Abstract
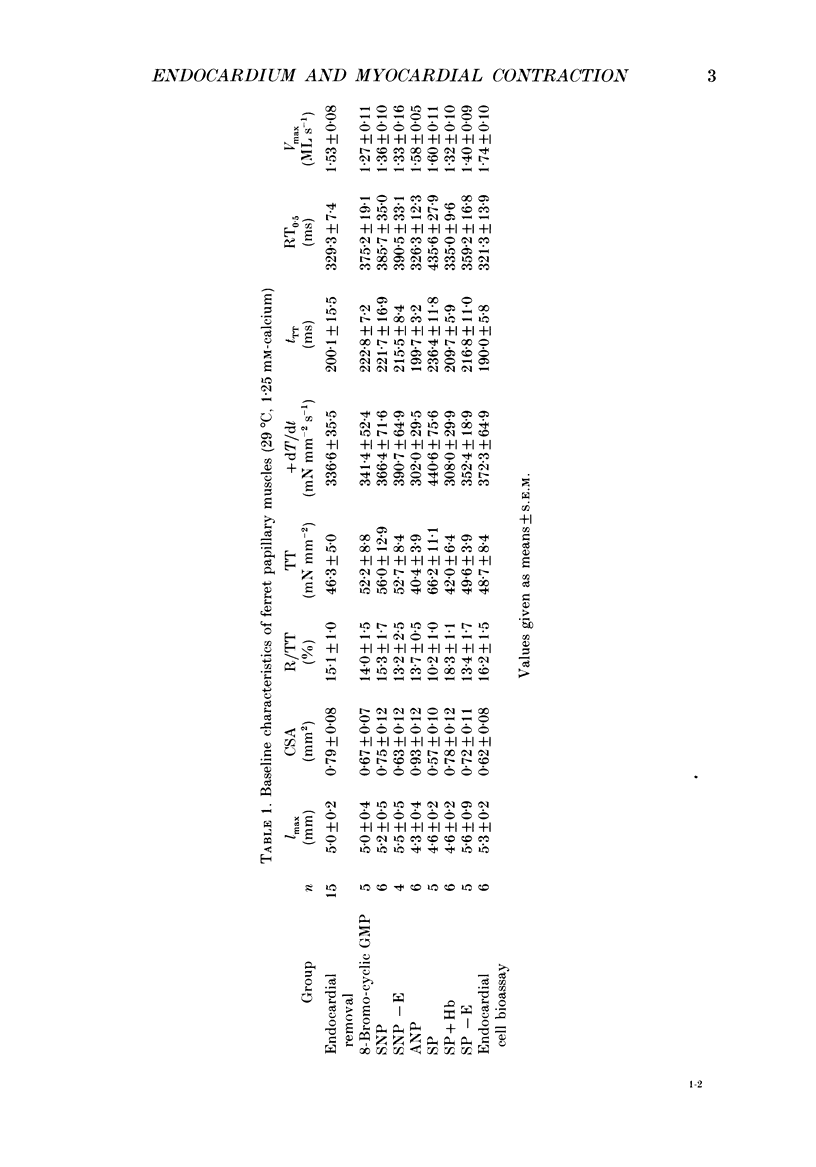
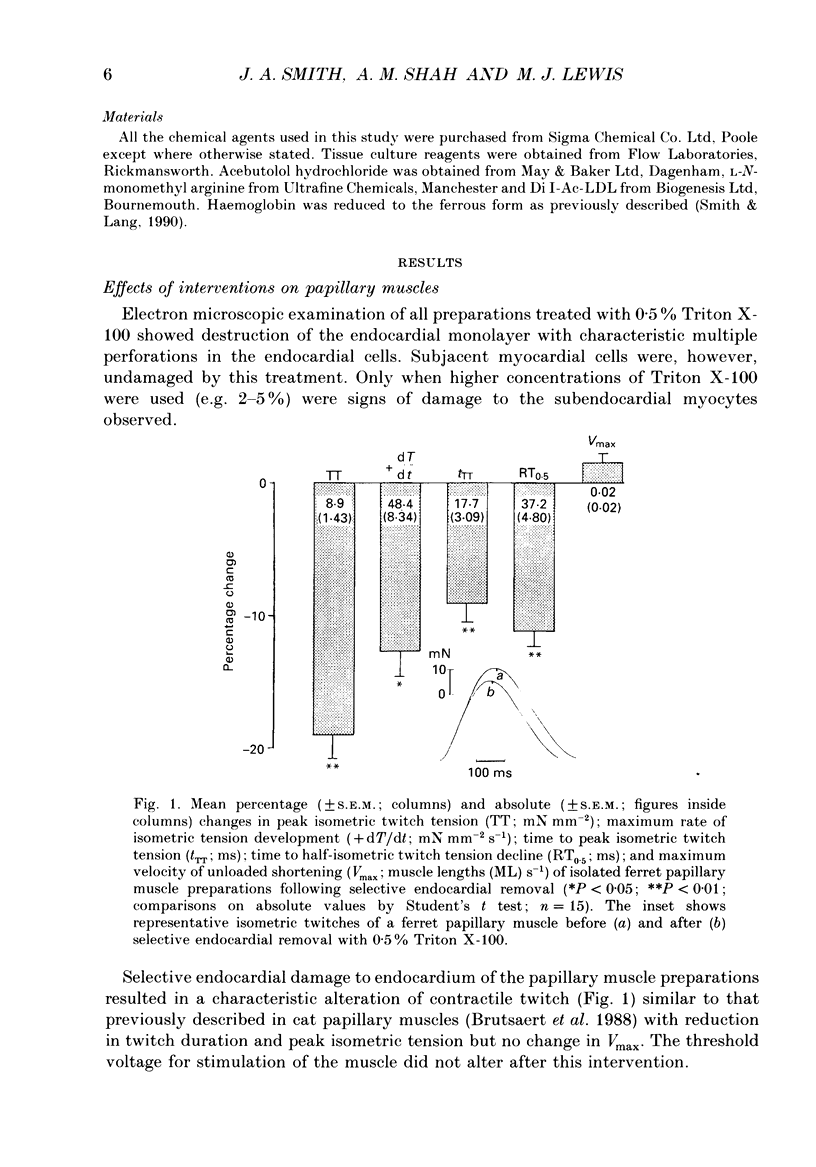
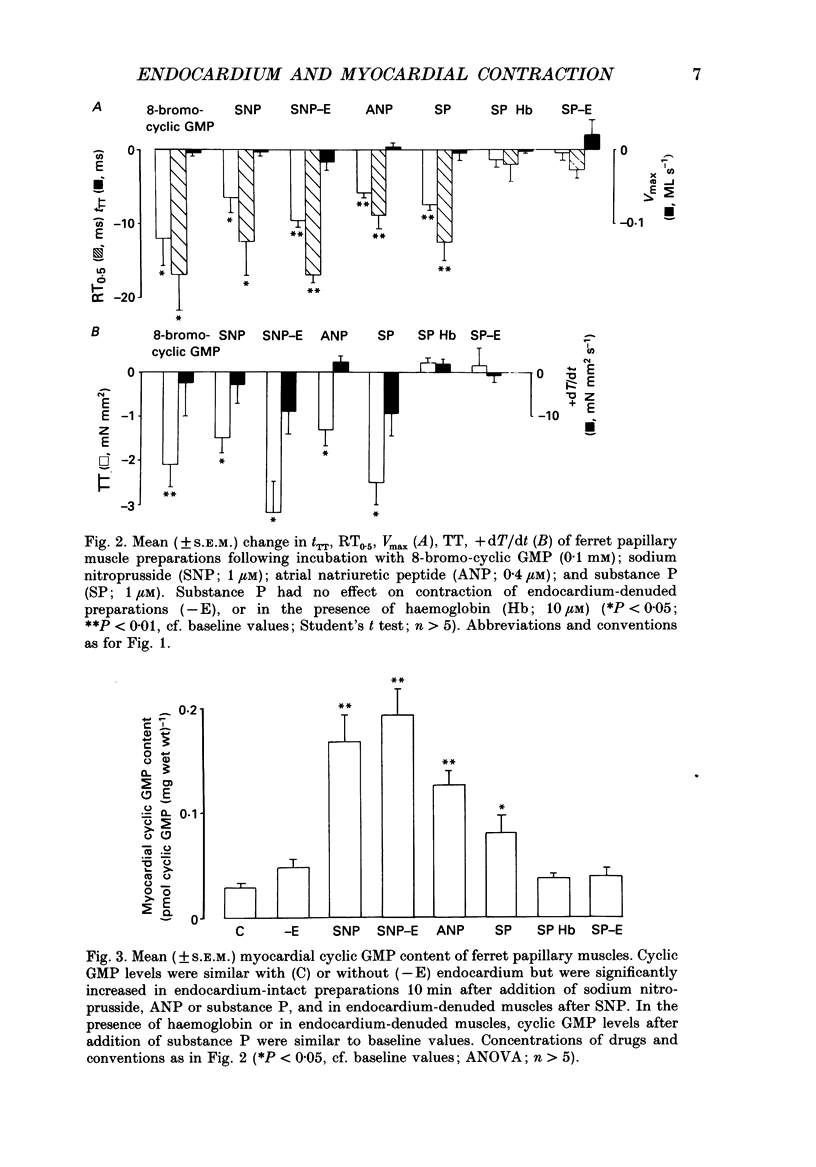
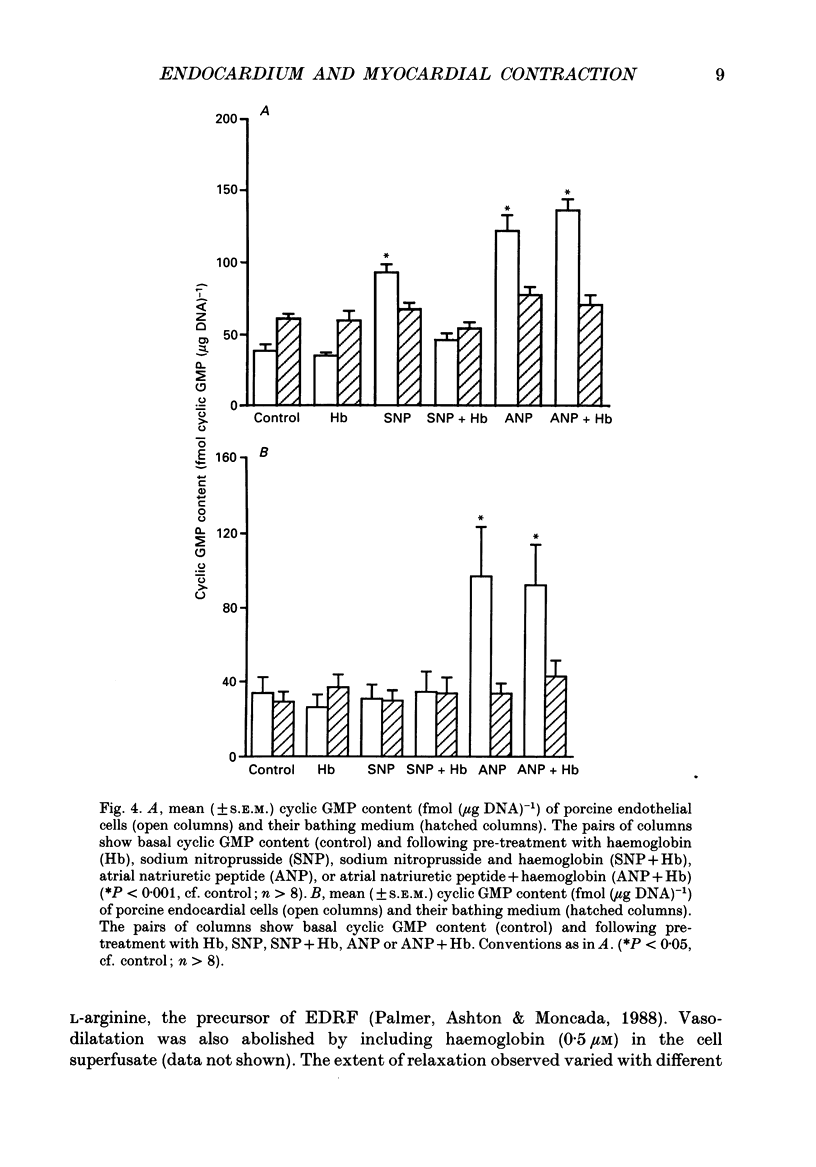
1. In isolated heart muscle preparations, selective removal of the endocardium results in a characteristic and unusual negative inotropic effect. Possible mechanisms for this effect were investigated in this study. 2. In endocardium-intact preparations of ferret papillary muscle, 8-bromo-cyclic GMP, sodium nitroprusside, atrial natriuretic peptide (ANP) and substance P each induced changes in contractile behaviour similar to selective endocardial removal, and each significantly elevated myocardial cyclic GMP levels. Substance P failed to elevate myocardial cyclic GMP levels following removal of endocardium or in the presence of haemoglobin, suggesting that it may act by releasing endothelium-derived relaxing factor (EDRF) from endocardium. However, there was no change in myocardial cyclic GMP levels following endocardium removal alone. 3. In cascade bioassay experiments, it was confirmed that porcine cultured endocardial cells released an unstable humoral agent whose effects on an endothelium-denuded pig coronary artery were indistinguishable from EDRF. 4. The negative inotropic effects of endocardium removal were reversed in bioassay experiments where an endocardium-denuded papillary muscle was exposed to the effluent from a column of porcine cultured endocardial cells on microcarrier beads. This demonstrates for the first time the release of a 'contraction prolonging factor' from endocardium, the tonic release of which would explain the negative inotropic effect of endocardium removal. 