Abstract
Purpose
To investigate the role of HER2 positivity in prognosis and unresponsiveness to anti-EGFR therapy for colorectal cancer.
Methods
Patients who underwent primary CRC tumor resection were included. HER2 status of CRC was confirmed by immunohistochemistry and fluorescence in situ hybridization tests. Comparison of survival analysis between HER2 positivity and negativity was evaluated by a stratified log-rank test and summarized with the use of Kaplan–Meier and Cox proportional hazards methods. The treatment effects of cetuximab were further compared in full subgroup analyses.
Result
1240 patients were enrolled, including 763 with stage I-III CRC and 477 with stage IV CRC. 57 (4.6%) CRC patients presented HER2 positivity in the entire cohort. The survival analysis showed that patients with HER2 positivity had significantly worse disease-free survival and overall survival in stage III and IV CRC. The multivariable analysis also confirmed that HER2 positivity was a significantly independent risk factor in stage III and IV CRC. Univariate and multivariable survival analysis showed no prognostic significance of HER2 positivity in stage I–II CRC patients. Prespecified subgroup analysis showed no favorable trends in progression-free survival and overall survival for cetuximab in the patients with HER2 positivity, KRAS/NRAS/BRAF wild-type metastatic CRC (interaction P values = 0.005 and 0.014).
Conclusion
For stage III and IV CRC patients, HER2 positivity was confirmed as an independent prognostic risk factor. It could help predict the unresponsiveness to anti-EGFR therapy for metastatic CRC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-021-03655-x.
Keywords: Colorectal cancer, HER2 positivity, Prognosis, Anti-EGFR therapy
Introduction
Globally, colorectal cancer (CRC) is the third most commonly diagnosed malignancy, and 1.8 million new CRC cases are diagnosed annually (Keum and Giovannucci 2019). In the era of precision medicine, validated biomarkers classify CRC patients according to their probable disease risk, prognosis, or response to treatment, and help determine optimal clinical management strategies. Recently, genome sequencing technology has made it possible to explore the characteristics of CRC at the signaling pathway molecular level (Dienstmann et al. 2017; Sveen et al. 2020). The RAS/RAF/MEK/ERK and PI3K/AKT/mTOR pathways play essential and diverse roles in both CRC oncogenesis and progression (Koveitypour et al. 2019; Nguyen et al. 2020). Activating hotspot mutations in KRAS, NRAS, and BRAF represent the most common mechanisms for RAS/RAF/MEK/ERK pathways activation (Dienstmann et al. 2017; Sveen et al. 2020). KRAS/NRAS/BRAF mutation has been proved to be associated with poor prognosis of stage II–IV CRC, even with resistance to anti-EGFR targeted therapy (Allegra et al. 2016; De Roock et al. 2011; Misale et al. 2014). Besides, activating alterations in ERBB family members, although less frequent events in CRC, have also been reported (Greally et al. 2018).
HER2 is a member of the ERBB family of receptor tyrosine kinases, which drive downstream RAS/RAF/MEK/ERK and PI3K/AKT/mTOR pathways (Oh and Bang 2020). Overexpression of HER2, most commonly driven by ERBB2 amplification, can result in HER2 homodimerization and ligand-independent activation(Oh and Bang 2020). HER2-positivity is an established therapeutic target and prognostic indicator in breast cancer and gastric cancer, with incidences of approximately 20% (Oh and Bang 2020; Waks and Winer, 2019). However, the incidence of HER2-positivity in colorectal cancer is only about 5% (Oh and Bang 2020). The numbers of HER2 overexpression cases reported in previous studies were very limited. Given the relative rarity of HER2-positive CRC, HER2-positivity has not been proved to have a definite prognostic significance. For stage II and III CRC, recent studies that evaluated HER2 expression of CRC have not demonstrated a clear association with prognosis (Nowak 2020). For stage IV CRC, evidence from phase 2 clinical trials suggests that HER2-positivity may predict lack of response to anti-EGFR therapy (Nowak 2020). Therefore, an additional large-scale study is necessary to determine whether there is a true prognostic significance to HER2-positivity in CRC.
