Abstract
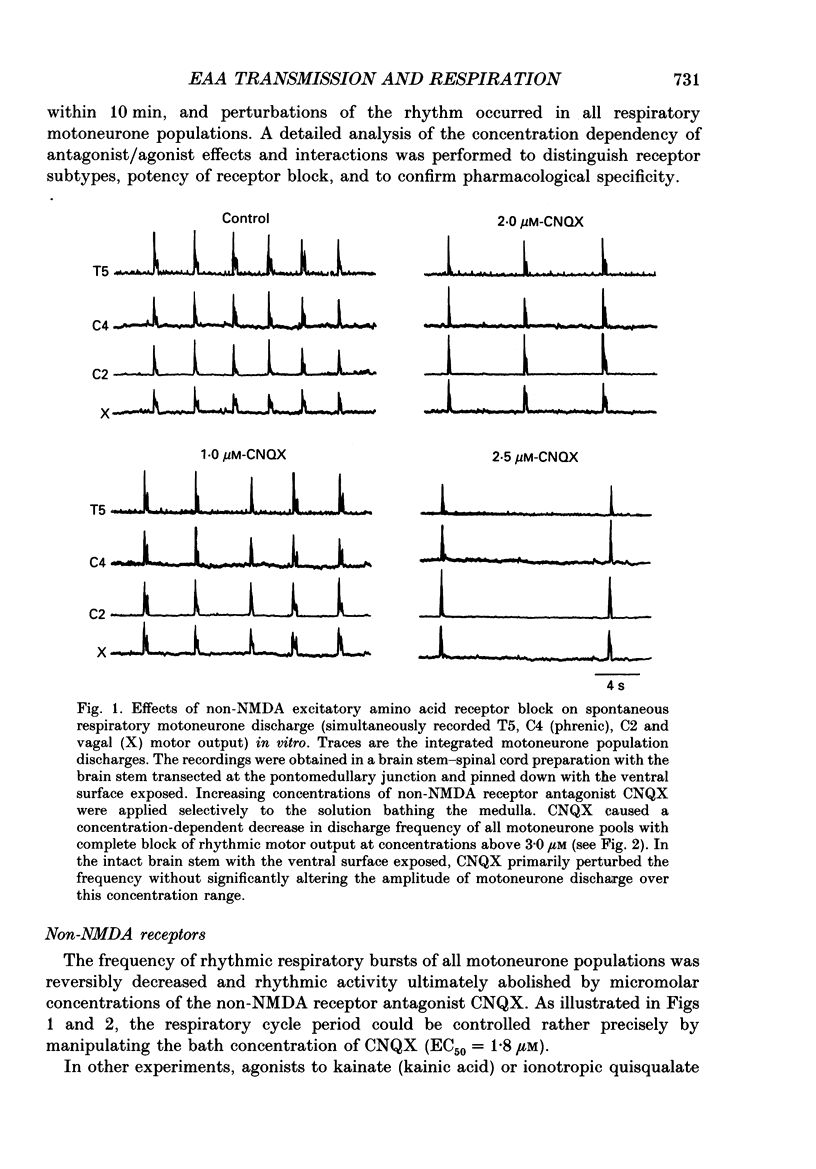
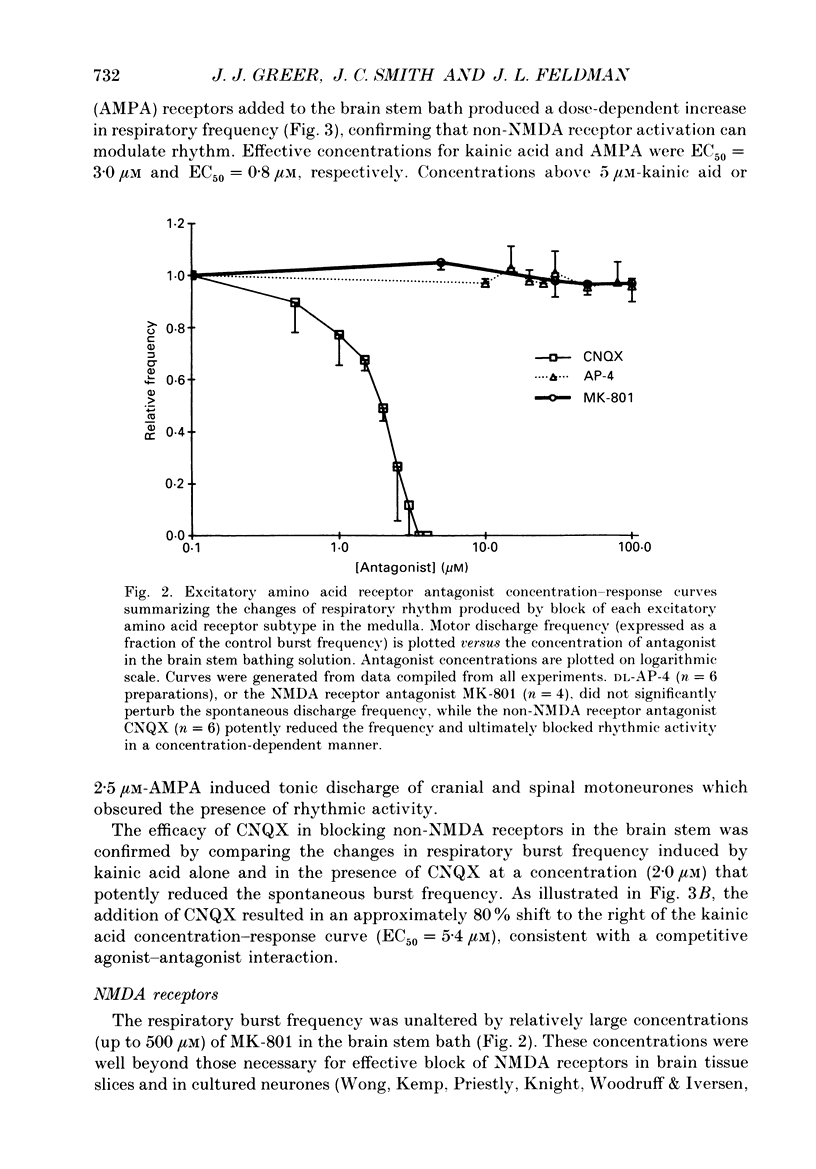
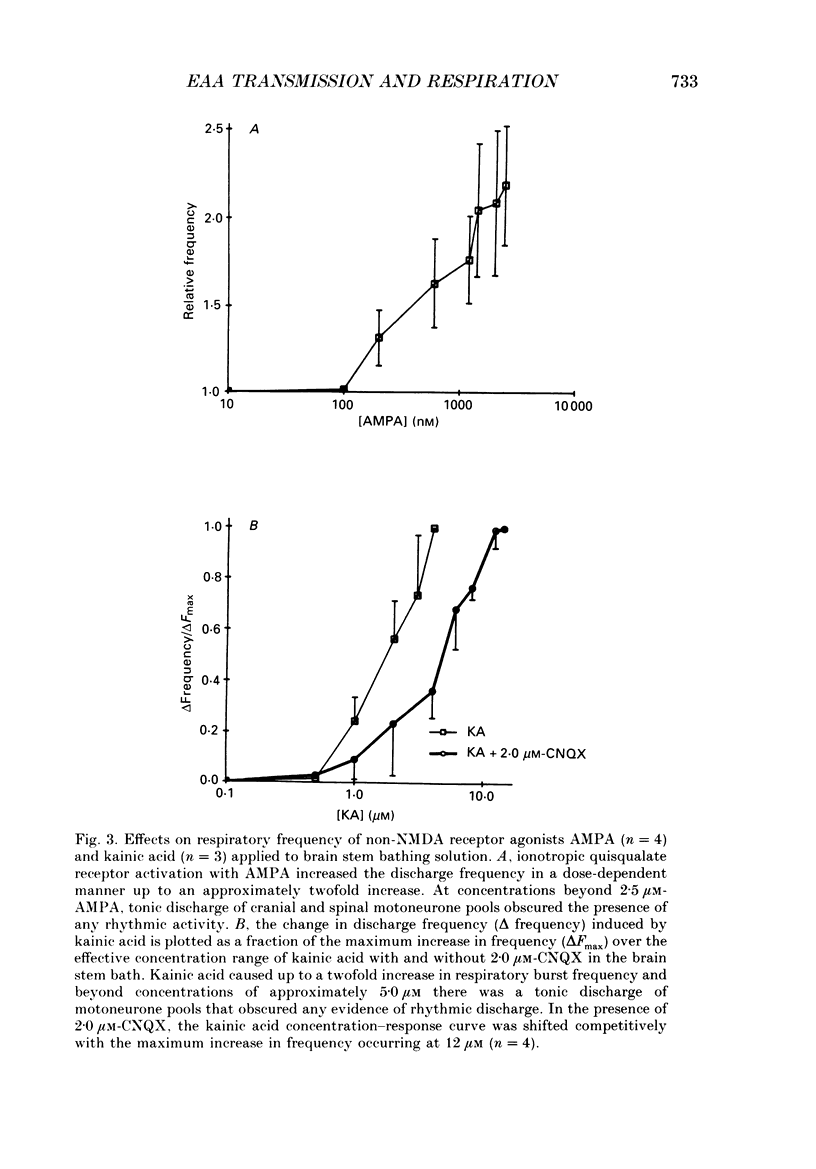
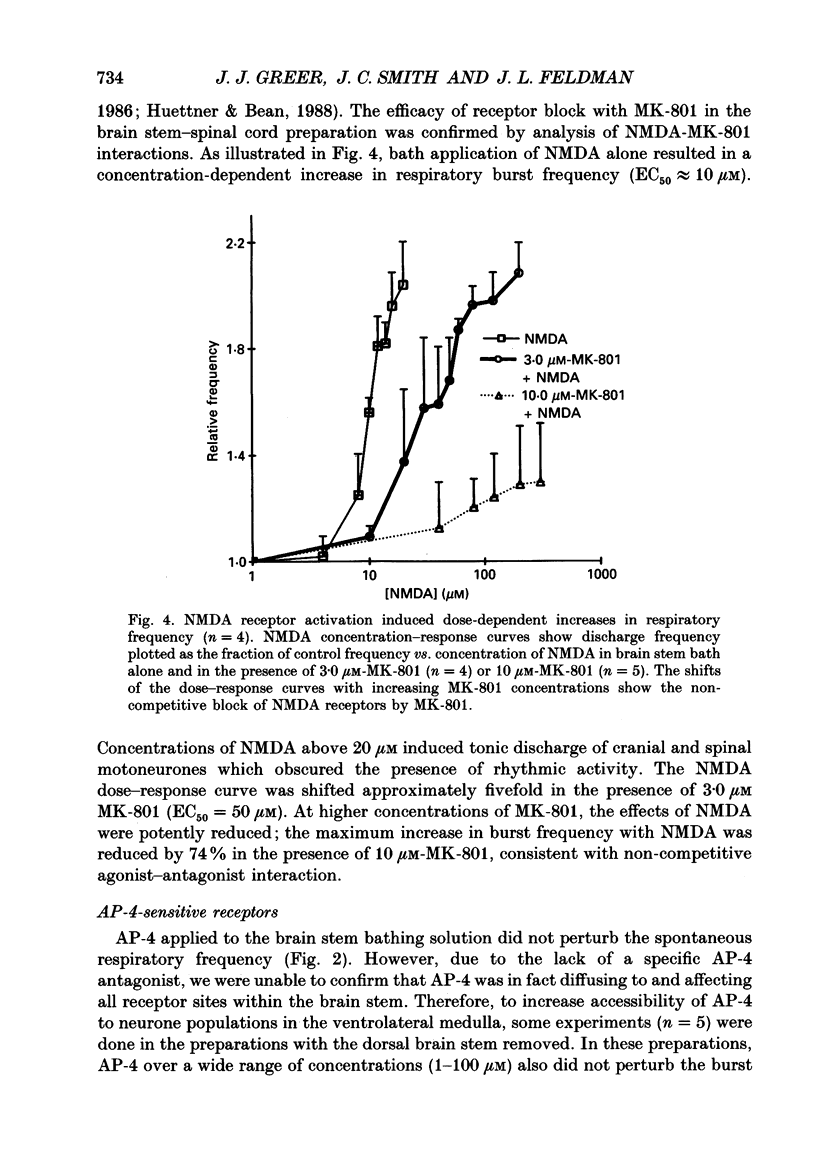
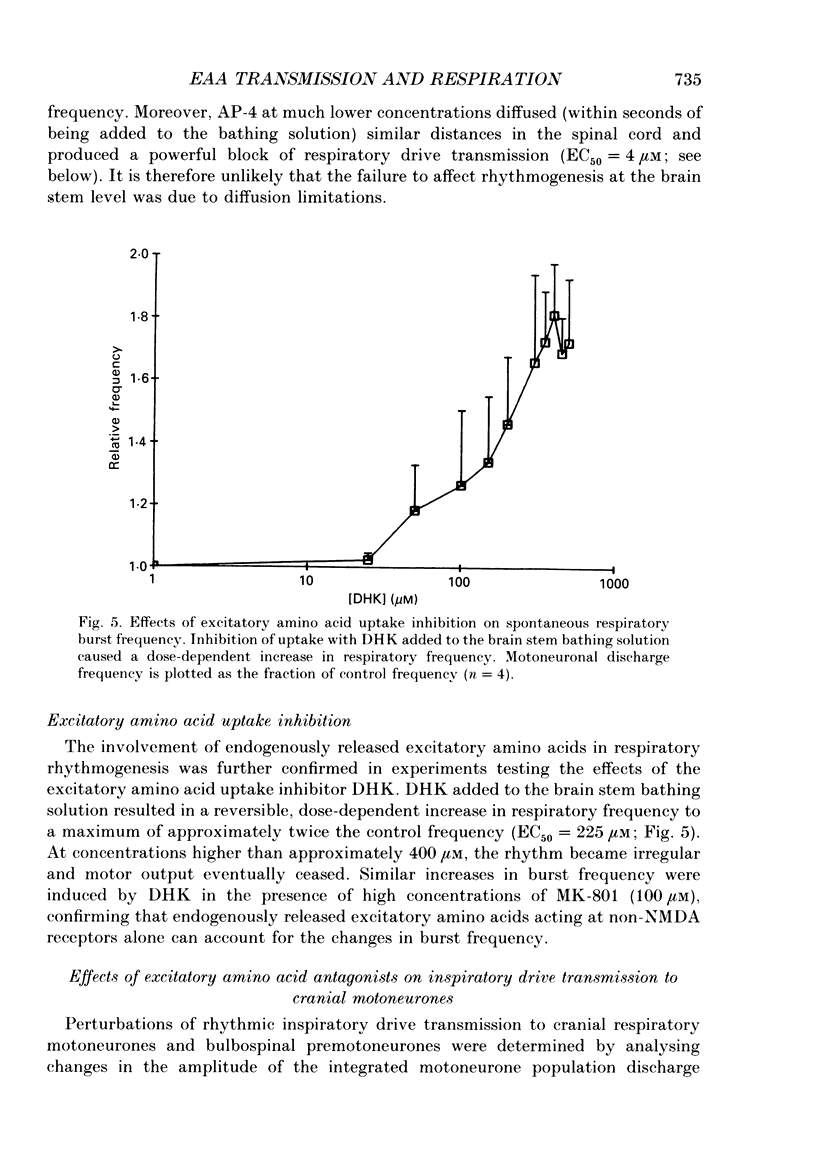
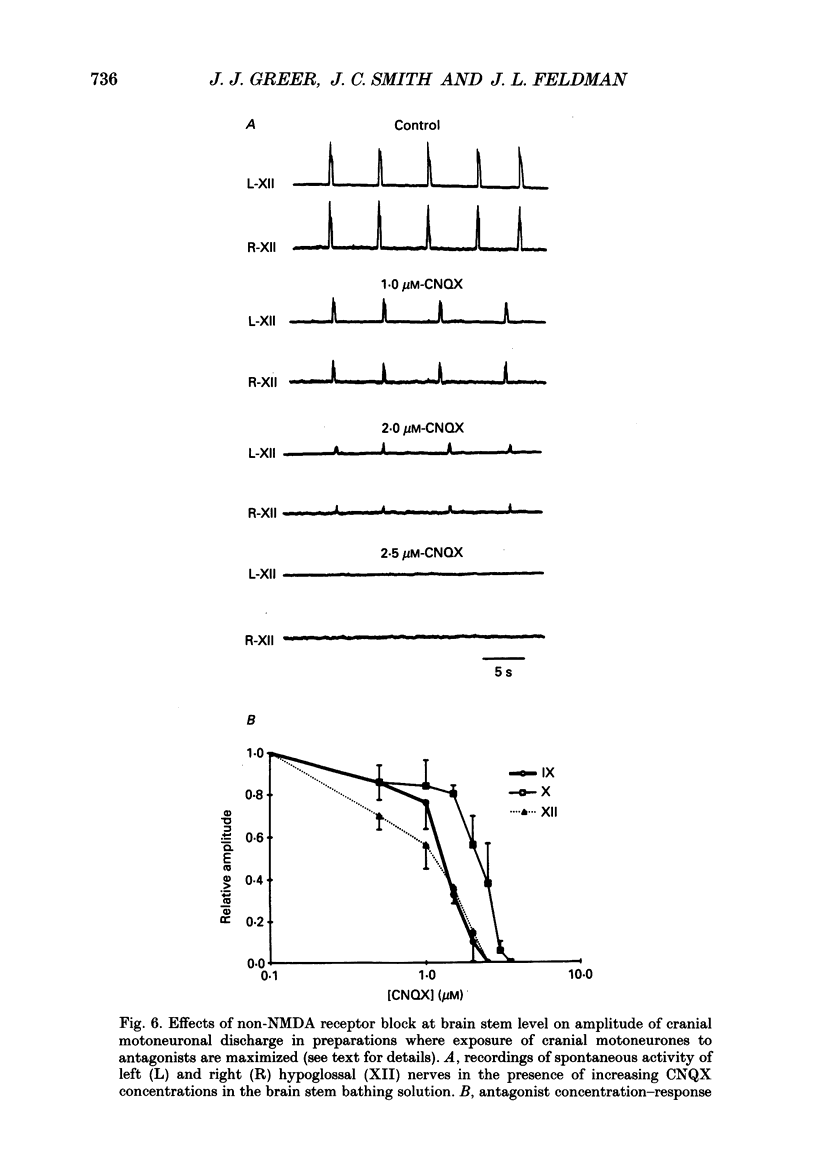
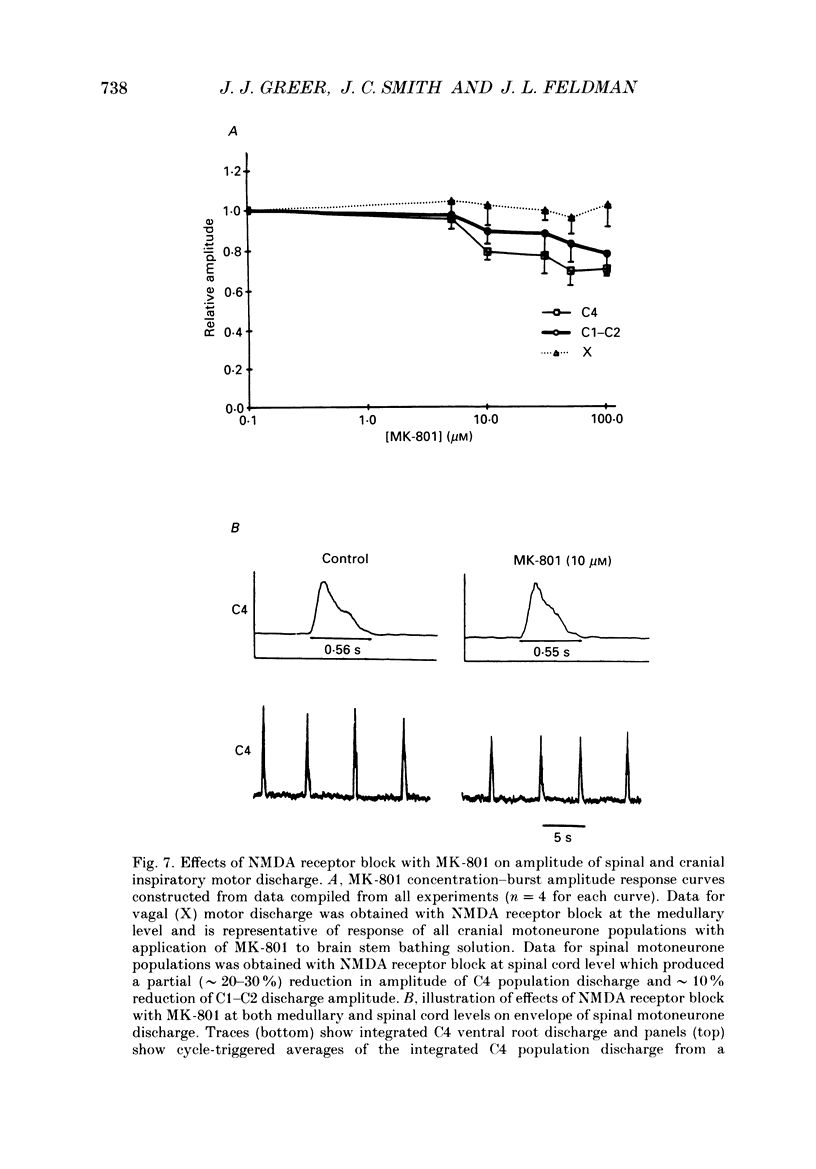
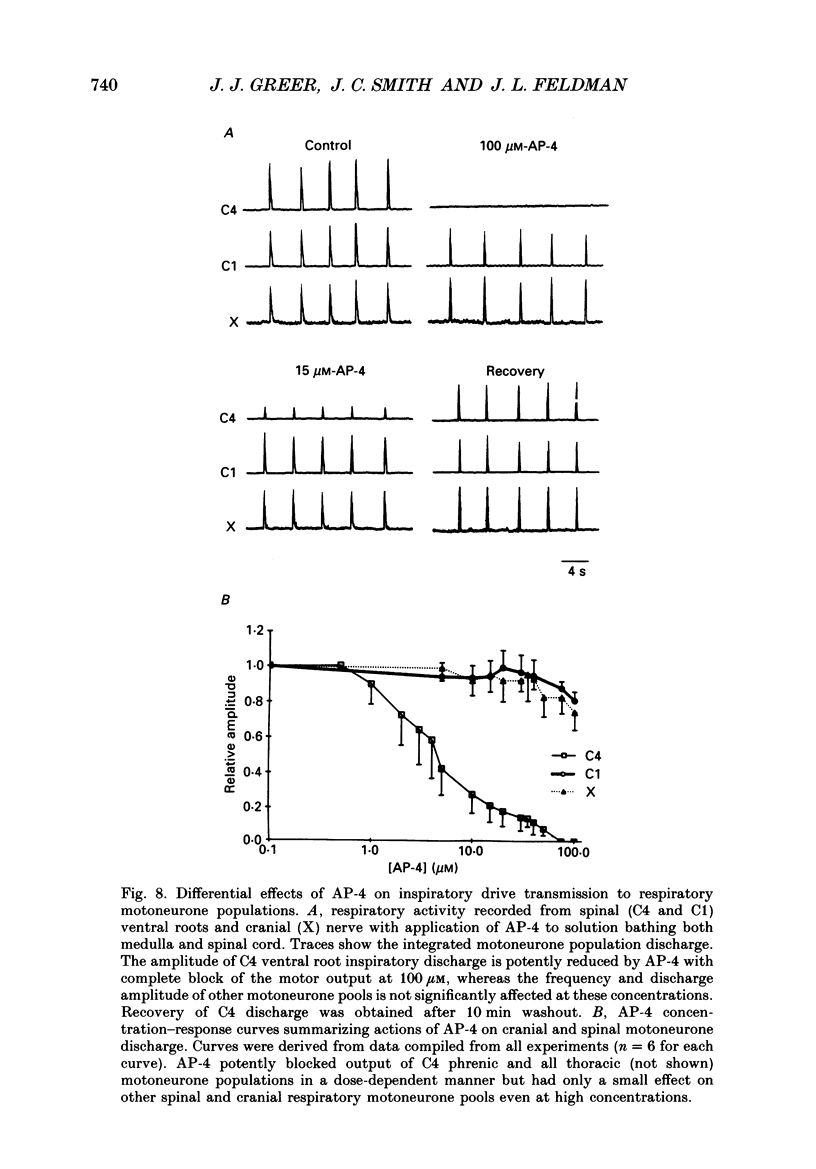
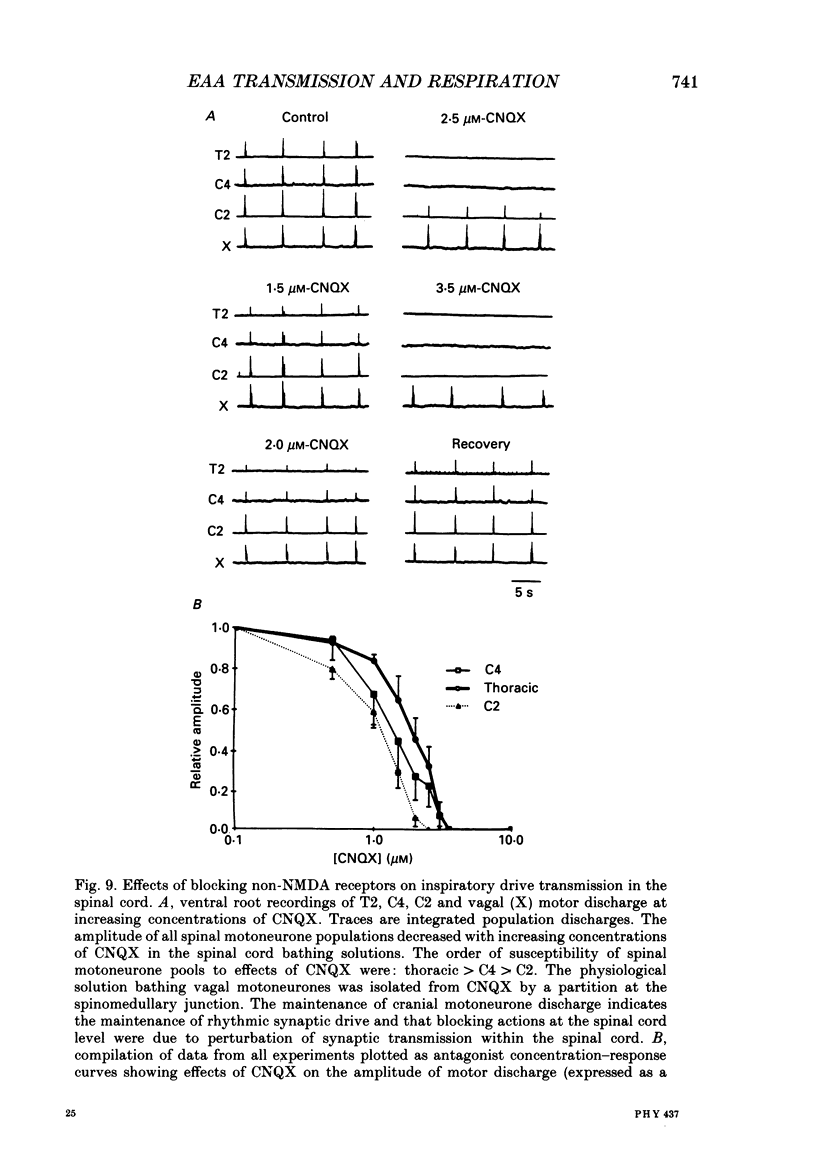
1. The involvement of excitatory amino acids in the generation and transmission of rhythmic respiratory drive was studied in an in vitro neonatal rat brain stem-spinal cord preparation. The subclasses of excitatory amino acid receptors studied included: (i) N-methyl-D-aspartate (NMDA) receptors, (ii) (R, S)-alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid hydrobromide (AMPA) and kainate (non-NMDA) receptors and (iii) 2-amino-4-phosphonobutyric acid (AP-4)-sensitive receptors. Respiratory motoneurone population discharge was recorded from glossopharyngeal (IX), vagus (X), and hypoglossal (XII) cranial nerves, as well as cervical (C1-C5) and thoracic (T2-T5) spinal ventral roots. This activity is generated in the motoneurone pools that transmit respiratory drive to upper airway, accessory, diaphragm and intercostal