5. It is concluded that elevation of ferret papillary muscle cyclic GMP (as for example with EDRF) produces changes in contractile performance similar to those induced by endocardium removal. We also demonstrate that superfused porcine cultured endocardial cells release a humoral agent (provisionally named 'endocardin') which causes reversal of the changes in mechanical properties seen after endocardial removal.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kentish J. C. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985 Sep;17(9):821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Lee J. A., Smith G. L. The consequences of simulated ischaemia on intracellular Ca2+ and tension in isolated ferret ventricular muscle. J Physiol. 1989 Mar;410:297–323. doi: 10.1113/jphysiol.1989.sp017534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. The effects of changes of pH on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1983 Feb;335:555–567. doi: 10.1113/jphysiol.1983.sp014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert D. L., Claes V. A. Onset of mechanical activation of mammalian heart muscle in calcium- and strontium-containing solutions. Circ Res. 1974 Sep;35(3):345–357. doi: 10.1161/01.res.35.3.345. [DOI] [PubMed] [Google Scholar]
- Brutsaert D. L., Meulemans A. L., Sipido K. R., Sys S. U. Effects of damaging the endocardial surface on the mechanical performance of isolated cardiac muscle. Circ Res. 1988 Feb;62(2):358–366. doi: 10.1161/01.res.62.2.358. [DOI] [PubMed] [Google Scholar]
- Chappell S., Henderson A., Lewis M. Characterization of the mechanical behavior of isolated papillary muscle preparations of the ferret. J Pharmacol Methods. 1986 Feb;15(1):35–49. doi: 10.1016/0160-5402(86)90004-5. [DOI] [PubMed] [Google Scholar]