Methods
Study population
This study retrospectively included CRC patients treated at General Surgery Department of Zhongshan Hospital, Fudan University (Shanghai, China) during May 2008 to December 2014. The inclusion criteria were as follows: radical resections of primary tumors; pathologically confirmed colorectal adenocarcinoma; no exposure to any treatment (chemotherapy, radiotherapy, or targeted therapy) before primary tumor resections, clinicopathological data available; enough formalin-fixed paraffin-embedded (FFPE) tissue of primary tumors. CRC stages were determined according to the 8th edition of AJCC TNM classification.
To further explore the predictability value of HER2 overexpression in the therapeutic efficacy of anti-EGFR, we studied the KRAS/NRAS/BRAF wild-type patients with colorectal liver metastasis (CRLM) in the general population. In the KRAS/NRAS/BRAF wild-type CRLM cohort, patients were treated with first-line chemotherapy with or without cetuximab. The detailed treatment information for KRAS/NRAS/BRAF wild-type CRLM patients was described as previously reported (Zheng et al. 2018).
This study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University. Informed consent was acquired from all patients of the primary cohort for the acquisition of clinical and pathological information and the use of surgical specimens.
Testing for HER2 status
The key steps to detect HER2 status were shown in Figure S1. Immunohistochemistry (IHC) was used to detect the HER2 expression. The IHC gave a score of 0 to 3 + that measures the amount of HER2 proteins in a CRC cancer tissue sample. If the score was 0 to 1 + , it was considered HER2-negative. If the score was 2 + , it was considered equivocal. A score of 3 + was considered HER2-positive. If the IHC test results were equivocal, a fluorescence in situ hybridization (FISH) test would be done on a sample of the cancer tissue to determine if the cancer was HER2-positive. If the FISH test showed an increased number of HER2 gene signals (red signals) relative to CEP17 (green signals) resulting in a calculated HER2/CE17 ratio of greater than 2, it was considered HER2-positive; otherwise, it was considered HER2-negative. The detailed procedure of the IHC was described as previously reported (Mao et al. 2018). The primary antibody was HER2/ErbB2 (29D8) rabbit monoclonal antibody (diluted 1:100, 2165, CST). The FISH test was performed in strict accordance with the protocol of PathVysion HER-2 DNA Probe Kit II (06N46, Abbott).
Follow-up
Patients were followed up mainly through outpatient clinic visits every 3 months for the first 2 years after the surgery, every 6 months for the next 3 years and once per year thereafter. Physical examination, serum CEA and CA19-9 tests, chest CT scan, and abdominal CT and MRI were performed at each follow-up, as well as colonoscopy once per year after the surgery. The starting point of follow-up was the initial primary tumor resection and the endpoint of follow-up was the evidence of tumor relapse or death until the deadline of December 1, 2020.
Statistical analysis
The association between clinicopathological features and HER2 status was accessed by Chi-square test or Fisher’s exact test as appropriate. Kaplan–Meier analysis and Log-rank test were performed to evaluate the relationship between HER2 status and survival outcome. Univariate cox regression analyses were performed to identify the independent prognostic factors among clinicopathological features and other information. Those factors with P < 0.10 in univariate cox regression analyses were included in the multivariate cox regression analysis. Hazard ratios (HR) and 95% confidence intervals (95% CI) were calculated using the Cox proportional hazards model. Heterogeneity among covariate levels in each subgroup was assessed in the subgroup analyses. For each subgroup, the Kaplan–Meier method was fitted. The interaction tests were performed for all subgroup analyses to assess whether the treatment effect varied according to subgroup (i.e., whether the effectiveness of anti-EGFR therapy differed in HER2 positive CRC patients compared to HER2 negative CRC patients). A two-sided P < 0.05 was considered statistically significant.
IBM SPSS software version 24.0 (IBM, Armonk, NJ, USA) and R software (https://www.r-project.org) were used for statistical analysis. The subgroup analysis of survival was conducted with the R package “Publish” (Version 2020.11.30). The forest plots were plotted with the R package “forestplot” (Version 1.10). The survival curve was plotted with the R package “survminer” (Version 0.4.8).
