Abstract
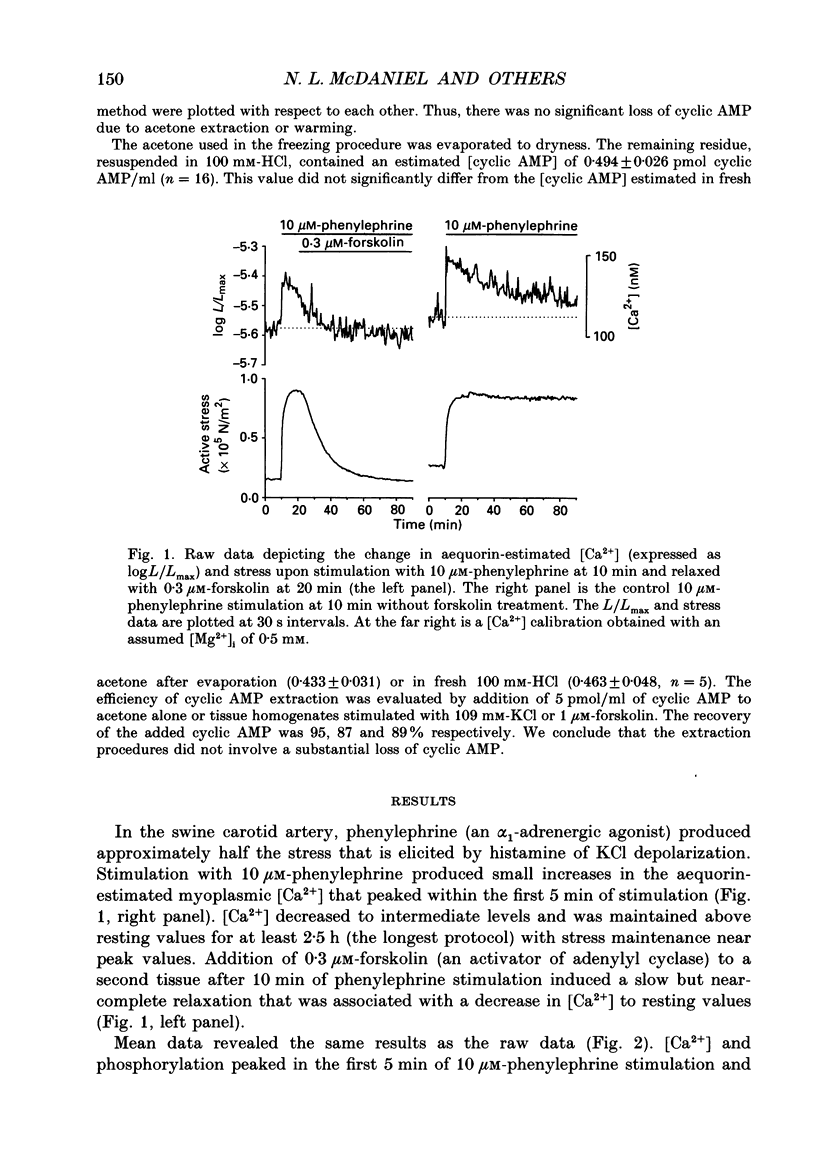
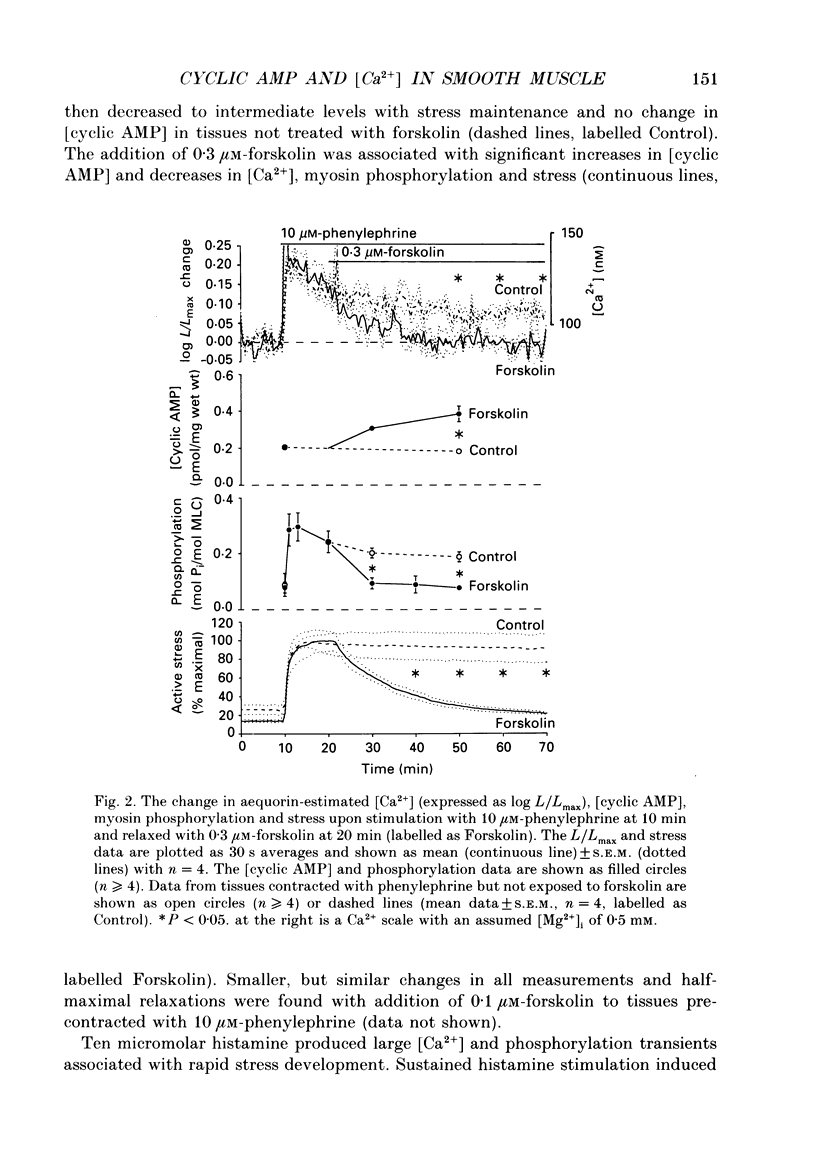
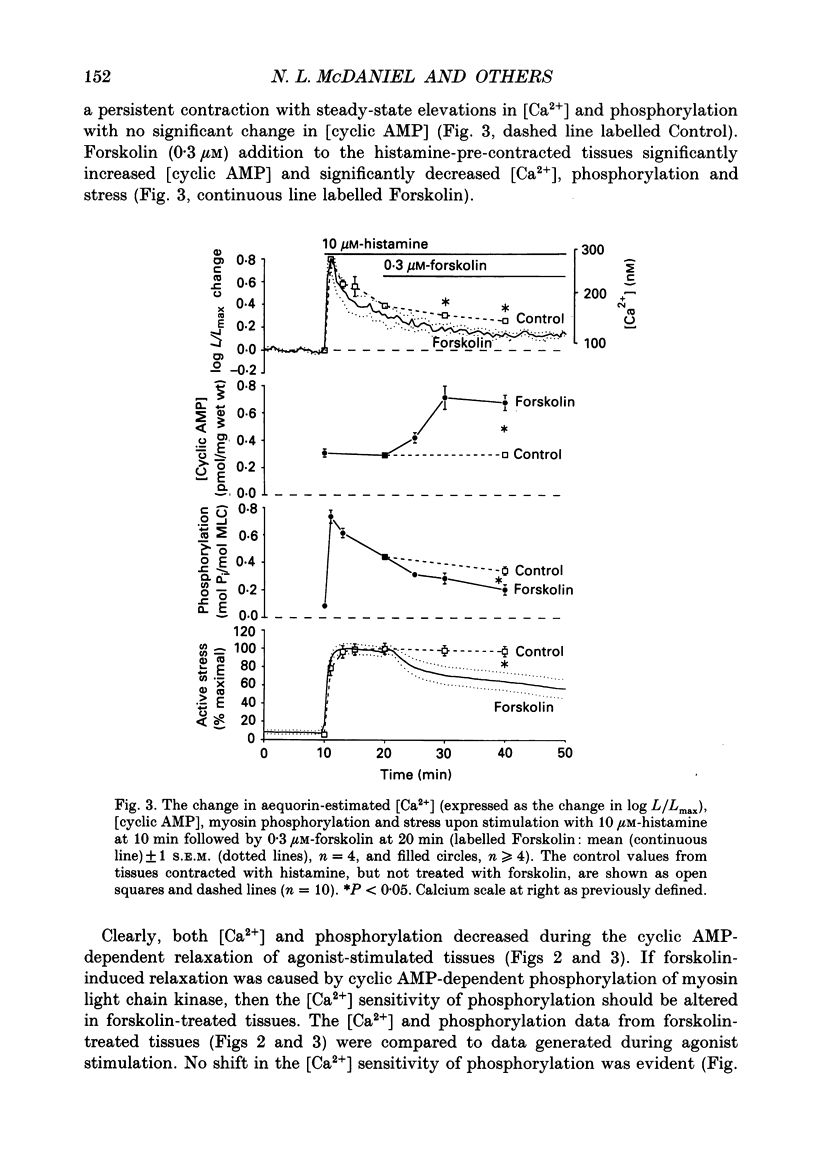
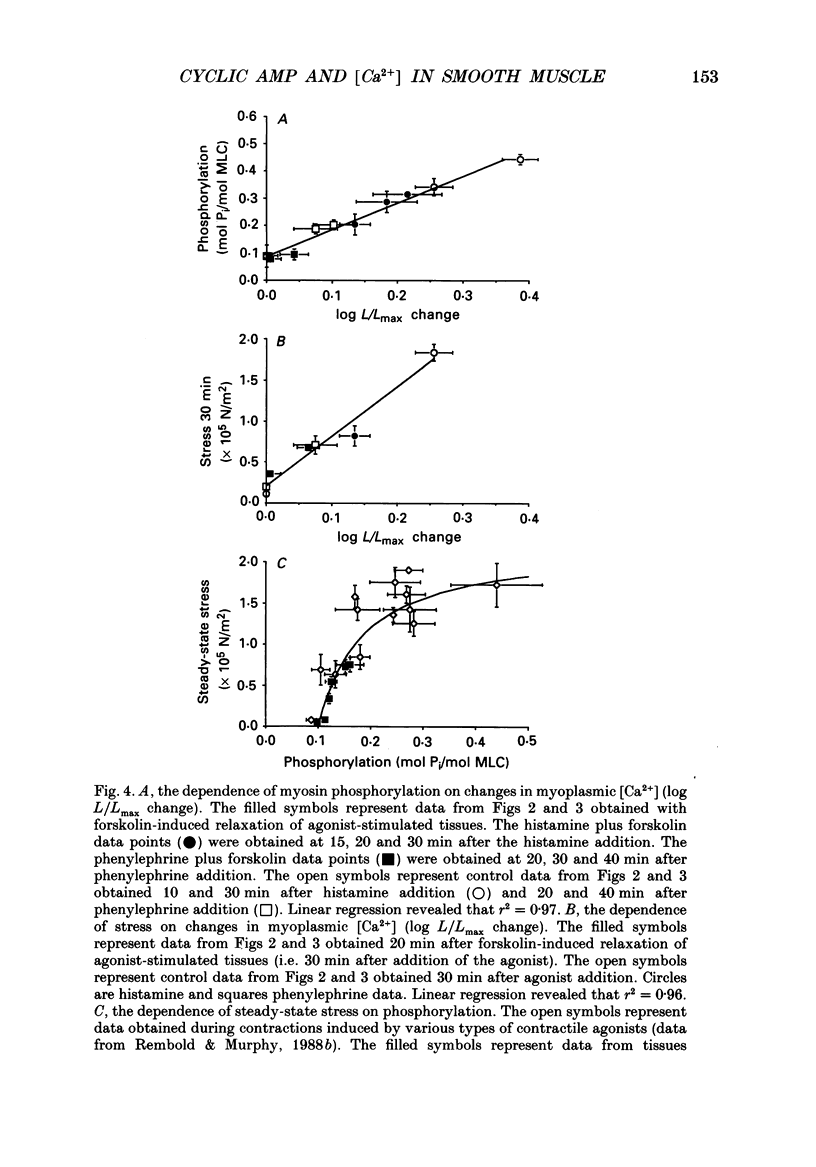
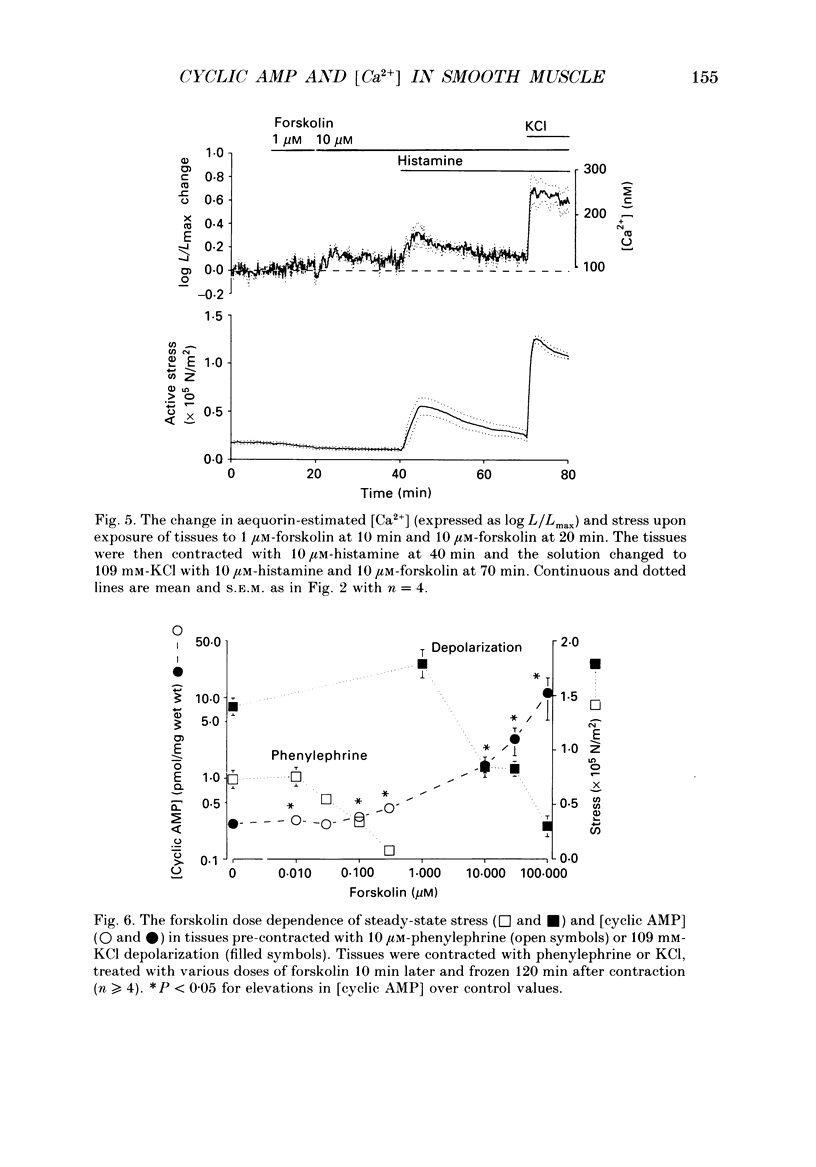
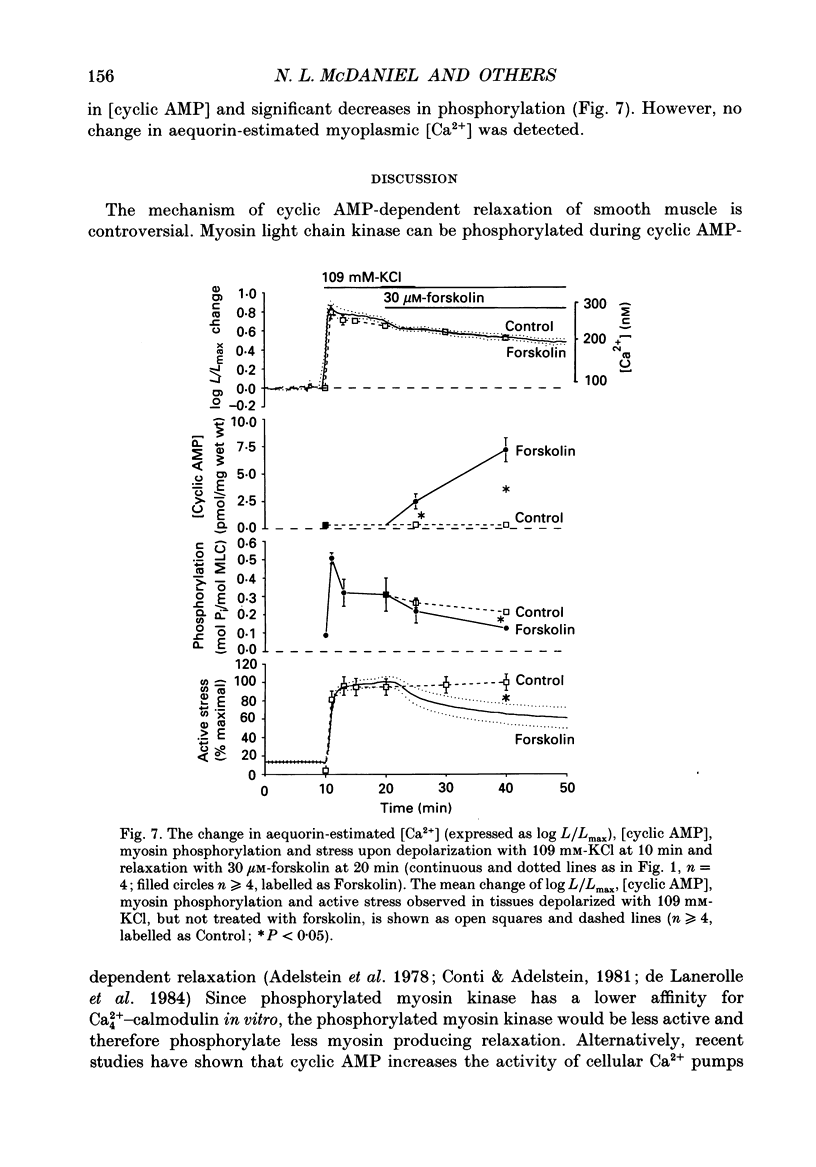
1. Our objective was to evaluate the mechanism of cyclic AMP-dependent arterial smooth muscle relaxation. Cyclic AMP-dependent relaxation has been proposed to result from either (a) a decrease in intracellular [Ca2+] or (b) a decrease in [Ca2+] sensitivity of myosin light chain kinase by protein kinase A-dependent phosphorylation of myosin kinase. 2. We evaluated these proposed mechanisms by examining forskolin-induced changes in aequorin-estimated myoplasmic [Ca2+], [cyclic AMP], myosin phosphorylation and stress generation in agonist-stimulated or KCl-depolarized swine common carotid media tissues. 3. Forskolin, an activator of adenylyl cyclase, increased [cyclic AMP] and reduced [Ca2+], myosin phosphorylation and stress in tissues pre-contracted with phenylephrine or histamine. This relaxation was not associated with an alteration of the [Ca2+] sensitivity of phosphorylation, nor the dependence of stress on phosphorylation. 4. Forskolin pre-treatment attenuated, but did not abolish, agonist-induced increases in [Ca2+] and stress. 5. These results suggest that cyclic AMP-induced relaxation of the agonist-stimulated swine carotid media is primarily caused by cyclic AMP-mediated decreases in myoplasmic [Ca2+].
