Abstract
1. The effects of bradykinin, ATP, adenosine, histamine and thrombin on the membrane potential of confluent monolayers of cultured bovine aortic endothelial cells (BAECs) and guinea-pig coronary endothelial cells (GCECs) were studied at 37 degrees C using the whole-cell mode of the patch-clamp technique. 2. The amplitude histogram of the resting potentials of BAEC monolayers showed a bimodal distribution with one peak around -25 mV and another peak around -85 mV. Transitions from one potential level to the other were observed. The bistable membrane potential can be explained by an N-shaped current-voltage relation of the endothelial cell membrane. 3. When BAECs with a low resting potential (-10 to -30 mV) were superfused with maximally effective concentrations of ATP (2-10 microM) an initial hyperpolarization of -80 to -90 mV was observed which decayed to a plateau of about -60 mV within 1 min. When ATP was removed after 2-3 min the membrane potential returned to control level within 1 min. This was followed by a second hyperpolarization of 10-20 mV, which decayed within 15 min. 4. In the absence of extracellular calcium, ATP produced only a brief transient hyperpolarization in aortic endothelium. The plateau and the secondary hyperpolarization were abolished. These findings are consistent with the idea that the changes in membrane potential reflect changes in intracellular free Ca2+ and that the initial peak is due to release of Ca2+ from intracellular stores, whereas the plateau and the secondary hyperpolarization depend on transmembrane Ca2+ influx. 