- Christie M. I., Lewis M. J. Vascular smooth muscle sensitivity to endothelium-derived relaxing factor is different in different arteries. Br J Pharmacol. 1988 Oct;95(2):630–636. doi: 10.1111/j.1476-5381.1988.tb11685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C. H., Poole-Wilson P. A. Effects of acid-base changes on excitation--contraction coupling in guinea-pig and rabbit cardiac ventricular muscle. J Physiol. 1981;313:141–160. doi: 10.1113/jphysiol.1981.sp013655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong B., Henderson A. H., Lewis M. J., Smith J. A. Endothelium-derived relaxing factor inhibits in vitro platelet aggregation. Br J Pharmacol. 1987 Apr;90(4):687–692. doi: 10.1111/j.1476-5381.1987.tb11221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Lewis M. J., Newby A. C., Henderson A. H. Endothelium-derived relaxing factor. J Am Coll Cardiol. 1988 Sep;12(3):797–806. doi: 10.1016/0735-1097(88)90324-5. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Henderson A. H., Brutsaert D. L. An analysis of the mechanical capabilities of heart muscle during hypoxia. Cardiovasc Res. 1973 Nov;7(6):763–776. doi: 10.1093/cvr/7.6.763. [DOI] [PubMed] [Google Scholar]
- Henderson A. H., Brutsaert D. L., Parmley W. W., Sonnenblick E. H. Myocardial mechanics in ppillary muscles of the rat and cat. Am J Physiol. 1969 Nov;217(5):1273–1279. doi: 10.1152/ajplegacy.1969.217.5.1273. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Jewell B. R. Calcium- and length-dependent force production in rat ventricular muscle. J Physiol. 