Abstract
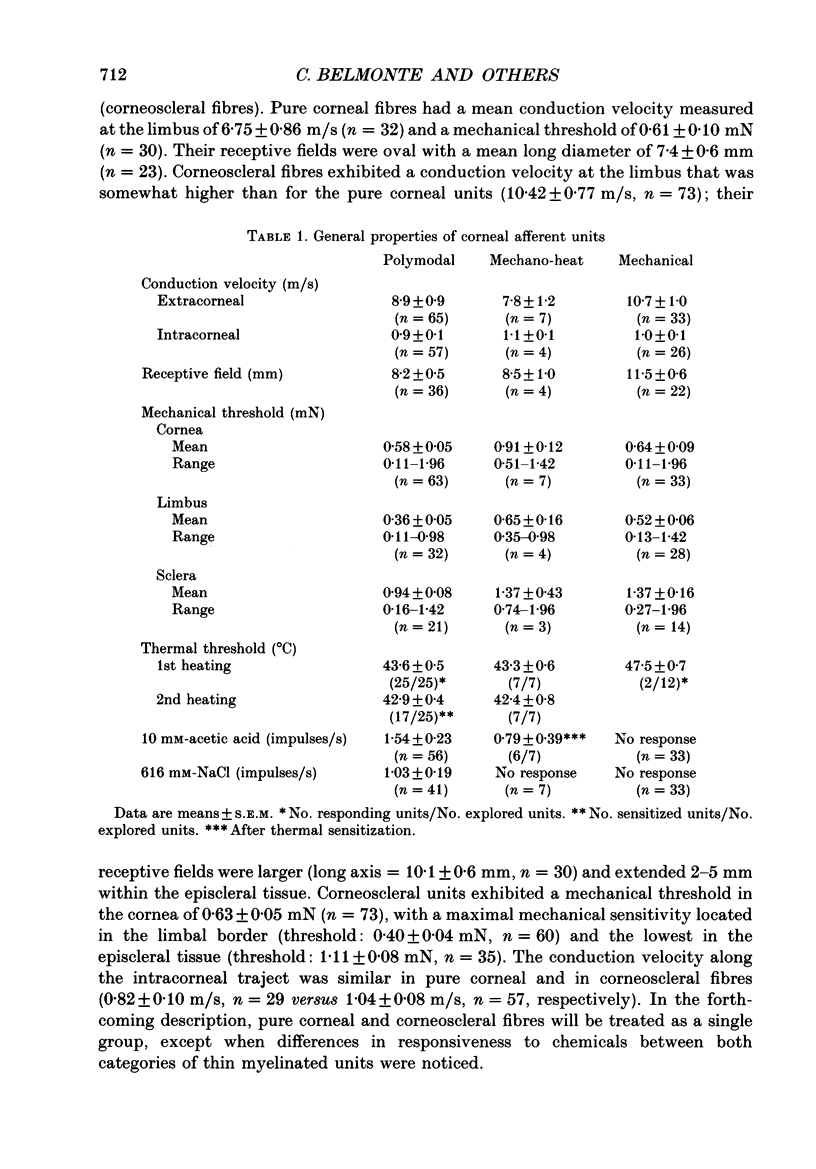
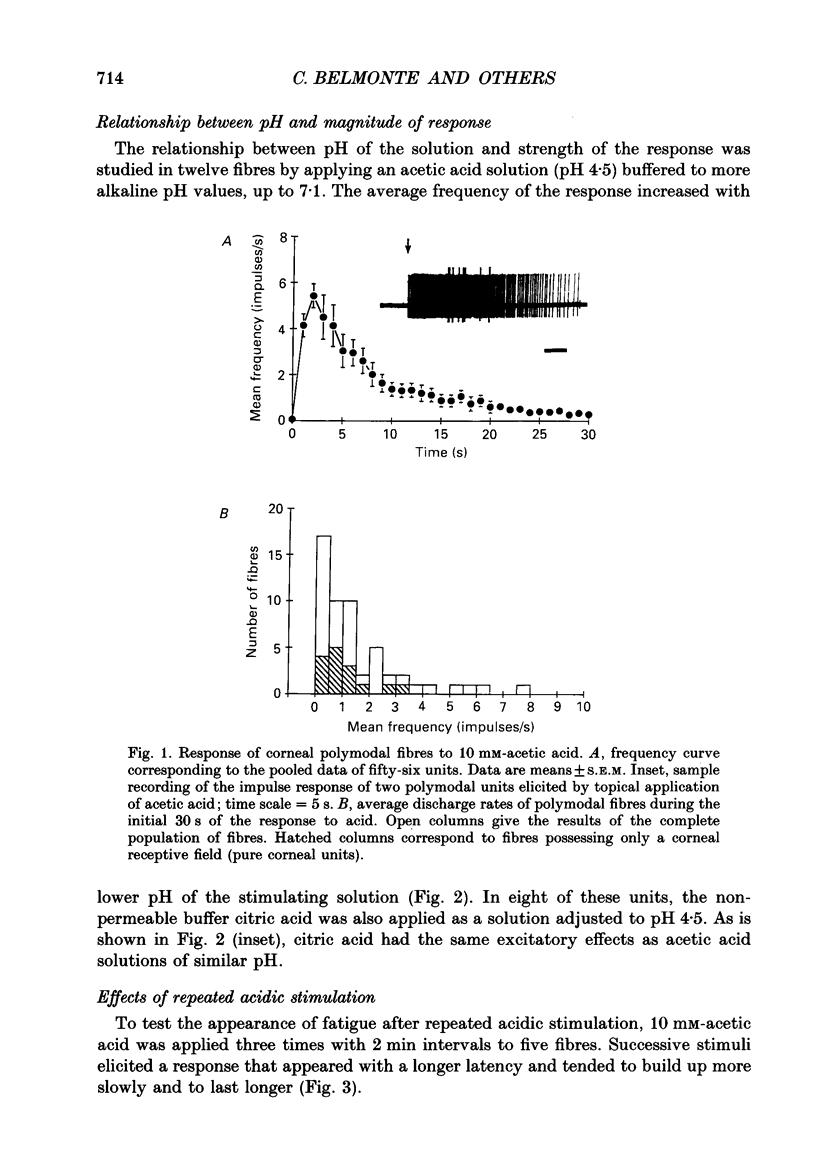
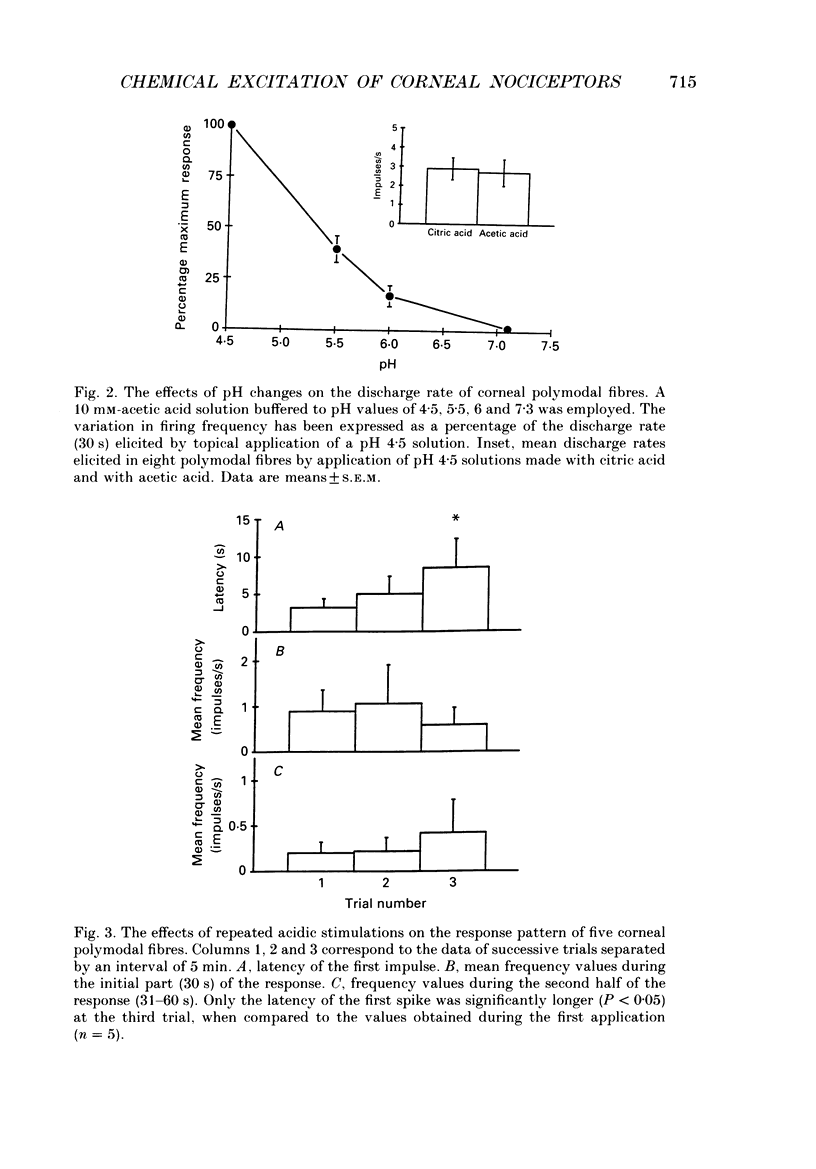
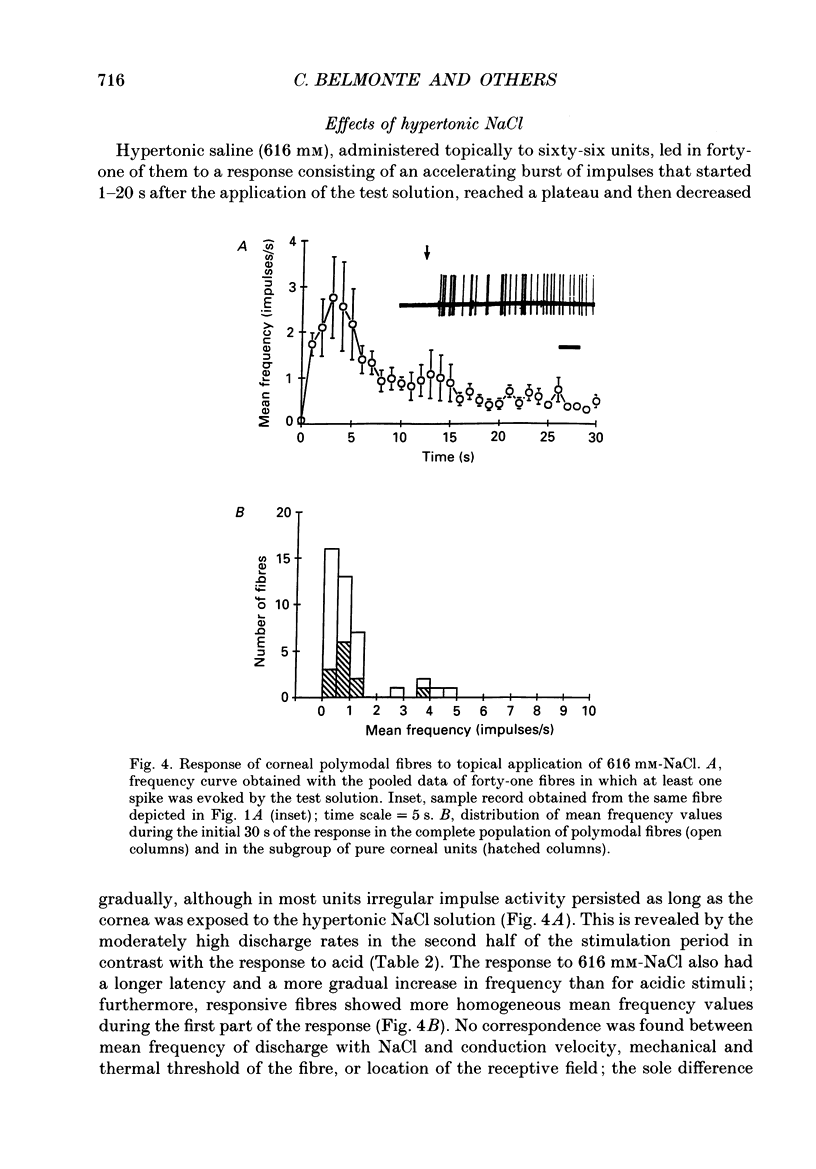
1. Single-unit electrical activity was recorded from thin myelinated sensory nerve fibres innervating the cornea of deeply anaesthetized cats. 2. Based on their responses to mechanical (calibrated von Frey hairs), chemical (10 mM-acetic acid and/or 616 mM-NaCl) and thermal (ice-cold or heat up to 51 degrees C) stimuli, corneal A delta fibres were classified as polymodal nociceptors (63%), high-threshold mechanoceptors (22%) and mechano-heat nociceptors (15%). Thin myelinated fibres responding only to cold were found in the limbus of the eye. 3. Application of 10 mM-acetic acid on the corneal surface for 30 s evoked in polymodal fibres a brisk discharge of impulses often followed by a low-frequency impulse activity. NaCl (616 mM) produced a more gradual and sustained firing response. 4. The responses of polymodal fibres to acid were proportional to extracellular pH values (pH range: 4.5-6.0). After sensitization to repeated heating, most mechano-heat units developed a sensitivity to acidic stimulation. 5. Topical 0.33 mM-capsaicin excited polymodal nociceptors of the cornea; 5 min after capsaicin about 15% of these fibres were inactivated to all subsequent stimuli. In the rest of the fibres, chemical and thermal sensitivity disappeared after 0.33-3.3 mM-capsaicin, but mechanosensitivity was preserved. 6. Corneal mechanoceptors and limbal cold receptors were not affected by capsaicin (up to 33 mM). 