Result
Patient characteristics
From May 2008 to December 2014, a total of 1240 patients were enrolled, including 763 patients with stage I-III CRC and 477 patients with stage IV CRC. There were 57 (4.6%) HER2 positive CRC patients in the entire cohort, including 19 (2.5%) in stage I–III cohort and 38 (8.0%) in stage IV cohort. Of the 477 patients with stage IV CRC, 216 patients receiving first-line chemotherapy with or without cetuximab (CTX) were included in KRAS/NRAS/BRAF wild-type CRLM cohort. The median follow-up time was 53.1 months (Quartiles = 24.8–91.0) for patients with stage I-III CRC and was 36.0 months (Quartiles = 20.5–54.0) for patients with stage IV CRC. Baseline clinical and pathological characteristics of the whole study population were shown in Table 1. The relationship between HER2 positivity and clinicopathological features was also presented in Table 1. In the KRAS/NRAS/BRAF wild-type CRLM cohort, patients were divided into two groups according to whether they received CTX or not. The baseline characteristics of the chemotherapy alone group and CTX plus chemotherapy group were compared in Table 2.
Table 1.
Baseline characteristics in the entire cohort
| Characteristics | Stage I–III CRC cohort | Stage IV CRC cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 763) |
HER2-NEG (n = 744) |
HER2-POS NAR (n = 19) |
P | Total (n = 477) |
HER2-NEG (n = 439) |
HER2-POS NAR (n = 38) |
P | |
| Patient characteristics, n (%) | ||||||||
| Age > 60 years | 403 (52.8) | 391 (52.6) | 12 (63.2) | 0.361 | 214 (44.9) | 199 (45.3) | 15 (39.5) | 0.486 |
| Female | 332 (43.5) | 324 (43.5) | 8 (42.1) | 0.900 | 158 (33.1) | 144 (32.8) | 14 (36.8) | 0.612 |
| Primary tumor characteristics, n (%) | ||||||||
| Right-sided CRC | 215 (28.2) | 209 (28.1) | 6 (31.6) | 0.739 | 143 (30.0) | 134 (30.5) | 9 (23.7) | 0.377 |
| Left-sided CRC | 548 (71.8) | 535 (71.9) | 13 (68.4) | 334 (70.0) | 305 (69.5) | 29 (76.3) | ||
| T stage: T1–T2 | 166 (21.8) | 161 (21.6) | 5 (26.3) | 0.837 | 23 (4.8) | 20 (4.6) | 3 (7.9) | 0.598 |
| T stage: T3–T4 | 597 (78.2) | 583 (78.4) | 14 (73.7) | 454 (95.2) | 419 (95.4) | 35 (92.1) | ||
| N stage: node negative | 483 (63.3) | 472 (63.4) | 11 (57.9) | 0.620 | 123 (25.8) | 110 (25.1) | 13 (34.2) | 0.216 |
| N stage: node positive | 280 (36.7) | 272 (36.6) | 8 (42.1) | 354 (74.2) | 329 (74.9) | 25 (65.8) | ||
| Differentiation: moderate | 550 (72.1) | 534 (71.8) | 16 (84.2) | 0.233 | 298 (62.5) | 271 (61.7) | 27 (71.1) | 0.255 |
| Differentiation: poor | 213 (27.9) | 210 (28.2) | 3 (15.8) | 179 (37.5) | 168 (38.3) | 11 (28.9) | ||
| Mucinous property | 115 (15.1) | 111 (14.9) | 4 (21.1) | 0.679 | 41 (8.6) | 41 (9.3) | 0 (0.0) | 0.085 |
| Preoperative factors, n (%) | ||||||||
| Preop. CEA > 5 ng/ml | 268 (35.1) | 262 (35.2) | 6 (31.6) | 0.743 | 379 (79.5) | 354 (80.6) | 25 (65.8) | 0.030 |
| Preop. CA19-9 > 37 U/ml | 95 (12.5) | 92 (12.4) | 3 (15.8) | 0.925 | 244 (51.4) | 226 (51.6) | 18 (48.6) | 0.730 |
| AJCC stage, n (%) | ||||||||
| I | 134 (17.6) | 130 (17.5) | 4 (21.1) | 0.727 | – | – | – | – |
| II | 349 (45.7) | 342 (46.0) | 7 (36.8) | – | – | – | – | |
| III | 280 (36.7) | 272 (36.6) | 8 (42.1) | – | – | – | – | |
| IV | – | – | – | – | 477 (100.0) | 439 (100.0) | 38 (100.0) | – |
| Metastases characteristics | ||||||||
| AJCC M stage | – | – | – | – | ||||
| 1a | – | – | – | – | 432 (90.6) | 395 (90.0) | 37 (97.4) | 0.220 |
| 1b | – | – | – | 44 (9.2) | 43 (9.8) | 1 (2.6) | ||
| 1c | – | – | – | 1 (0.2) | 1 (0.1) | 0 (0.0) | ||
| Liver metastasis only | – | – | – | – | 403 (84.5) | 367 (83.6) | 36 (94.7) | 0.069 |
Table 2.
