Abstract
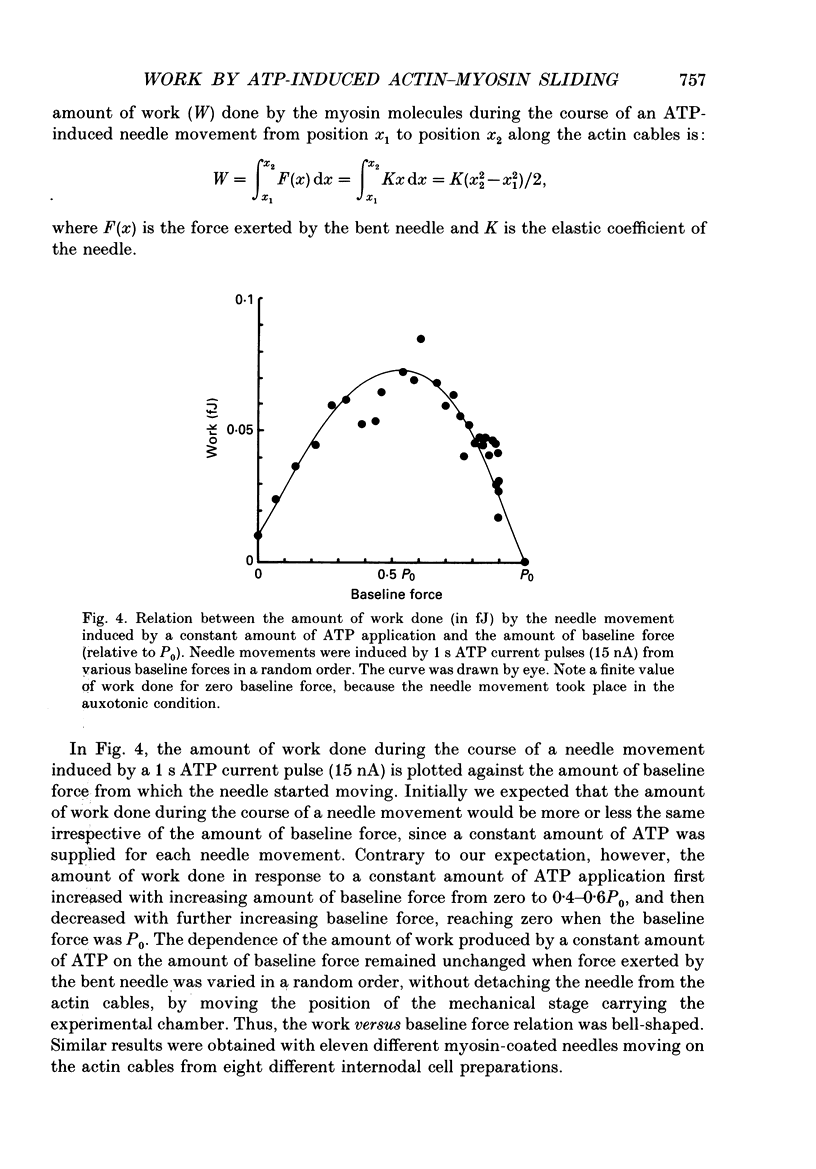
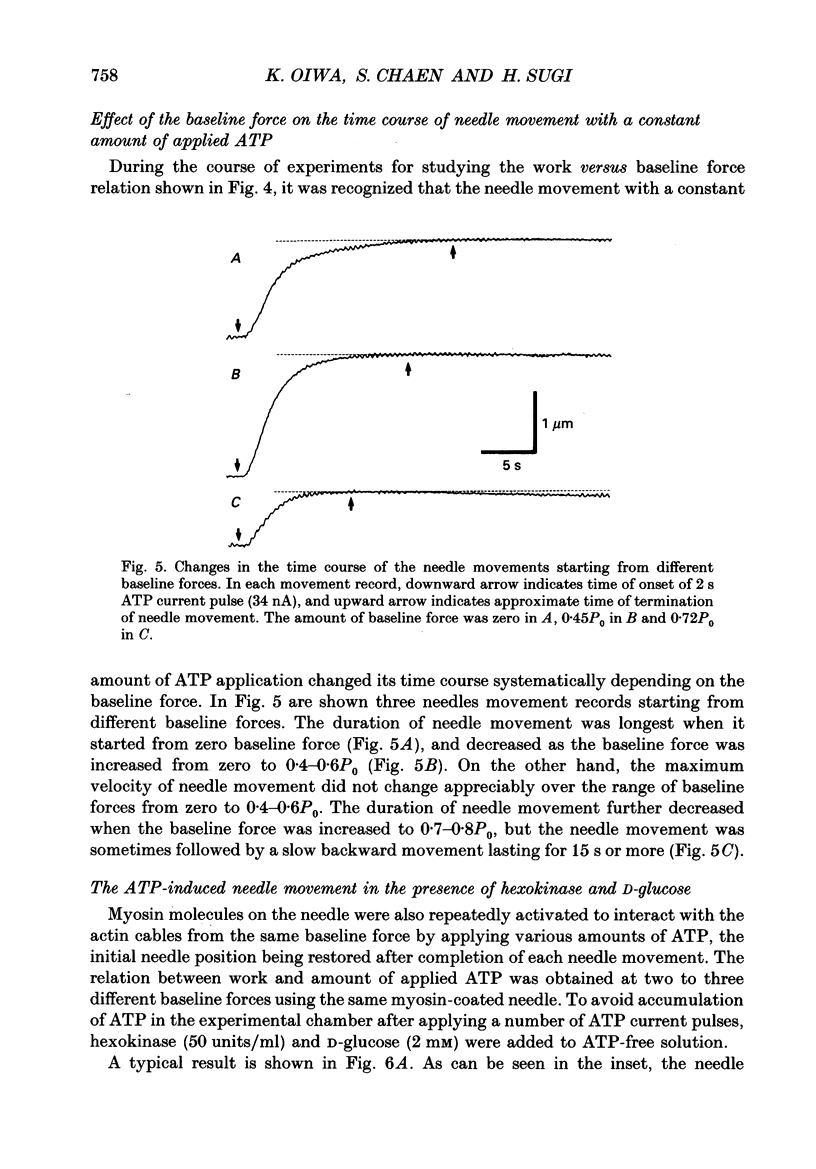
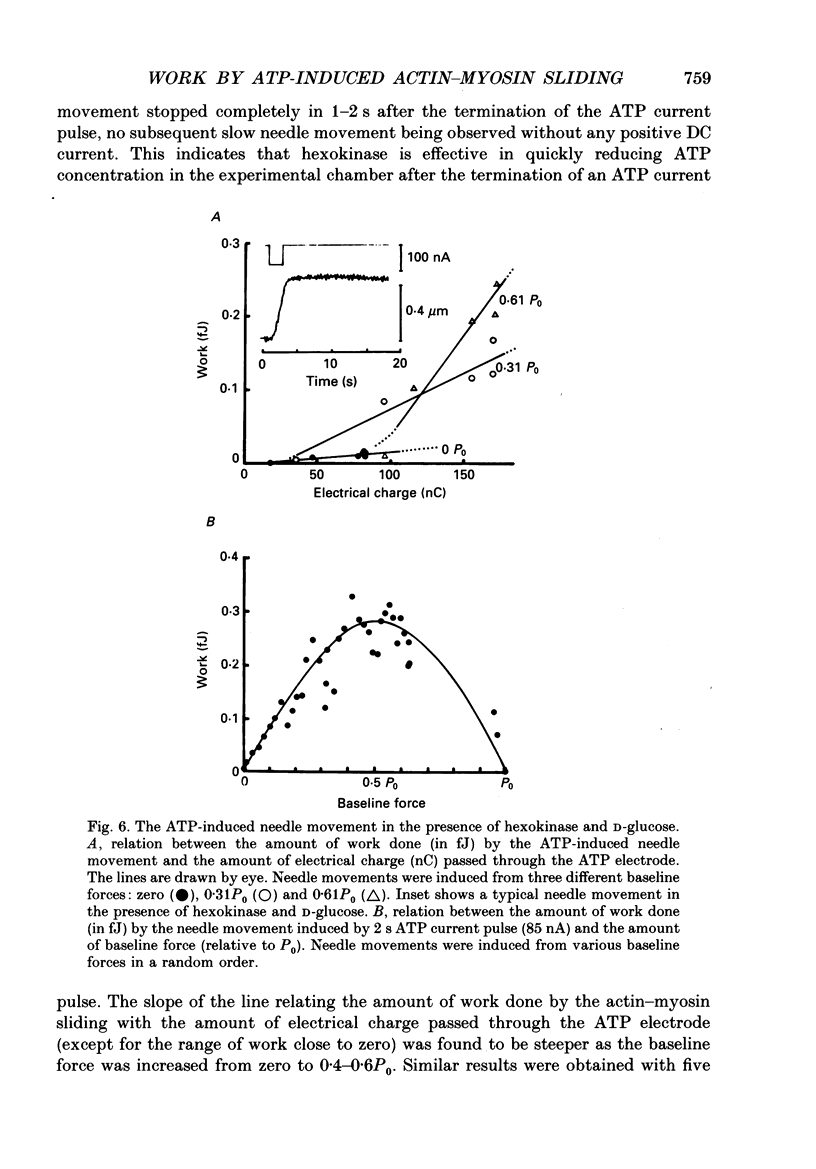
1. The basic properties of the ATP-dependent actin-myosin interaction responsible for muscle contraction were studied using an in vitro force-movement assay system, in which a glass microneedle coated with rabbit skeletal muscle myosin was made to slide on the actin filament arrays (actin cables) in the internodal cell of an alga Nitellopsis obtusa with ionophoretic application of ATP. 2. In response to an ATP current pulse (intensity, 5-85 nA; duration, 0.5-10 s), the myosin-coated needle moved for a distance and eventually stopped, indicating reformation of rigor actin-myosin linkages to prevent elastic recoil of the bent needle. A subsequent ATP