5. Bradykinin evoked potential changes similar to ATP in BAECs, except that the secondary hyperpolarization during wash-out was absent. When the membrane potential was more negative than -80 mV, ATP and bradykinin induced only a small initial hyperpolarization followed by a depolarization of up to 20 mV. 6. In aortic endothelium, ADP (10 microM) evoked a much smaller response than ATP. Adenosine (10 microM), thrombin (2 units/ml), acetylcholine (10 microM) and histamine (10 microM) had only a very small effect on the membrane potential, if any. 7. The amplitude histogram of the membrane potential of GCECs showed only one peak around -35 mV. In coronary endothelium, application of bradykinin, ATP, histamine, thrombin, acetylcholine and adenosine all evoked a transient hyperpolarization of 10-40 mV lasting 1 min or less, which then turned into a depolarization. 8. The K+ channel openers cromakalim (BRL 34915) and lemakalim (BRL 38227) did not affect the membrane potential of GCECs or BAECs.(ABSTRACT TRUNCATED AT 400 WORDS)
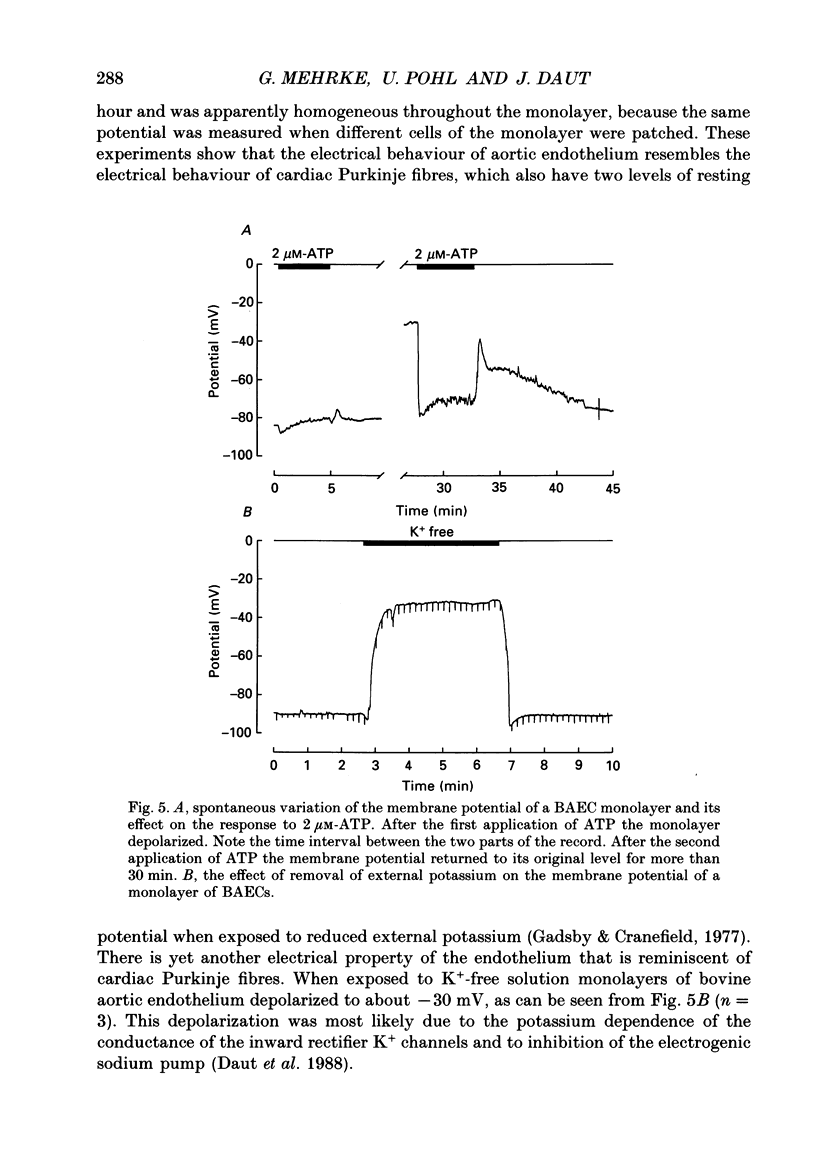
Full text
PDF














Selected References
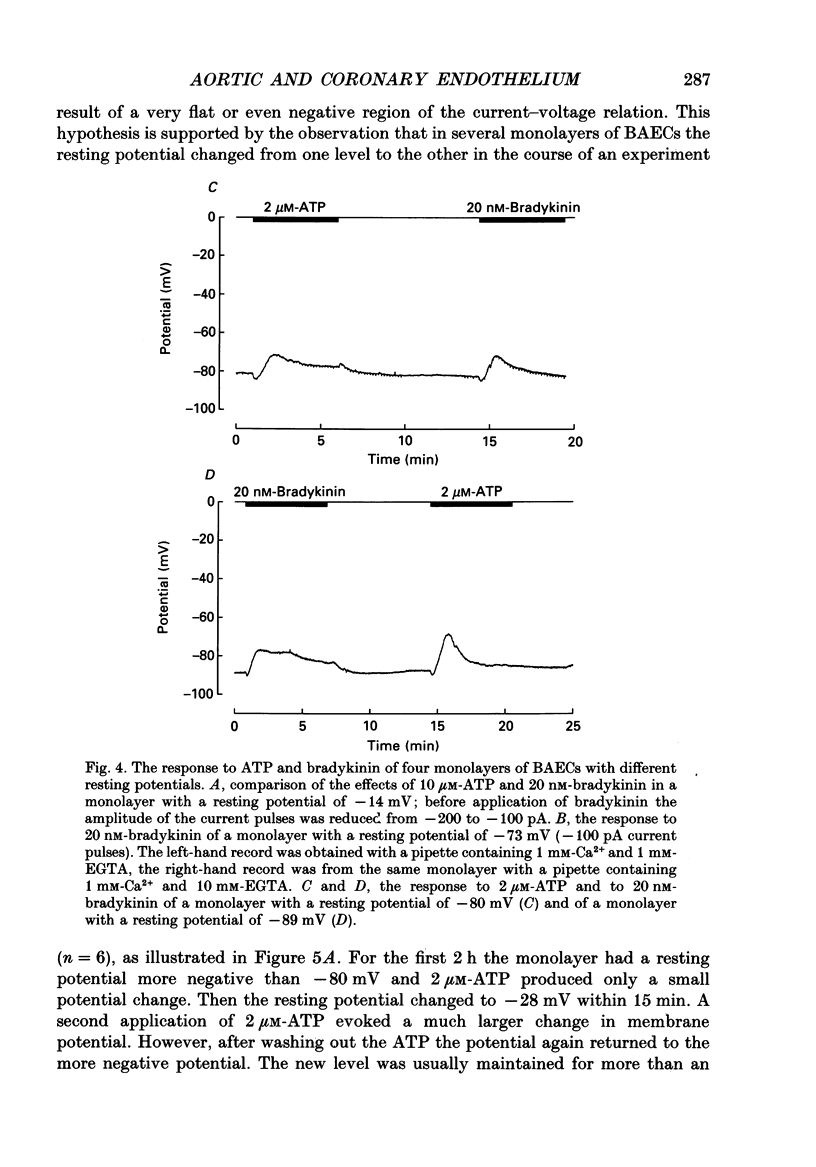
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Barakeh J., Laskey R., Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989 Oct;3(12):2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
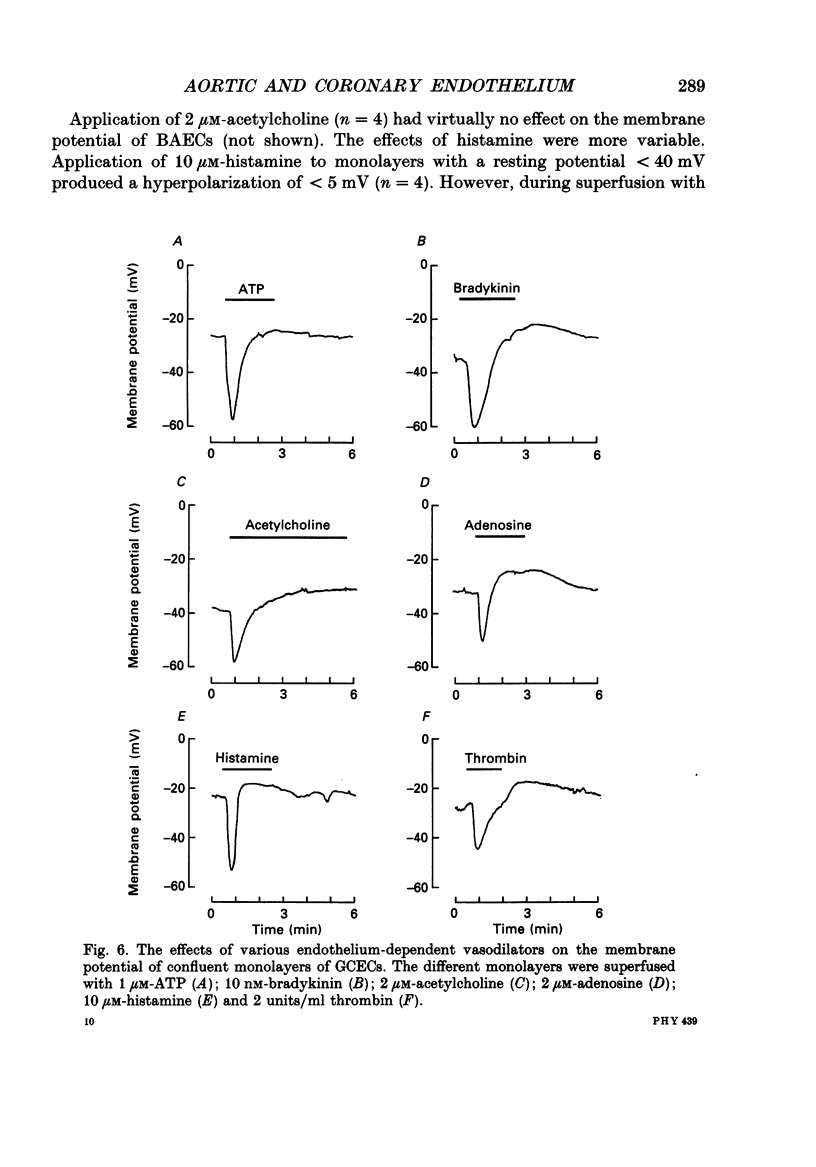
- Alheid U., Reichwehr I., Förstermann U. Human endothelial cells inhibit platelet aggregation by separately stimulating platelet cyclic AMP and cyclic GMP. Eur J Pharmacol. 1989 May 2;164(1):103–110. doi: 10.1016/0014-2999(89)90236-7. [DOI] [PubMed] [Google Scholar]
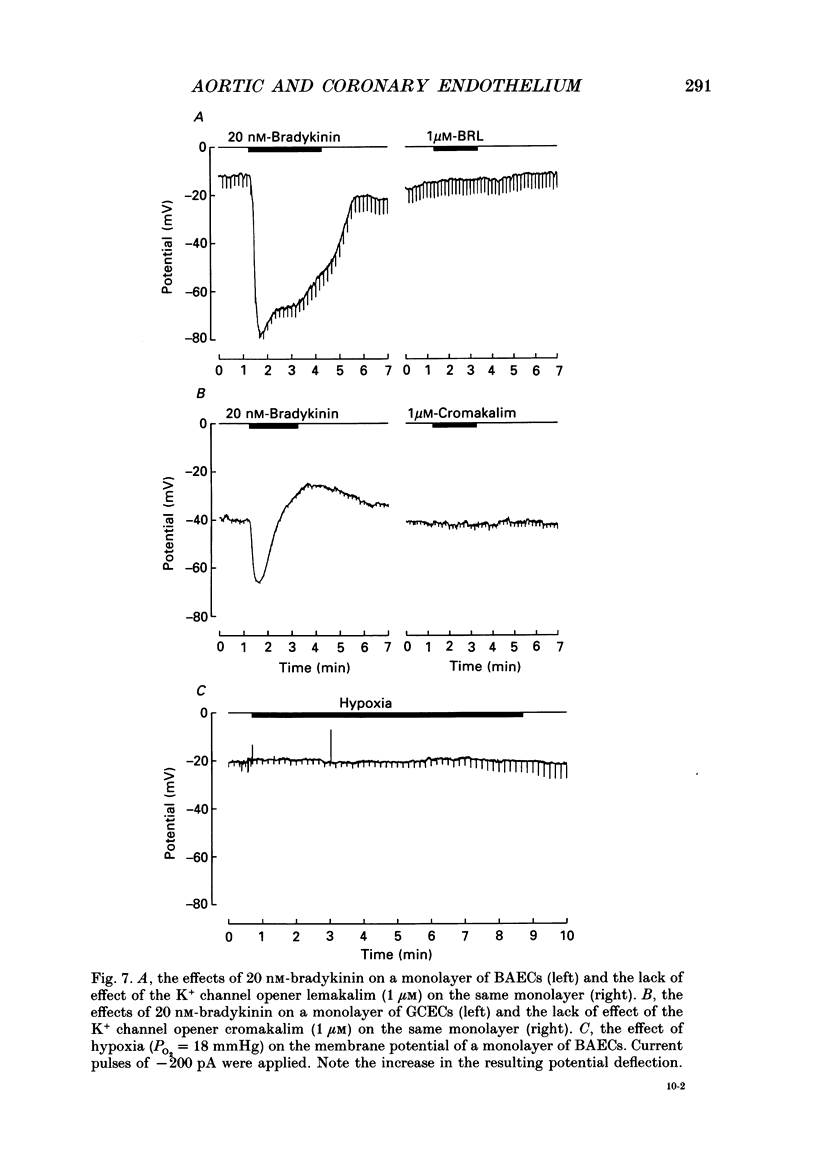