Baseline characteristics in the KRAS/NRAS/BRAF wild-type CRLM cohort
| Total (n = 216) |
Chemo. Alone (n = 113) |
CTX plus Chemo (n = 103) |
P | |
|---|---|---|---|---|
| Patient characteristics, n (%) | ||||
| Age > 60 years | 92 (42.6) | 47 (41.6) | 45 (43.7) | 0.756 |
| Female | 67 (31.0) | 33 (29.2) | 34 (33.0) | 0.546 |
| Primary tumor characteristics, n (%) | ||||
| Right-sided CRC | 60 (27.8) | 33 (29.2) | 27 (26.2) | 0.624 |
| Left-sided CRC | 156 (72.2) | 80 (70.8) | 76 (73.8) | |
| T stage: T1–T2 | 7 (3.2) | 5 (4.4) | 2 (1.9) | 0.519 |
| T stage: T3–T4 | 209 (96.8) | 108 (95.6) | 101 (98.1) | |
| N stage: node negative | 57 (26.4) | 31 (27.4) | 26 (25.2) | 0.715 |
| N stage: node positive | 159 (73.6) | 82 (72.6) | 77 (74.8) | |
| Preoperative factors, n (%) | ||||
| Preop. CEA > 5 ng/ml | 175 (81.0) | 90 (79.6) | 85 (82.5) | 0.590 |
| Preop. CA19-9 > 37 U/ml | 115 (53.2) | 68 (60.2) | 47 (45.6) | 0.032 |
| CRLM characteristics, n (%) | ||||
| Number of CRLM | ||||
| 1 | 34 (15.7) | 22 (19.5) | 12 (11.7) | 0.386 |
| 2–3 | 61 (28.2) | 29 (25.7) | 32 (31.1) | |
| 4–5 | 32 (14.8) | 15 (13.3) | 17 (16.5) | |
| > 5 | 89 (41.2) | 47 (41.6) | 42 (40.8) | |
| Size of largest CRLM ≥ 5 cm | 86 (39.8) | 47 (41.6) | 39 (37.9) | 0.576 |
| Bilateral CRLM | 139 (64.4) | 68 (60.2) | 71 (68.9) | 0.180 |
| HER2 Positivity, n (%) | 19 (8.8) | 9 (8.0) | 10 (9.7) | 0.651 |
CRLM colorectal liver metastasis, CTX cetuximab
HER2 positivity as prognostic biomarker in stage III and IV CRC
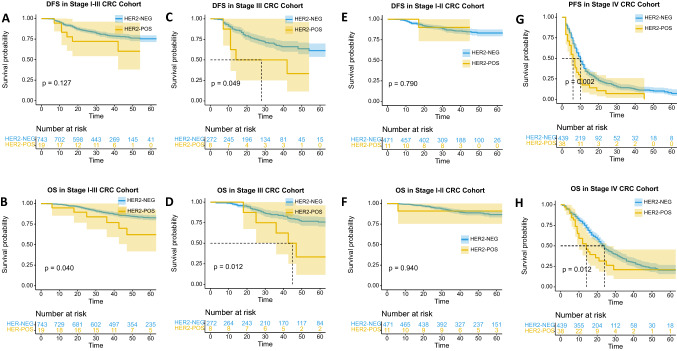
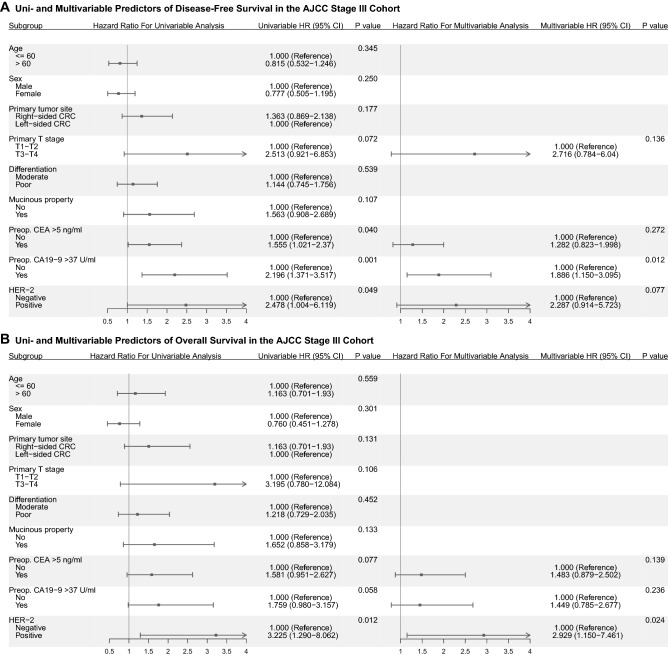
In stage I-III cohort, there was no significant difference in disease-free survival (DFS) between HER2 positive and HER2 negative patients (P = 0.127, Fig. 1a), but there was a difference in overall survival (OS) (P = 0.040, Fig. 1b). Multivariable Cox regression analysis in stage I-III cohort showed that HER2 positivity was not an independent risk factor in DFS, but a borderline risk factor in OS (Table S1 and S2). We further stratified the stage I–II and stage III cohorts. In stage III cohort, patients with HER2 positivity had significantly worse DFS and OS (P = 0.049; P = 0.012, as shown in Fig. 1c and d). Multivariable Cox regression analysis in stage III cohort showed that HER2 positivity was a borderline risk factor in DFS (Fig. 2a, Table S3) and an independent risk factor in OS (Fig. 2b, Table S4). However, in stage I-II cohort, no significant difference was observed in DFS and OS between HER2 positive and HER2 negative patients (Fig. 1e and f). As for stage IV cohort, patients with HER2 positivity had significantly worse progression-free survival (PFS) and OS (P = 0.002; P = 0.012, as shown in Fig. 1g and h). Multivariable Cox regression analysis in stage IV cohort showed that HER2 positivity was an independent risk factor in PFS and OS (Fig. 3, Table S5 and S6).