muscles. Perturbations of motor nerve discharge were analysed after excitatory amino acid receptor antagonists or agonists were added to bathing solutions surrounding either the spinal cord or brain stem. The excitatory amino acid receptor antagonists included: (i) NMDA receptor antagonist (+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imin-H-maleate (MK-801) and (ii) non-NMDA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). The agonists included: (i) NMDA, (ii) non-NMDA receptor agonists AMPA and kainic acid. The effects of perturbations of AP-4-sensitive receptors with AP-4, and of inhibiting excitatory amino acid uptake with dihydrokainic acid (DHK) were also studied. 2. Block of non-NMDA receptors in the medulla by CNQX resulted in an antagonist concentration-dependent decrease in the respiratory motoneuronal burst frequency. Non-NMDA receptor activation with kainic acid or AMPA caused a concentration-dependent increase in burst frequency, with competitive interactions with CNQX. 3. Inhibition of excitatory amino acid uptake in the medulla with DHK resulted in a reversible, dose-dependent increase in respiratory frequency. A similar increase in respiratory frequency was induced by DHK when medullary NMDA receptors were blocked with MK-801, confirming that endogenously released excitatory amino acids act at non-NMDA receptors to modulate rhythm. 4. Non-NMDA receptor block reduced and ultimately abolished the amplitude of integrated cranial and spinal respiratory motoneuronal discharge when added to the solution bathing the medulla and spinal cord, respectively. 5. NMDA receptor block in the medulla with MK-801 did not perturb the spontaneous respiratory burst frequency, although bath application of NMDA produced a dose-dependent increase in frequency, with non-competitive interactions with MK-801. MK-801 also did not perturb the amplitude of cranial or bulbospinal premotoneurone discharge.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers G. W., Goldberg M. P., Choi D. W. N-methyl-D-aspartate antagonists: ready for clinical trial in brain ischemia? Ann Neurol. 1989 Apr;25(4):398–403. doi: 10.1002/ana.410250412. [DOI] [PubMed] [Google Scholar]
- Barry M. J., O'Donovan M. J. The effects of excitatory amino acids and their antagonists on the generation of motor activity in the isolated chick spinal cord. Brain Res. 1987 Dec 1;433(2):271–276. doi: 10.1016/0165-3806(87)90030-7. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Flatman J. A., Ganong A. H., Perkins M. N. Effects of excitatory amino acid antagonists on evoked and spontaneous excitatory potentials in guinea-pig hippocampus. J Physiol. 1986 Sep;378:403–415. doi: 10.1113/jphysiol.1986.sp016227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Roberts A. Excitatory amino acid receptors in Xenopus embryo spinal cord and their role in the activation of swimming. J Physiol. 1984 Mar;348:527–543. doi: 10.1113/jphysiol.1984.sp015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982 Mar 11;235(2):378–386. doi: 10.1016/0006-8993(82)91017-4. [DOI] [PubMed] [Google Scholar]
- Ellenberger H. H., Feldman J. L. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol. 1988 Mar 1;269(1):47–57. doi: 10.1002/cne.902690104. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Gautier H. Interaction of pulmonary afferents and pneumotaxic center in control of respiratory pattern in cats. J Neurophysiol. 1976 Jan;39(1):31–44. doi: 10.1152/jn.1976.39.1.31. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Loewy A. D., Speck D. F. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci. 1985 Aug;5(8):1993–2000. doi: 10.1523/JNEUROSCI.05-08-01993.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. L., Smith J. C. Cellular mechanisms underlying modulation of breathing pattern in mammals. Ann N Y Acad Sci. 1989;563:114–130. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Smith J. C., Ellenberger H. H., Connelly C. A., Liu G. S., Greer J. J., Lindsay A. D., Otto M. R. Neurogenesis of respiratory rhythm and pattern: emerging concepts. Am J Physiol. 1990 Nov;259(5 Pt 2):R879–R886. doi: 10.1152/ajpregu.1990.259.5.R879. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Clements J. D. Presynaptic glutamate receptors depress excitatory monosynaptic transmission between mouse hippocampal neurones. J Physiol. 1990 Oct;429:1–16. doi: 10.1113/jphysiol.1990.sp018240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foutz A. S., Champagnat J., Denavit-Saubié M. Respiratory effects of the N-methyl-D-aspartate (NMDA) antagonist, MK-801, in intact and vagotomized chronic cats. Eur J Pharmacol. 1988 Sep 13;154(2):179–184. doi: 10.1016/0014-2999(88)90095-7. [DOI] [PubMed] [Google Scholar]
