Sir2p, but not the other Sir proteins (19, 59). To our knowledge, no silencer has been identified in the rDNA locus. Given the role of Reb1p in Kluyveromyces silencing, it would appear to be worth the effort to test the role of Reb1p in rDNA silencing in Saccharomyces.
Further study of the K. lactis HMLα and HMRa silencers promises to be a fruitful avenue of research to learn more about characteristics of and variations among these DNA elements. It is likely that the A and C boxes correspond to protein binding sites, and the identification of the proteins binding to these sites is a high priority. This study shows that two evolutionarily related yeasts have quite different silencer elements to impart silencing of their cryptic mating-type loci. Furthermore, the K. lactis silencers cannot functionally complement S. cerevisiae HMR-E (A. Ehrenhofer-Murray, personal communication). Learning more about Kluyveromyces silencers should further our understanding about how silencer elements work in general.
Acknowledgments
J. Warner is acknowledged for plasmids and strains. We thank U. Sauer for help with predicting the structure of the Reb1p Myb domain.
S.U.Å. is supported by grants from the Swedish Natural Science Research Council (B-AA/BU 11279-306).
REFERENCES
- 1.Adams, A., D. E. Gottschling, C. A. Kaiser, and T. Stearns. 1998. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 2.Alani, E., L. Cao, and N. Kleckner. 1987. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics 116:541-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aparicio, O. M., B. L. Billington, and D. E. Gottschling. 1991. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 66:1279-1287. [DOI] [PubMed] [Google Scholar]
- 4.Åström, S. U., A. Kegel, J. O. Sjöstrand, and J. Rine. 2000. Kluyveromyces lactis Sir2p regulates cation sensitivity and maintains a specialized chromatin structure at the cryptic alpha-locus. Genetics 156:81-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Åström, S. U., and J. Rine. 1998. Theme and variation among silencing proteins in Saccharomyces cerevisiae and Kluyveromyces lactis. Genetics 148:1021-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 2002. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 7.Barns, S. M., D. J. Lane, M. L. Sogin, C. Bibeau, and W. G. Weisburg. 1991. Evolutionary relationships among pathogenic Candida species and relatives. J. Bacteriol. 173:2250-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell, S. P., and B. Stillman. 1992. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 357:128-134. [DOI] [PubMed] [Google Scholar]
- 9.Brand, A. H., L. Breeden, J. Abraham, R. Sternglanz, and K. Nasmyth. 1985. Characterization of a “silencer” in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell 41:41-48. [DOI] [PubMed] [Google Scholar]
- 10.Brand, A. H., G. Micklem, and K. Nasmyth. 1987. A yeast silencer contains sequences that can promote autonomous plasmid replication and transcriptional activation. Cell 51:709-719. [DOI] [PubMed] [Google Scholar]
- 11.Braunstein, M., A. B. Rose, S. G. Holmes, C. D. Allis, and J. R. Broach. 1993. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 7:592-604. [DOI] [PubMed] [Google Scholar]
- 12.Bryk, M., M. Banerjee, M. Murphy, K. E. Knudsen, D. J. Garfinkel, and M. J. Curcio. 1997. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 11:255-269. [DOI] [PubMed] [Google Scholar]