Fig. 1.
Kaplan–Meier estimates of DFS and OS stratified by the HER2 status. a and b: DFS and OS curves for HER2 positivity and negativity in stage I–III CRC cohort. c and d: DFS and OS curves for HER2 positivity and negativity in stage III CRC cohort. e and f: DFS and OS curves for HER2 positivity and negativity in stage I–II CRC cohort. g and h: PFS and OS curves for HER2 positivity and negativity in stage IV CRC cohort. The shaded part of the survival curve represents 95% confidence interval
Fig. 2.
a: Uni- and Multivariable Predictors of Disease-Free Survival in the AJCC Stage III Cohort; b: Uni- and Multivariable Predictors of Overall Survival in the AJCC Stage III Cohort
Fig. 3.
a: Uni- and Multivariable Predictors of Disease-Free Survival in the AJCC Stage IV Cohort; b: Uni- and Multivariable Predictors of Overall Survival in the AJCC Stage IV Cohort
HER2 positivity predicts unresponsiveness to anti-EGFR therapy
Results for PFS and OS in predefined subgroups of the KRAS/NRAS/BRAF wild-type CRLM cohort were generally consistent with those in the overall population of the KRAS/NRAS/BRAF wild-type CRLM cohort (Fig. 4). In these subgroups, HER2 status groups showed evidence of heterogeneity, with significant tests for interaction in PFS and OS (P values for interaction, 0.005; 0.014). In the KRAS/NRAS/BRAF wild-type CRLM cohort, whether PFS or OS, patients generally benefit from CTX (both P values were less than 0.001, Fig. 4, Fig. 5a and b). Similar results were found in HER2 negative and KRAS/NRAS/BRAF wild-type CRLM patients (both P values were less than 0.001, Fig. 4, Fig. 5c and d). However, for patients with HER2 positivity, there was no difference between the chemotherapy alone group and CTX plus chemotherapy group in PFS and OS (P = 0.169, P = 0.199, Figs. 4, 5e and f), which reflects the unresponsiveness of CTX.
Fig. 4.
Hazard ratios for PFS and OS in predefined subgroups of KRAS/NRAS/BRAF wild-type CRLM cohort. For each subgroup in the forest plots, the square represents the point estimate of the treatment effect and the horizontal line represents the 95% confidence interval
Fig. 5.