1982 Aug;329:527–540. doi: 10.1113/jphysiol.1982.sp014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Lee J. A., Allen D. G. Comparison of the effects of inotropic interventions on isometric tension and shortening in isolated ferret ventricular muscle. Cardiovasc Res. 1989 Sep;23(9):748–755. doi: 10.1093/cvr/23.9.748. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- Shah A. M., Lewis M. J., Henderson A. H. Inotropic effects of endothelin in ferret ventricular myocardium. Eur J Pharmacol. 1989 Apr 25;163(2-3):365–367. doi: 10.1016/0014-2999(89)90208-2. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Lang D. Release of endothelium-derived relaxing factor from pig cultured aortic endothelial cells, as assessed by changes in endothelial cell cyclic GMP content, is inhibited by a phorbol ester. Br J Pharmacol. 1990 Mar;99(3):565–571. doi: 10.1111/j.1476-5381.1990.tb12969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Katz A. M. Phosphorylation of the sarcoplasmic reticulum and sarcolemma. Annu Rev Physiol. 1982;44:401–423. doi: 10.1146/annurev.ph.44.030182.002153. [DOI] [PubMed] [Google Scholar]
- Tyberg J. V., Yeatman L. A., Parmley W. W., Urschel C. W., Sonnenblick E. H. Effects of hypoxia on mechanics of cardiac contraction. Am J Physiol. 1970 Jun;218(6):1780–1788. doi: 10.1152/ajplegacy.1970.218.6.1780. [DOI] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984 Dec 10;259(23):14332–14334. [PubMed] [Google Scholar]
- Winegrad S. Regulation of cardiac contractile proteins. Correlations between physiology and biochemistry. Circ Res. 1984 Nov;55(5):565–574. doi: 10.1161/01.res.55.5.565. [DOI] [PubMed] [Google Scholar]