- Getting P. A. Emerging principles governing the operation of neural networks. Annu Rev Neurosci. 1989;12:185–204. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- Grillner S., McClellan A., Sigvardt K., Wallén P., Wilén M. Activation of NMDA-receptors elicits "fictive locomotion" in lamprey spinal cord in vitro. Acta Physiol Scand. 1981 Dec;113(4):549–551. doi: 10.1111/j.1748-1716.1981.tb06937.x. [DOI] [PubMed] [Google Scholar]
- Hilaire G., Monteau R., Errchidi S. Possible modulation of the medullary respiratory rhythm generator by the noradrenergic A5 area: an in vitro study in the newborn rat. Brain Res. 1989 Apr 24;485(2):325–332. doi: 10.1016/0006-8993(89)90577-5. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A. H., Chernick V. Development of respiratory control. Physiol Rev. 1983 Apr;63(2):437–483. doi: 10.1152/physrev.1983.63.2.437. [DOI] [PubMed] [Google Scholar]
- Liu G., Feldman J. L., Smith J. C. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J Neurophysiol. 1990 Aug;64(2):423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- Marlot D., Duron B. Postnatal maturation of phrenic, vagus, and intercostal nerves in the kitten. Biol Neonate. 1979;36(5-6):264–272. doi: 10.1159/000241238. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- McCrimmon D. R., Smith J. C., Feldman J. L. Involvement of excitatory amino acids in neurotransmission of inspiratory drive to spinal respiratory motoneurons. J Neurosci. 