Kaplan–Meier estimates of PFS and OS stratified by whether or not to receive cetuximab. a and b: PFS and OS curves in KRAS/NRAS/BRAF wild-type CRLM cohort (the entire cohort). c and d: PFS and OS curves for patients with KRAS/NRAS/BRAF wild-type and HER2 negative CRLM (the HER2-Negative cohort). e and f: PFS and OS curves for patients with KRAS/NRAS/BRAF wild-type but HER2 positive CRLM (the HER2-Positive cohort)
Discussion
In this study, a real-world large-scale cohort was constructed to determine the prognostic significance of HER2 in CRC patients. Although the prognostic value of HER2 status was not detected in stage I–II CRC, the negative association between HER2-positivity and OS and DFS was determined in stage III CRC. Furthermore, we confirmed that HER2-positivity could predict the unresponsiveness to anti-EGFR therapy in stage IV CRC.
Stratified analysis in our study suggested that stage III CRC patients with HER2-positivity had a more poor prognosis. Although there have been a number of studies on HER2-positive CRC, the prognostic role of HER2 in CRC is still subject to debate. Early studies have demonstrated that HER2-positivity was associated with a worse prognosis of CRC (Kapitanovic et al. 1997; Osako et al. 1998). However, subsequent series studies have not confirmed that HER2 had a significant association with DFS or OS in CRC (Conradi et al. 2013; Ingold Heppner et al. 2014; Kruszewski et al. 2010; Richman et al. 2016; Sawada et al. 2018; Song et al. 2014). In a retrospective study involving 1645 patients with stage I–IV CRC, Heppner et al. found a trend toward worse OS in HER2-positive CRC, but not statistically significant (Ingold Heppner et al. 2014). Another large study performed a pool analysis of 3256 CRC patients enrolled in three RCTs (QUASAR, FOCUS and PICCOLO), and did not found any significant associations of HER2 with PFS or OS in stage II–IV CRC (Richman et al. 2016). Almost the previous studies did not explore stage III CRC alone. In the current study, HER2 positivity in stage III CRC was associated with worse survival, but not in stage I–II CRC. After all, the prognosis of stage I–II CRC is good, and the prognostic significance of HER2 in stage I–II CRC research requires a large sample size, to make a statistical significance. The stage III CRC means potential micrometastasis and residual tumor. The mechanism of HER2 as a prognostic indicator in stage III CRC patients could be explained by previous studies. HER2 positivity, as an oncogenic driver, can promote the malignant phenotype by triggering numerous down-stream pathways, such as RAS/RAF/MEK/ERK and PI3K/AKT/mTOR pathways (Oh and Bang 2020). HER2 positivity could activate the early dissemination and metastasis of tumor cells (Harper et al. 2016). Mangiapane et al. found that high HER2 expression levels were associated with an activation of the PI3K/AKT pathway, which promoted the characteristics of CRC stem cells (Mangiapane et al. 2021). Another recent study suggested that high HER2 expression levels might be associated with the characteristics of a quiescent subgroup of Paneth cells and Lgr5-high signature (Lupo et al. 2020). Therefore, HER2 positive in colorectal cancer may promote tumor progression and metastasis, and lead to resistance to therapeutics.
To our knowledge, the activating mutations in KRAS, NRAS, and BRAF serve as predictors of resistance to anti-EGFR therapy (Sveen et al. 2020). Given the ability of HER2 overexpression/amplification to also activate the RAS/RAF/MEK/ERK pathway, it seems reasonable that HER2 positivity can also predict the unresponsiveness to anti-EGFR therapy. The study in patient-derived xenografts found HER2 positivity enrichment in cetuximab-resistant cases (Bertotti et al., 2011). In a cohort study of 233 patients, a subset of colorectal cancer patients (n = 13) who exhibit either primary or acquired resistance to cetuximab-based therapy has HER2 positivity (Yonesaka et al. 2011). HER2 positivity, as one of the indicators of PRESSING panel, is a negative selection for patients with RAS/BRAF wild-type metastatic CRC receiving panitumumab-based maintenance therapy (Morano et al. 2019). In the HERACLES-A trial, none of HER2-positive CRC patients who received anti-EGFR therapy achieved an objective response (Sartore-Bianchi et al. 2016). In the phase 2 basket MyPathway trial, dual HER2-targeted therapy (pertuzumab plus trastuzumab) could represent a therapeutic opportunity for treatment-refractory patients with HER2-positive CRC (Meric-Bernstam et al. 2019). As for the current study, we supported such an association that HER2 positivity could predict the lack of response to anti-EGFR therapy in KRAS/NRAS/BRAF wild-type CRC.