- Burnstock G., Kennedy C. A dual function for adenosine 5'-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986 Mar;58(3):319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Sage S. O. Bradykinin-evoked changes in cytosolic calcium and membrane currents in cultured bovine pulmonary artery endothelial cells. J Physiol. 1989 Dec;419:555–568. doi: 10.1113/jphysiol.1989.sp017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Possani L. D., Kunze D. L. Bradykinin-induced potassium current in cultured bovine aortic endothelial cells. J Membr Biol. 1990 Jul;116(3):227–238. doi: 10.1007/BF01868462. [DOI] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Daut J., Mehrke G., Nees S., Newman W. H. Passive electrical properties and electrogenic sodium transport of cultured guinea-pig coronary endothelial cells. J Physiol. 1988 Aug;402:237–254. doi: 10.1113/jphysiol.1988.sp017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decking U. K., Juengling E., Kammermeier H. Interstitial transudate concentration of adenosine and inosine in rat and guinea pig hearts. Am J Physiol. 1988 Jun;254(6 Pt 2):H1125–H1132. doi: 10.1152/ajpheart.1988.254.6.H1125. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Two levels of resting potential in cardiac Purkinje fibers. J Gen Physiol. 1977 Dec;70(6):725–746. doi: 10.1085/jgp.70.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Evidence that agonists stimulate bivalent-cation influx into human endothelial cells. Biochem J. 1988 Oct 1;255(1):179–184. doi: 10.1042/bj2550179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Influx of bivalent cations can be independent of receptor stimulation in human endothelial cells. Biochem J. 1989 Apr 1;259(1):125–129. doi: 10.1042/bj2590125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J Physiol. 