7. These experiments demonstrate that the cornea of the cat is innervated by polymodal as well as mechanoceptive A delta nociceptors. In polymodal nociceptive fibres, mechanical and chemical sensitivities appear to be subserved by separate transduction mechanisms.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adriaensen H., Gybels J., Handwerker H. O., Van Hees J. Response properties of thin myelinated (A-delta) fibers in human skin nerves. J Neurophysiol. 1983 Jan;49(1):111–122. doi: 10.1152/jn.1983.49.1.111. [DOI] [PubMed] [Google Scholar]
- Beck P. W., Handwerker H. O. Bradykinin and serotonin effects on various types of cutaneous nerve fibers. Pflugers Arch. 1974 Mar 11;347(3):209–222. doi: 10.1007/BF00592598. [DOI] [PubMed] [Google Scholar]
- Beitel R. E., Dubner R. Response of unmyelinated (C) polymodal nociceptors to thermal stimuli applied to monkey's face. J Neurophysiol. 1976 Nov;39(6):1160–1175. doi: 10.1152/jn.1976.39.6.1160. [DOI] [PubMed] [Google Scholar]
- Belmonte C., Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol. 1981 Dec;321:355–368. doi: 10.1113/jphysiol.1981.sp013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Perl E. R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Bevan S., Szolcsányi J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Pharmacol Sci. 1990 Aug;11(8):330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- Burgess P. R., Perl E. R. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967 Jun;190(3):541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. N., Meyer R. A., LaMotte R. H. Sensitization of myelinated nociceptive afferents that innervate monkey hand. J Neurophysiol. 1979 Nov;42(6):1669–1679. doi: 10.1152/jn.1979.42.6.1669. [DOI] [PubMed] [Google Scholar]