However, a couple of limitations of this study must be noticed. On the one hand, our study is a retrospective one. To further validate our conclusion, a prospective study with data from multiple centers is necessary. On the other hand, considering the rarity of HER2 positivity, the number of HER2-positive CRC patients in this study is less, which limits the statistical interpretation.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
(1) Conception/design: WH, YC, WC, LR, YW, JX; (2) Provision of study material or patients: WH, YC, WC, LR, YW, JX; (3) Collection and/or assembly of data: WH, YC, WC, LR, WT, PZ, QW; (4) Data analysis and interpretation: WH, YC, PZ, QW, TL, YL; (5) Manuscript writing: WH, YC, WC, LR; (6) Final approval of manuscript: WH, YC, WC, LR, WT, PZ, QW, TL, YL, YW, JX.
Funding
Supported by The National Natural Science Foundation of China (82072678, 82072653, 81372315); Shanghai Science and Technology Committee Project (19511121301, 17411951300); Shanghai Engineering Research Center of Colorectal Cancer Minimally Invasive (17DZ2252600); The National Key R&D Program of China (2017YFC0908200).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declaration
Conflict of interest
All authors declared no competing interests.
Ethical approval
Approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University.
Informed consent
Informed consents were acquired from all patients for the acquisition of clinical and pathological information and the use of surgical specimens.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenbai Huang, Yijiao Chen, Wenju Chang and Li Ren contributed equally.
Contributor Information
Ye Wei, Email: 13818661815@126.com.
Jianmin Xu, Email: xujmin@aliyun.com.
References
- Allegra CJ, Rumble RB, Hamilton SR, Mangu PB, Roach N, Hantel A, Schilsky RL (2016) Extended RAS gene mutation testing in metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy: American society of clinical oncology provisional clinical opinion update 2015. J Clin Oncol 34(2):179–185. 10.1200/JCO.2015.63.9674 [DOI] [PubMed] [Google Scholar]
- Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, Trusolino L (2011) A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 1(6):508–523. 10.1158/2159-8290.CD-11-0109 [DOI] [PubMed] [Google Scholar]
- Conradi LC, Styczen H, Sprenger T, Wolff HA, Rodel C, Nietert M, Liersch T (2013) Frequency of HER-2 positivity in rectal cancer and prognosis. Am J Surg Pathol 37(4):522–531. 10.1097/PAS.0b013e318272ff4d [DOI] [PubMed] [Google Scholar]
- De Roock W, De Vriendt V, Normanno N, Ciardiello F, Tejpar S (2011) KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol 12(6):594–603. 10.1016/S1470-2045(10)70209-6 [DOI] [PubMed] [Google Scholar]
- Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J (2017) Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer 17(2):79–92. 10.1038/nrc.2016.126 [DOI] [PubMed] [Google Scholar]
- Greally M, Kelly CM, Cercek A (2018) HER2: an emerging target in colorectal cancer. Curr Probl Cancer 42(6):560–571. 10.1016/j.currproblcancer.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, Aguirre-Ghiso JA (2016) Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature 540(7634):588–592. 10.1038/nature20609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold Heppner B, Behrens HM, Balschun K, Haag J, Kruger S, Becker T, Rocken C (2014) HER2/neu testing in primary colorectal carcinoma. Br J Cancer 111(10):1977–1984. 10.1038/bjc.2014.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitanovic S, Radosevic S, Kapitanovic M, Andelinovic S, Ferencic Z, Tavassoli M, Spaventi R (1997) The expression of p185(HER-2/neu) correlates with the stage of disease and survival in colorectal cancer. Gastroenterology 112(4):1103–1113. 10.1016/s0016-5085(97)70120-3 [DOI] [PubMed] [Google Scholar]
- Keum N, Giovannucci E (2019) Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 16(12):713–732. 10.1038/s41575-019-0189-8 [DOI] [PubMed] [Google Scholar]