current pulse again produced the needle movement starting from the baseline force attained by the preceding needle movement. 3. With a constant amount of ATP application, the amount of work done by the ATP-induced actin-myosin sliding first increased with increasing baseline force from zero to 0.4-0.6P0, and then decreased with further increasing baseline force, thus giving a bell-shaped work versus baseline force relation. 4. With increasing amount of ATP application, the amount of work done by the actin-myosin sliding increased more steeply as the baseline force was increased from zero to 0.4-0.6P0. 5. These results are discussed in connection with the basic properties of the actin-myosin sliding in muscle contraction.
Full text
PDF

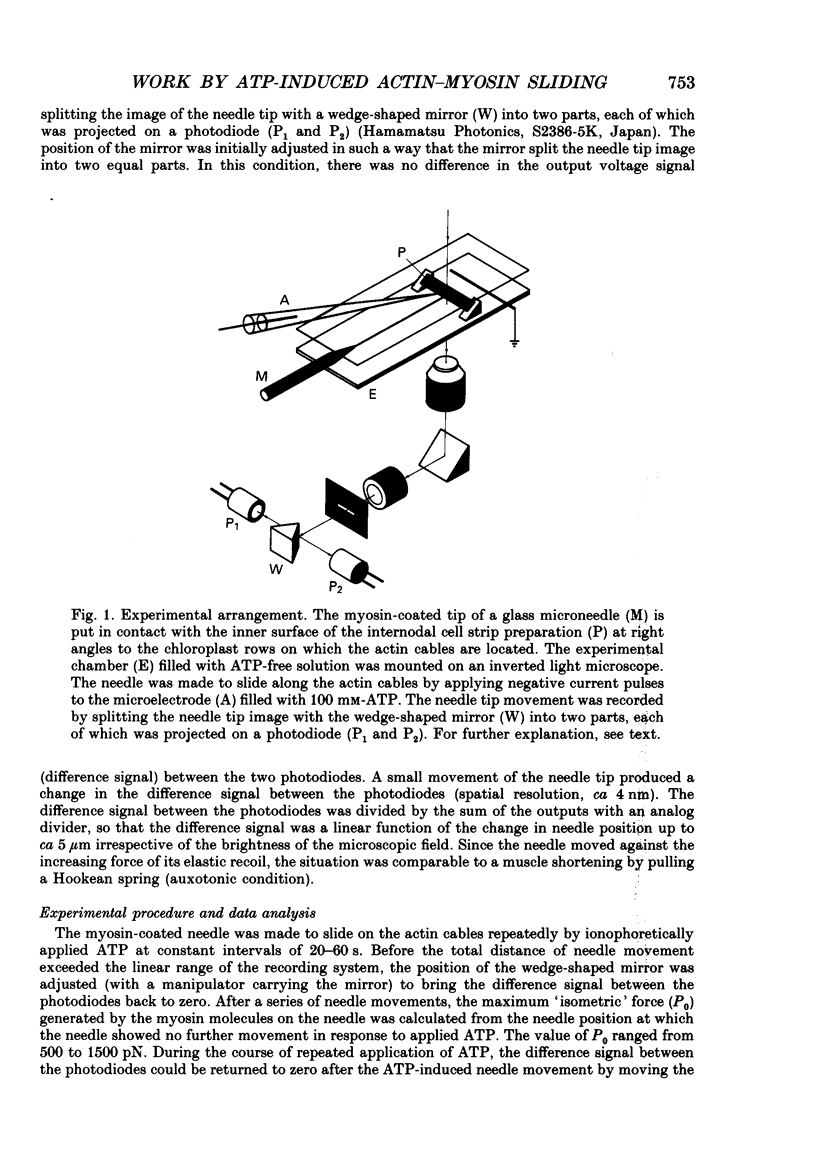