1985 Sep;366:365–385. doi: 10.1113/jphysiol.1985.sp015803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., Johns A., Rubanyi G. M., van Breemen C. Membrane potential and Na(+)-K+ pump activity modulate resting and bradykinin-stimulated changes in cytosolic free calcium in cultured endothelial cells from bovine atria. J Biol Chem. 1990 Feb 15;265(5):2613–2619. [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R., Winter I., Bassenge E. Characterization of vascular relaxant factor released from cultured endothelial cells. Hypertension. 1987 Mar;9(3):295–303. doi: 10.1161/01.hyp.9.3.295. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Pohl U., Mülsch A., Busse R. Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988 Sep;95(1):189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G., Daut J. The electrical response of cultured guinea-pig coronary endothelial cells to endothelium-dependent vasodilators. J Physiol. 1990 Nov;430:251–272. doi: 10.1113/jphysiol.1990.sp018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Moncada S., Radomski M. W., Palmer R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988 Jul 1;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Bassenge E., Busse R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium-dependent and a calcium-independent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- Nees S., Herzog V., Becker B. F., Böck M., Des Rosiers Ch, Gerlach E. The coronary endothelium: a highly active metabolic barrier for adenosine. Basic Res Cardiol. 1985 Sep-Oct;80(5):515–529. doi: 10.1007/BF01907915. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Olesen S. P. An electrophysiological study of microvascular permeability and its modulation by chemical mediators. Acta Physiol Scand Suppl. 1989;579:1–28. [PubMed] [Google Scholar]