- 13.Buchman, A. R., and R. D. Kornberg. 1990. A yeast ARS-binding protein activates transcription synergistically in combination with other weak activating factors. Mol. Cell. Biol. 10:887-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan, C. S., L. Rastelli, and V. Pirrotta. 1994. A Polycomb response element in the Ubx gene that determines an epigenetically inherited state of repression. EMBO J. 13:2553-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, X. J. 1996. Low- and high-copy-number shuttle vectors for replication in the budding yeast Kluyveromyces lactis. Gene 172:131-136. [DOI] [PubMed] [Google Scholar]
- 16.Chen, X.-J., and G. D. Clark-Walker. 1994. sir2 mutants of Kluyveromyces lactis are hypersensitive to DNA-targeting drugs. Mol. Cell. Biol. 14:4501-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper, J. P., E. R. Nimmo, R. C. Allshire, and T. R. Cech. 1997. Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature 385:744-747. [DOI] [PubMed] [Google Scholar]
- 18.Fox, C. A., A. E. Ehrenhofer-Murray, S. Loo, and J. Rine. 1997. The origin recognition complex, SIR1, and the S phase requirement for silencing. Science 276:1547-1551. [DOI] [PubMed] [Google Scholar]
- 19.Fritze, C. E., K. Verschueren, R. Strich, and R. Easton Esposito. 1997. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 16:6495-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaillardin, C., G. Duchateau-Nguyen, F. Tekaia, B. Llorente, S. Casaregola, C. Toffano-Nioche, M. Aigle, F. Artiguenave, G. Blandin, M. Bolotin-Fukuhara, E. Bon, P. Brottier, J. de Montigny, B. Dujon, P. Durrens, A. Lepingle, A. Malpertuy, C. Neuveglise, O. Ozier-Kalogeropoulos, S. Potier, W. Saurin, M. Termier, M. Wesolowski-Louvel, P. Wincker, J. Souciet, and J. Weissenbach. 2000. Genomic exploration of the hemiascomycetous yeasts. 21. Comparative functional classification of genes. FEBS Lett. 487:134-149. [DOI] [PubMed] [Google Scholar]
- 21.Goldmark, J. P., T. G. Fazzio, P. W. Estep, G. M. Church, and T. Tsukiyama. 2000. The Isw2 chromatin remodeling complex represses early meiotic genes upon recruitment by Ume6p. Cell 103:423-433. [DOI] [PubMed] [Google Scholar]
- 22.Gottschling, D. E., O. M. Aparicio, B. L. Billington, and V. A. Zakian. 1990. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63:751-762. [DOI] [PubMed] [Google Scholar]
- 23.Grigliatti, T. 1991. Position-effect variegation—an assay for nonhistone chromosomal proteins and chromatin assembly and modifying factors. Methods Cell Biol. 35:587-627. [PubMed] [Google Scholar]
- 24.Hecht, A., T. Laroche, S. Strahl-Bolsinger, S. M. Gasser, and M. Grunstein. 1995. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell 80:583-592. [DOI] [PubMed] [Google Scholar]
- 25.Imai, S., C. M. Armstrong, M. Kaeberlein, and L. Guarente. 2000. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403:795-800. [DOI] [PubMed] [Google Scholar]
- 26.Ivy, J. M., A. J. S. Klar, and J. B. Hicks. 1986. Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Mol. Cell. Biol. 6:688-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju, Q., B. E. Morrow, and J. R. Warner. 1990. REB1, a yeast DNA-binding protein with many targets, is essential for growth and bears some resemblance to the oncogene myb. Mol. Cell. Biol. 10:5226-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadosh, D., and K. Struhl. 1997. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell 89:365-371. [DOI] [PubMed] [Google Scholar]
- 29.Klar, A. J., J. N. Strathern, J. R. Broach, and J. B. Hicks. 1981. Regulation of transcription in expressed and unexpressed mating type cassettes of yeast. Nature 289:239-244. [DOI] [PubMed] [Google Scholar]
- 30.Krauskopf, A., and E. H. Blackburn. 1996. Control of telomere growth by interactions of RAP1 with the most distal telomeric repeats. Nature 383:354-357. [DOI] [PubMed] [Google Scholar]