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe A., Karaki H. Effect of forskolin on cytosolic Ca++ level and contraction in vascular smooth muscle. J Pharmacol Exp Ther. 1989 Jun;249(3):895–900. [PubMed] [Google Scholar]
- Adelstein R. S., Conti M. A., Hathaway D. R., Klee C. B. Phosphorylation of smooth muscle myosin light chain kinase by the catalytic subunit of adenosine 3': 5'-monophosphate-dependent protein kinase. J Biol Chem. 1978 Dec 10;253(23):8347–8350. [PubMed] [Google Scholar]
- Aksoy M. O., Mras S., Kamm K. E., Murphy R. A. Ca2+, cAMP, and changes in myosin phosphorylation during contraction of smooth muscle. Am J Physiol. 1983 Sep;245(3):C255–C270. doi: 10.1152/ajpcell.1983.245.3.C255. [DOI] [PubMed] [Google Scholar]
- Brooker G., Terasaki W. L., Price M. G. Gammaflow: a completely automated radioimmunoassay system. Science. 1976 Oct 15;194(4262):270–276. doi: 10.1126/science.184530. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Yatani A., Imoto Y., Codina J., Mattera R., Birnbaumer L. Direct G-protein regulation of Ca2+ channels. Ann N Y Acad Sci. 1989;560:373–386. doi: 10.1111/j.1749-6632.1989.tb24116.x. [DOI] [PubMed] [Google Scholar]
- Conti M. A., Adelstein R. S. The relationship between calmodulin binding and phosphorylation of smooth muscle myosin kinase by the catalytic subunit of 3':5' cAMP-dependent protein kinase. J Biol Chem. 1981 Apr 10;256(7):3178–3181. [PubMed] [Google Scholar]
- Driska S. P., Aksoy M. O., Murphy R. A. Myosin light chain phosphorylation associated with contraction in arterial smooth muscle. Am J Physiol. 1981 May;240(5):C222–C233. doi: 10.1152/ajpcell.1981.240.5.C222. [DOI] [PubMed] [Google Scholar]
- Francis S. H., Noblett B. D., Todd B. W., Wells J. N., Corbin J. D. Relaxation of vascular and tracheal smooth muscle by cyclic nucleotide analogs that preferentially activate purified cGMP-dependent protein kinase. Mol Pharmacol. 1988 Oct;34(4):506–517. [PubMed] [Google Scholar]
- Furukawa K., Tawada Y., Shigekawa M. Regulation of the plasma membrane Ca2+ pump by cyclic nucleotides in cultured vascular smooth muscle cells. J Biol Chem. 1988 Jun 15;263(17):8058–8065. [PubMed] [Google Scholar]
- Gerthoffer W. T., Trevethick M. A., Murphy R. A. Myosin phosphorylation and cyclic adenosine 3',5'-monophosphate in relaxation of arterial smooth muscle by vasodilators. Circ Res. 1984 Jan;54(1):83–89. doi: 10.1161/01.res.54.1.83. [DOI] [PubMed] [Google Scholar]
- Hai C. M., Murphy R. A. Cross-bridge dephosphorylation and relaxation of vascular smooth muscle. Am J Physiol. 1989 Feb;256(2 Pt 1):C282–C287. doi: 10.1152/ajpcell.1989.256.2.C282. [DOI] [PubMed] [Google Scholar]
- Hai C. M., Murphy R. A. Cross-bridge phosphorylation and regulation of latch state in smooth muscle. Am J Physiol. 1988 Jan;254(1 Pt 1):C99–106. doi: 10.1152/ajpcell.1988.254.1.C99. [DOI] [PubMed] [Google Scholar]
- Hall I. P., Hill S. J. Beta-adrenoceptor stimulation inhibits histamine-stimulated inositol phospholipid hydrolysis in bovine tracheal smooth muscle. Br J Pharmacol. 1988 Dec;95(4):1204–1212. doi: 10.1111/j.1476-5381.1988.tb11757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Garber S. S., Aldrich R. W. Effect of forskolin on voltage-gated K+ channels is independent of adenylate cyclase activation. Science. 1988 Jun 17;240(4859):1652–1655. doi: 10.1126/science.2454506. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Kimura M., Kimura I., Kobayashi S. Relationship between cyclic AMP-dependent protein kinase activation and Ca uptake increase of sarcoplasmic reticulum fraction of hog biliary muscles relaxed by cholecystokinin-C-terminal peptides. Biochem Pharmacol. 1982 Oct 1;31(19):3077–3083. doi: 10.1016/0006-2952(82)90083-1. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L., Taylor A. E. cGMP-dependent protein kinase mediates the reduction of Ca2+ by cAMP in vascular smooth muscle cells. Am J Physiol. 1990 Mar;258(3 Pt 1):C399–C407. doi: 10.1152/ajpcell.1990.258.3.C399. [DOI] [PubMed] [Google Scholar]