- Olesen S. P., Davies P. F., Clapham D. E. Muscarinic-activated K+ current in bovine aortic endothelial cells. Circ Res. 1988 Jun;62(6):1059–1064. doi: 10.1161/01.res.62.6.1059. [DOI] [PubMed] [Google Scholar]
- Olsson R. A., Bünger R. Metabolic control of coronary blood flow. Prog Cardiovasc Dis. 1987 Mar-Apr;29(5):369–387. doi: 10.1016/0033-0620(87)90003-x. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Adams D. J., van Breemen C. Synchronized oscillations in cytoplasmic free calcium concentration in confluent bradykinin-stimulated bovine pulmonary artery endothelial cell monolayers. J Biol Chem. 1989 Jan 5;264(1):6–9. [PubMed] [Google Scholar]
- Sauve R., Parent L., Simoneau C., Roy G. External ATP triggers a biphasic activation process of a calcium-dependent K+ channel in cultured bovine aortic endothelial cells. Pflugers Arch. 1988 Oct;412(5):469–481. doi: 10.1007/BF00582535. [DOI] [PubMed] [Google Scholar]
- Schilling W. P. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. Am J Physiol. 1989 Sep;257(3 Pt 2):H778–H784. doi: 10.1152/ajpheart.1989.257.3.H778. [DOI] [PubMed] [Google Scholar]
- Schilling W. P., Ritchie A. K., Navarro L. T., Eskin S. G. Bradykinin-stimulated calcium influx in cultured bovine aortic endothelial cells. Am J Physiol. 1988 Aug;255(2 Pt 2):H219–H227. doi: 10.1152/ajpheart.1988.255.2.H219. [DOI] [PubMed] [Google Scholar]
- Takeda K., Schini V., Stoeckel H. Voltage-activated potassium, but not calcium currents in cultured bovine aortic endothelial cells. Pflugers Arch. 1987 Nov;410(4-5):385–393. doi: 10.1007/BF00586515. [DOI] [PubMed] [Google Scholar]