- 31.Kulkens, T., C. A. van der Sande, A. F. Dekker, H. van Heerikhuizen, and R. J. Planta. 1992. A system to study transcription by yeast RNA polymerase I within the chromosomal context: functional analysis of the ribosomal DNA enhancer and the RBP1/REB1 binding sites. EMBO J. 11:4665-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunkel, T. A. 1985. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc. Natl. Acad. Sci. USA 82:488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyrion, G., K. Liu, C. Liu, and A. J. Lustig. 1993. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 7:1146-1159. [DOI] [PubMed] [Google Scholar]
- 34.Landry, J., A. Sutton, S. T. Tafrov, R. C. Heller, J. Stebbins, L. Pillus, and R. Sternglanz. 2000. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl. Acad. Sci. USA 97:5807-5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang, W. H., B. E. Morrow, Q. Ju, J. R. Warner, and R. H. Reeder. 1994. A model for transcription termination by RNA polymerase I. Cell 79:527-534. [DOI] [PubMed] [Google Scholar]
- 36.Lang, W. H., and R. H. Reeder. 1993. The REB1 site is an essential component of a terminator for RNA polymerase I in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:649-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liaw, P. C., and C. J. Brandl. 1994. Defining the sequence specificity of the Saccharomyces cerevisiae DNA binding protein REB1p by selecting binding sites from random-sequence oligonucleotides. Yeast 10:771-787. [DOI] [PubMed] [Google Scholar]
- 38.Longtine, M. S., N. M. Wilson, M. E. Petracek, and J. Berman. 1989. A yeast telomere binding activity binds to two related telomere sequence motifs and is indistinguishable from RAP1. Curr. Genet. 16:225-239. [DOI] [PubMed] [Google Scholar]
- 39.Loo, S., and J. Rine. 1994. Silencers and domains of generalized repression. Science 264:1768-1771. [DOI] [PubMed] [Google Scholar]
- 40.Lustig, A. J. 1998. Mechanisms of silencing in Saccharomyces cerevisiae. Curr. Opin. Genet. Dev. 8:233-239. [DOI] [PubMed] [Google Scholar]
- 41.Lustig, A. J., S. Kurtz, and D. Shore. 1990. Involvement of the silencer and UAS binding protein RAP1 in regulation of telomere length. Science 250:549-553. [DOI] [PubMed] [Google Scholar]
- 42.Moazed, D., A. Kistler, A. Axelrod, J. Rine, and A. D. Johnson. 1997. Silent information regulator protein complexes in Saccharomyces cerevisiae: a SIR2/SIR4 complex and evidence for a regulatory domain in SIR4 that inhibits its interaction with SIR3. Proc. Natl. Acad. Sci. USA 94:2186-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moretti, P., K. Freeman, L. Coodly, and D. Shore. 1994. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev. 8:2257-2269. [DOI] [PubMed] [Google Scholar]
- 44.Morrow, B. E., Q. Ju, and J. R. Warner. 1993. A bipartite DNA-binding domain in yeast Reb1p. Mol. Cell. Biol. 13:1173-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasmyth, K. A., K. Tatchell, B. D. Hall, C. Astell, and M. Smith. 1981. A position effect in the control of transcription at yeast mating type loci. Nature 289:244-250. [DOI] [PubMed] [Google Scholar]
- 46.Ogata, K., H. Hojo, S. Aimoto, T. Nakai, H. Nakamura, A. Sarai, S. Ishii, and Y. Nishimura. 1992. Solution structure of a DNA-binding unit of Myb: a helix-turn-helix-related motif with conserved tryptophans forming a hydrophobic core. Proc. Natl. Acad. Sci. USA 89:6428-6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogata, K., H. Kanai, T. Inoue, A. Sekikawa, M. Sasaki, A. Nagadoi, A. Sarai, S. Ishii, and Y. Nishimura. 1993. Solution structures of Myb DNA-binding domain and its complex with DNA. Nucleic Acids Symp. Ser. 29:201-202. [PubMed] [Google Scholar]