- Croze S., Duclaux R., Kenshalo D. R. The thermal sensitivity of the polymodal nociceptors in the monkey. J Physiol. 1976 Dec;263(3):539–562. doi: 10.1113/jphysiol.1976.sp011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON W. W. Chemical stimulation of the peripheral trigeminal nerve. Nature. 1962 Oct 27;196:341–345. doi: 10.1038/196341a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Lynn B. The sensitization of high threshold mechanoreceptors with myelinated axons by repeated heating. J Physiol. 1977 Feb;265(2):549–563. doi: 10.1113/jphysiol.1977.sp011730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. W., Ramage A. G. The action of some chemical irritants on somatosensory receptors of the cat. Neuropharmacology. 1981 Feb;20(2):191–198. doi: 10.1016/0028-3908(81)90203-3. [DOI] [PubMed] [Google Scholar]
- Gallego R., Eyzaguirre C., Monti-Bloch L. Thermal and osmotic responses of arterial receptors. J Neurophysiol. 1979 May;42(3):665–680. doi: 10.1152/jn.1979.42.3.665. [DOI] [PubMed] [Google Scholar]
- Georgopoulos A. P. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. J Neurophysiol. 1976 Jan;39(1):71–83. doi: 10.1152/jn.1976.39.1.71. [DOI] [PubMed] [Google Scholar]
- Giraldez F., Geijo E., Belmonte C. Response characteristics of corneal sensory fibers to mechanical and thermal stimulation. Brain Res. 1979 Nov 30;177(3):571–576. doi: 10.1016/0006-8993(79)90475-x. [DOI] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyes A. D., Barber P. Ultrastructure of the corneal nerves in the rat. Cell Tissue Res. 1976 Sep 6;172(1):133–144. doi: 10.1007/BF00226054. [DOI] [PubMed] [Google Scholar]