1989 Jun;9(6):1910–1921. doi: 10.1523/JNEUROSCI.09-06-01910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteau R., Errchidi S., Gauthier P., Hilaire G., Rega P. Pneumotaxic centre and apneustic breathing: interspecies differences between rat and cat. Neurosci Lett. 1989 May 8;99(3):311–316. doi: 10.1016/0304-3940(89)90465-5. [DOI] [PubMed] [Google Scholar]
- Monteau R., Gauthier P., Rega P., Hilaire G. Effects of N-methyl-D-aspartate (NMDA) antagonist MK-801 on breathing pattern in rats. Neurosci Lett. 1990 Feb 5;109(1-2):134–139. doi: 10.1016/0304-3940(90)90551-j. [DOI] [PubMed] [Google Scholar]
- Olney J., Price M., Salles K. S., Labruyere J., Frierdich G. MK-801 powerfully protects against N-methyl aspartate neurotoxicity. Eur J Pharmacol. 1987 Sep 23;141(3):357–361. doi: 10.1016/0014-2999(87)90552-8. [DOI] [PubMed] [Google Scholar]
- Onimaru H., Homma I. Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1987 Feb 17;403(2):380–384. doi: 10.1016/0006-8993(87)90080-1. [DOI] [PubMed] [Google Scholar]
- Saether K., Hilaire G., Monteau R. Dorsal and ventral respiratory groups of neurons in the medulla of the rat. Brain Res. 1987 Sep 1;419(1-2):87–96. doi: 10.1016/0006-8993(87)90571-3. [DOI] [PubMed] [Google Scholar]
- Schwarzacher S. W., Wilhelm Z., Anders K., Richter D. W. The medullary respiratory network in the rat. J Physiol. 1991 Apr;435:631–644. doi: 10.1113/jphysiol.1991.sp018529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Hollyday M. The development and postnatal organization of motor nuclei in the rat thoracic spinal cord. J Comp Neurol. 1983 Oct 10;220(1):16–28. doi: 10.1002/cne.902200104. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Feldman J. L. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987 Oct;21(2-4):321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Feldman J. L., Schmidt B. J. Neural mechanisms generating locomotion studied in mammalian brain stem-spinal cord in vitro. FASEB J. 1988 Apr;2(7):2283–2288. doi: 10.1096/fasebj.2.7.2450802. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Greer J. J., Liu G. S., Feldman J. L. Neural mechanisms generating respiratory pattern in mammalian brain stem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J Neurophysiol. 1990 Oct;64(4):1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984 Sep;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]