- Koveitypour Z, Panahi F, Vakilian M, Peymani M, Seyed Forootan F, Nasr Esfahani MH, Ghaedi K (2019) Signaling pathways involved in colorectal cancer progression. Cell Biosci 9:97. 10.1186/s13578-019-0361-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszewski WJ, Rzepko R, Ciesielski M, Szefel J, Zielinski J, Szajewski M, Wojtacki J (2010) Expression of HER2 in colorectal cancer does not correlate with prognosis. Dis Markers 29(5):207–212. 10.3233/DMA-2010-0742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo B, Sassi F, Pinnelli M, Galimi F, Zanella ER, Vurchio V, Trusolino L (2020) Colorectal cancer residual disease at maximal response to EGFR blockade displays a druggable Paneth cell-like phenotype. Sci Transl Med. 10.1126/scitranslmed.aax8313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiapane LR, Nicotra A, Turdo A, Gaggianesi M, Bianca P, Di Franco S, Stassi G (2021) PI3K-driven HER2 expression is a potential therapeutic target in colorectal cancer stem cells. Gut. 10.1136/gutjnl-2020-323553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Feng Q, Zheng P, Yang L, Zhu D, Chang W, Xu J (2018) Low tumor infiltrating mast cell density confers prognostic benefit and reflects immunoactivation in colorectal cancer. Int J Cancer 143(9):2271–2280. 10.1002/ijc.31613 [DOI] [PubMed] [Google Scholar]
- Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, Hainsworth J (2019) Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 20(4):518–530. 10.1016/S1470-2045(18)30904-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A (2014) Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov 4(11):1269–1280. 10.1158/2159-8290.CD-14-0462 [DOI] [PubMed] [Google Scholar]
- Morano F, Corallo S, Lonardi S, Raimondi A, Cremolini C, Rimassa L, Pietrantonio F (2019) Negative hyperselection of patients With RAS and BRAF wild-type metastatic colorectal cancer who received panitumumab-based maintenance therapy. J Clin Oncol 37(33):3099–3110. 10.1200/JCO.19.01254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Goel A, Chung DC (2020) Pathways of colorectal carcinogenesis. Gastroenterology 158(2):291–302. 10.1053/j.gastro.2019.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JA (2020) HER2 in colorectal carcinoma: are we there yet? Surg Pathol Clin 13(3):485–502. 10.1016/j.path.2020.05.007 [DOI] [PubMed] [Google Scholar]
- Oh DY, Bang YJ (2020) HER2-targeted therapies—a role beyond breast cancer. Nat Rev Clin Oncol 17(1):33–48. 10.1038/s41571-019-0268-3 [DOI] [PubMed] [Google Scholar]
- Osako T, Miyahara M, Uchino S, Inomata M, Kitano S, Kobayashi M (1998) Immunohistochemical study of c-erbB-2 protein in colorectal cancer and the correlation with patient survival. Oncology 55(6):548–555. 10.1159/000011911 [DOI] [PubMed] [Google Scholar]
- Richman SD, Southward K, Chambers P, Cross D, Barrett J, Hemmings G, Quirke P (2016) HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol 238(4):562–570. 10.1002/path.4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Siena S (2016) Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 17(6):738–746. 10.1016/S1470-2045(16)00150-9 [DOI] [PubMed] [Google Scholar]
- Sawada K, Nakamura Y, Yamanaka T, Kuboki Y, Yamaguchi D, Yuki S, Fujii S (2018) Prognostic and predictive value of HER2 amplification in patients with metastatic colorectal cancer. Clin Colorectal Cancer 17(3):198–205. 10.1016/j.clcc.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Song Z, Deng Y, Zhuang K, Li A, Liu S (2014) Immunohistochemical results of HER2/neu protein expression assessed by rabbit monoclonal antibodies SP3 and 4B5 in colorectal carcinomas. Int J Clin Exp Pathol 7(7): 4454–4460. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25120833 [PMC free article] [PubMed]
- Sveen A, Kopetz S, Lothe RA (2020) Biomarker-guided therapy for colorectal cancer: strength in complexity. Nat Rev Clin Oncol 17(1):11–32. 10.1038/s41571-019-0241-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waks AG, Winer EP (2019) Breast cancer treatment: a review. JAMA 321(3):288–300. 10.1001/jama.2018.19323 [DOI] [PubMed] [Google Scholar]
- Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, Janne PA (2011) Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 3(99):99ra86. 10.1126/scitranslmed.3002442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Liang C, Ren L, Zhu D, Feng Q, Chang W, Xu J (2018) Additional biomarkers beyond RAS that impact the efficacy of cetuximab plus chemotherapy in mCRC: a retrospective biomarker analysis. J Oncol 2018:5072987. 10.1155/2018/5072987 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.