- IGGO A. Cutaneous mechanoreceptors with afferent C fibres. J Physiol. 1960 Jul;152:337–353. doi: 10.1113/jphysiol.1960.sp006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. The role of sensory nerve endings in neurogenic inflammation induced in human skin and in the eye and paw of the rat. Br J Pharmacol Chemother. 1968 May;33(1):32–41. doi: 10.1111/j.1476-5381.1968.tb00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenins P. Responses of single nerve fibres to capsaicin applied to the skin. Neurosci Lett. 1982 Mar 17;29(1):83–88. doi: 10.1016/0304-3940(82)90369-x. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Lux H. D., Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987 May;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. Chemical responses of polymodal receptors of the scrotal contents in dogs. J Physiol. 1980 Feb;299:219–231. doi: 10.1113/jphysiol.1980.sp013121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. Mechanical and thermal responses of polymodal receptors recorded from the superior spermatic nerve of dogs. J Physiol. 1980 Feb;299:233–245. doi: 10.1113/jphysiol.1980.sp013122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977 Dec;273(1):179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LELE P. P., WEDDELL G. Sensory nerves of the cornea and cutaneous sensibility. Exp Neurol. 1959 Oct;1:334–359. doi: 10.1016/0014-4886(59)90025-1. [DOI] [PubMed] [Google Scholar]
- LELE P. P., WEDDELL G. The relationship between neurohistology and corneal sensibility. Brain. 1956 Mar;79(1):119–154. doi: 10.1093/brain/79.1.119. [DOI] [PubMed] [Google Scholar]