- 48.Park, H. D., R. M. Luche, and T. G. Cooper. 1992. The yeast UME6 gene product is required for transcriptional repression mediated by the CAR1 URS1 repressor binding site. Nucleic Acids Res. 20:1909-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rastan, S. 1994. X chromosome inactivation and the Xist gene. Curr. Opin. Genet. Dev. 4:292-297. [DOI] [PubMed] [Google Scholar]
- 50.Rine, J., and I. Herskowitz. 1987. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics 116:9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rine, J., J. N. Strathern, J. B. Hicks, and I. Herskowitz. 1979. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics 93:877-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivier, D. H., and J. Rine. 1992. An origin of DNA replication and a transcription silencer require a common element. Science 256:659-663. [DOI] [PubMed] [Google Scholar]
- 53.Scherer, S., and R. W. Davis. 1979. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc. Natl. Acad. Sci. USA 76:4951-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shore, D. 1997. Telomeres. Different means to common ends. Nature 385:676-677. [DOI] [PubMed] [Google Scholar]
- 55.Shore, D., and K. Nasmyth. 1987. Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell 51:721-732. [DOI] [PubMed] [Google Scholar]
- 56.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simon, J., A. Chiang, W. Bender, M. J. Shimell, and M. O'Connor. 1993. Elements of the Drosophila bithorax complex that mediate repression by Polycomb group products. Dev. Biol. 158:131-144. [DOI] [PubMed] [Google Scholar]
- 58.Singh, J., and A. J. Klar. 1992. Active genes in budding yeast display enhanced in vivo accessibility to foreign DNA methylases: a novel in vivo probe for chromatin structure of yeast. Genes Dev. 6:186-196. [DOI] [PubMed] [Google Scholar]
- 59.Smith, J. S., and J. D. Boeke. 1997. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 11:241-254. [DOI] [PubMed] [Google Scholar]
- 60.Smith, J. S., C. B. Brachmann, I. Celic, M. A. Kenna, S. Muhammad, V. J. Starai, J. L. Avalos, J. C. Escalante-Semerena, C. Grubmeyer, C. Wolberger, and J. D. Boeke. 2000. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl. Acad. Sci. USA 97:6658-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Souciet, J., M. Aigle, F. Artiguenave, G. Blandin, M. Bolotin-Fukuhara, E. Bon, P. Brottier, S. Casaregola, J. de Montigny, B. Dujon, P. Durrens, C. Gaillardin, A. Lepingle, B. Llorente, A. Malpertuy, C. Neuveglise, O. Ozier-Kalogeropoulos, S. Potier, W. Saurin, F. Tekaia, C. Toffano-Nioche, M. Wesolowski-Louvel, P. Wincker, and J. Weissenbach. 2000. Genomic exploration of the hemiascomycetous yeasts. 1. A set of yeast species for molecular evolution studies. FEBS Lett. 487:3-12. [DOI] [PubMed] [Google Scholar]
- 62.Strahl-Bolsinger, S., A. Hecht, K. Luo, and M. Grunstein. 1997. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev. 11:83-93. [DOI] [PubMed] [Google Scholar]
- 63.Strich, R., R. T. Surosky, C. Steber, E. Dubois, F. Messenguy, and R. E. Esposito. 1994. UME6 is a key regulator of nitrogen repression and meiotic development. Genes Dev. 8:796-810. [DOI] [PubMed] [Google Scholar]
- 64.Thon, G., K. P. Bjerling, and I. S. Nielsen. 1999. Localization and properties of a silencing element near the mat3-M mating-type cassette of Schizosaccharomyces pombe. Genetics 151:945-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Triolo, T., and R. Sternglanz. 1996. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature 381:251-253. [DOI] [PubMed] [Google Scholar]
- 66.van Steensel, B., and T. de Lange. 1997. Control of telomere length by the human telomeric protein TRF1. Nature 385:740-743. [DOI] [PubMed] [Google Scholar]
- 67.Wertman, K. F., D. G. Drubin, and D. Botstein. 1992. Syst. mutational analysis of the yeast ACT1 gene. Genetics 132:337-350. [DOI] [PMC free article] [PubMed] [Google Scholar]