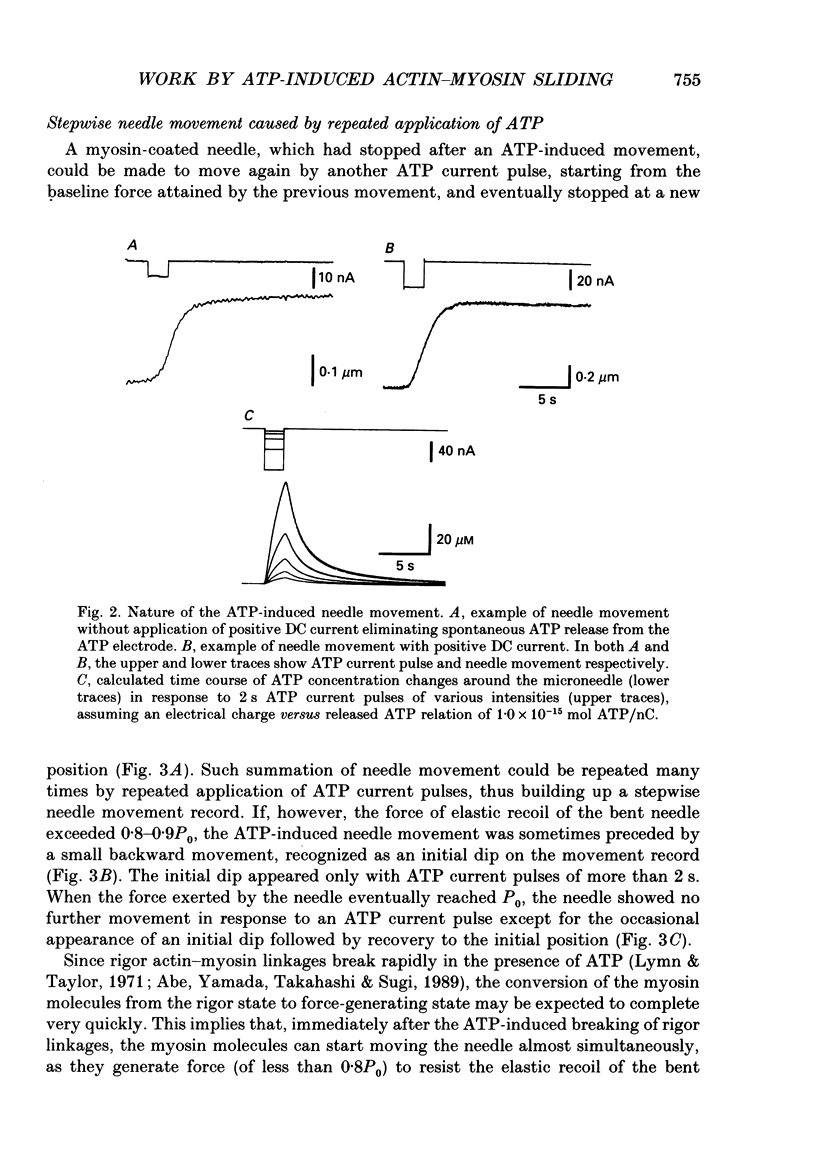
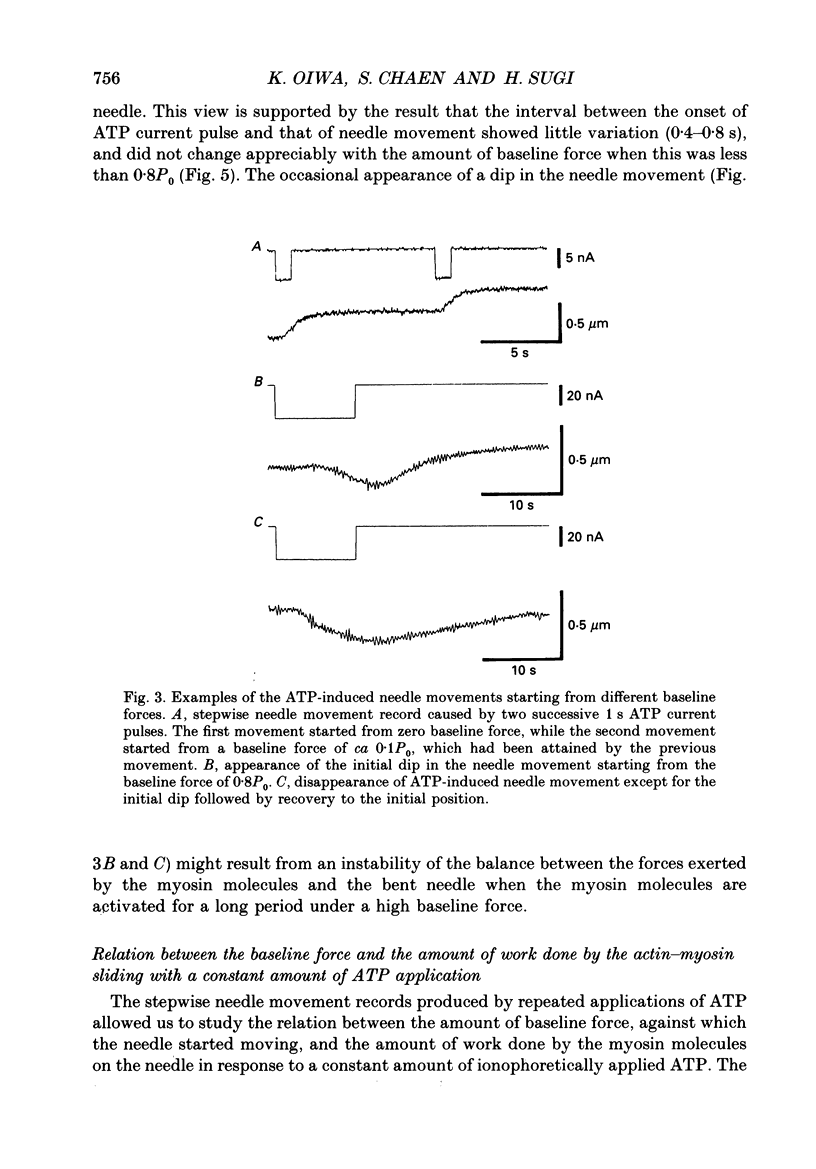




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOWEN W. J., MARTIN H. L. THE DIFFUSION OF ADENOSINE TRIPHOSPHATE THROUGH AQUEOUS SOLUTIONS. Arch Biochem Biophys. 1964 Jul;107:30–36. doi: 10.1016/0003-9861(64)90265-6. [DOI] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C. Relationship between the nerve action potential and transmitter release from sympathetic postganglionic nerve terminals. Nature. 1987 Apr 9;326(6113):605–607. doi: 10.1038/326605a0. [DOI] [PubMed] [Google Scholar]
- Chaen S., Oiwa K., Shimmen T., Iwamoto H., Sugi H. Simultaneous recordings of force and sliding movement between a myosin-coated glass microneedle and actin cables in vitro. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1510–1514. doi: 10.1073/pnas.86.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn W. O. A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J Physiol. 1923 Dec 28;58(2-3):175–203. doi: 10.1113/jphysiol.1923.sp002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hill T. L. Theoretical formalism for the sliding filament model of contraction of striated muscle. Part I. Prog Biophys Mol Biol. 1974;28:267–340. doi: 10.1016/0079-6107(74)90020-0. [DOI] [PubMed] [Google Scholar]
- Iwamoto H., Sugaya R., Sugi H. Force-velocity relation of frog skeletal muscle fibres shortening under continuously changing load. J Physiol. 1990 Mar;422:185–202. doi: 10.1113/jphysiol.1990.sp017979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron S. J., Spudich J. A. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Oiwa K., Chaen S., Kamitsubo E., Shimmen T., Sugi H. Steady-state force-velocity relation in the ATP-dependent sliding movement of myosin-coated beads on actin cables in vitro studied with a centrifuge microscope. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7893–7897. doi: 10.1073/pnas.87.20.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Spudich J. A. Movement of myosin-coated fluorescent beads on actin cables in vitro. Nature. 1983 May 5;303(5912):31–35. doi: 10.1038/303031a0. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Weeds A. G. Determination of the association of myosin subfragment 1 with actin in the presence of ATP. Biochemistry. 1979 May 29;18(11):2260–2266. doi: 10.1021/bi00578a020. [DOI] [PubMed] [Google Scholar]