- Lansman J. B., Hallam T. J., Rink T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? 1987 Feb 26-Mar 4Nature. 325(6107):811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Marsh S. J., Stansfeld C. E., Brown D. A., Davey R., McCarthy D. The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience. 1987 Oct;23(1):275–289. doi: 10.1016/0306-4522(87)90289-2. [DOI] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977 May;267(1):75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsche U., Fleischer E., Lembeck F., Handwerker H. O. The effect of capsaicin application to a peripheral nerve on impulse conduction in functionally identified afferent nerve fibres. Brain Res. 1983 Apr 18;265(2):233–240. doi: 10.1016/0006-8993(83)90337-2. [DOI] [PubMed] [Google Scholar]
- Schaible H. G., Schmidt R. F. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol. 1985 Nov;54(5):1109–1122. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J., Anton F., Reeh P. W., Handwerker H. O. Selective excitation by capsaicin of mechano-heat sensitive nociceptors in rat skin. Brain Res. 1988 Apr 19;446(2):262–268. doi: 10.1016/0006-8993(88)90885-2. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J., Jancśo-Gábor A., JOO F. Functional and fine structural characteristics of the sensory neuron blocking effect of capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 1975;287(2):157–169. doi: 10.1007/BF00510447. [DOI] [PubMed] [Google Scholar]
- Tanelian D. L., Beuerman R. W. Responses of rabbit corneal nociceptors to mechanical and thermal stimulation. Exp Neurol. 1984 Apr;84(1):165–178. doi: 10.1016/0014-4886(84)90013-x. [DOI] [PubMed] [Google Scholar]