- Miller J. R., Silver P. J., Stull J. T. The role of myosin light chain kinase phosphorylation in beta-adrenergic relaxation of tracheal smooth muscle. Mol Pharmacol. 1983 Sep;24(2):235–242. [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Alteration of cytoplasmic ionized calcium levels in smooth muscle by vasodilators in the ferret. J Physiol. 1984 Dec;357:539–551. doi: 10.1113/jphysiol.1984.sp015516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch. 1982 Oct;395(1):75–77. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- Parker I., Ito Y., Kuriyama H., Miledi R. Beta-adrenergic agonists and cyclic AMP decrease intracellular resting free-calcium concentration in ileum smooth muscle. Proc R Soc Lond B Biol Sci. 1987 Mar 23;230(1259):207–214. doi: 10.1098/rspb.1987.0016. [DOI] [PubMed] [Google Scholar]
- Ratz P. H., Hai C. M., Murphy R. A. Dependence of stress on cross-bridge phosphorylation in vascular smooth muscle. Am J Physiol. 1989 Jan;256(1 Pt 1):C96–100. doi: 10.1152/ajpcell.1989.256.1.C96. [DOI] [PubMed] [Google Scholar]
- Rembold C. M. Desensitization of swine arterial smooth muscle to transplasmalemmal Ca2+ influx. J Physiol. 1989 Sep;416:273–290. doi: 10.1113/jphysiol.1989.sp017760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold C. M. Modulation of the [Ca2+] sensitivity of myosin phosphorylation in intact swine arterial smooth muscle. J Physiol. 1990 Oct;429:77–94. doi: 10.1113/jphysiol.1990.sp018245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold C. M., Murphy R. A. Latch-bridge model in smooth muscle: [Ca2+]i can quantitatively predict stress. Am J Physiol. 1990 Aug;259(2 Pt 1):C251–C257. doi: 10.1152/ajpcell.1990.259.2.C251. [DOI] [PubMed] [Google Scholar]
- Rembold C. M., Murphy R. A. Myoplasmic [Ca2+] determines myosin phosphorylation in agonist-stimulated swine arterial smooth muscle. Circ Res. 1988 Sep;63(3):593–603. doi: 10.1161/01.res.63.3.593. [DOI] [PubMed] [Google Scholar]
- Rembold C. M., Murphy R. A. [Ca2+]-dependent myosin phosphorylation in phorbol diester stimulated smooth muscle contraction. Am J Physiol. 1988 Dec;255(6 Pt 1):C719–C723. doi: 10.1152/ajpcell.1988.255.6.C719. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Takuwa N., Rasmussen H. The effects of isoproterenol on intracellular calcium concentration. J Biol Chem. 1988 Jan 15;263(2):762–768. [PubMed] [Google Scholar]
- Tawada Y., Furukawa K., Shigekawa M. Cyclic AMP enhances inositol trisphosphate-induced mobilization of intracellular Ca2+ in cultured aortic smooth muscle cells. J Biochem. 1988 Nov;104(5):795–800. doi: 10.1093/oxfordjournals.jbchem.a122552. [DOI] [PubMed] [Google Scholar]
- Wagoner P. K., Pallotta B. S. Modulation of acetylcholine receptor desensitization by forskolin is independent of cAMP. Science. 1988 Jun 17;240(4859):1655–1657. doi: 10.1126/science.2454507. [DOI] [PubMed] [Google Scholar]
- de Lanerolle P., Nishikawa M., Yost D. A., Adelstein R. S. Increased phosphorylation of myosin light chain kinase after an increase in cyclic AMP in intact smooth muscle. Science. 1984 Mar 30;223(4643):1415–1417. doi: 10.1126/science.6322302. [DOI] [PubMed] [Google Scholar]


