Abstract
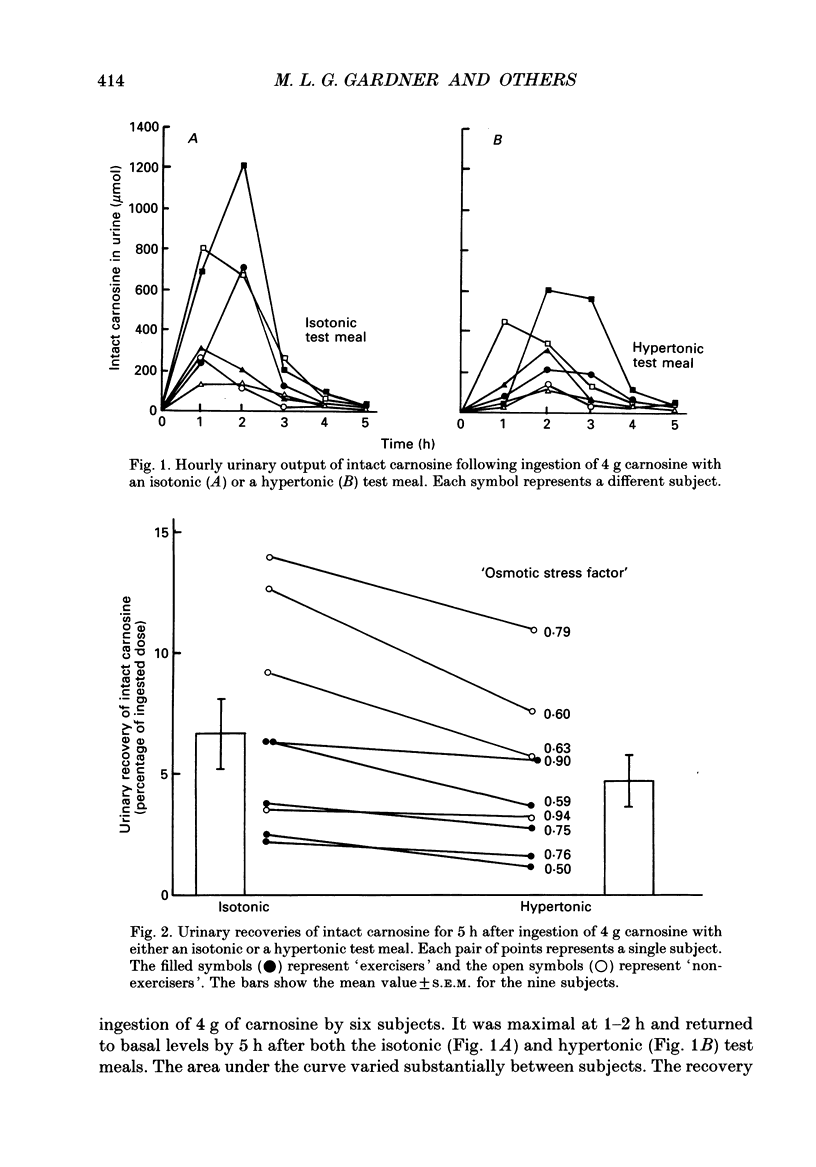
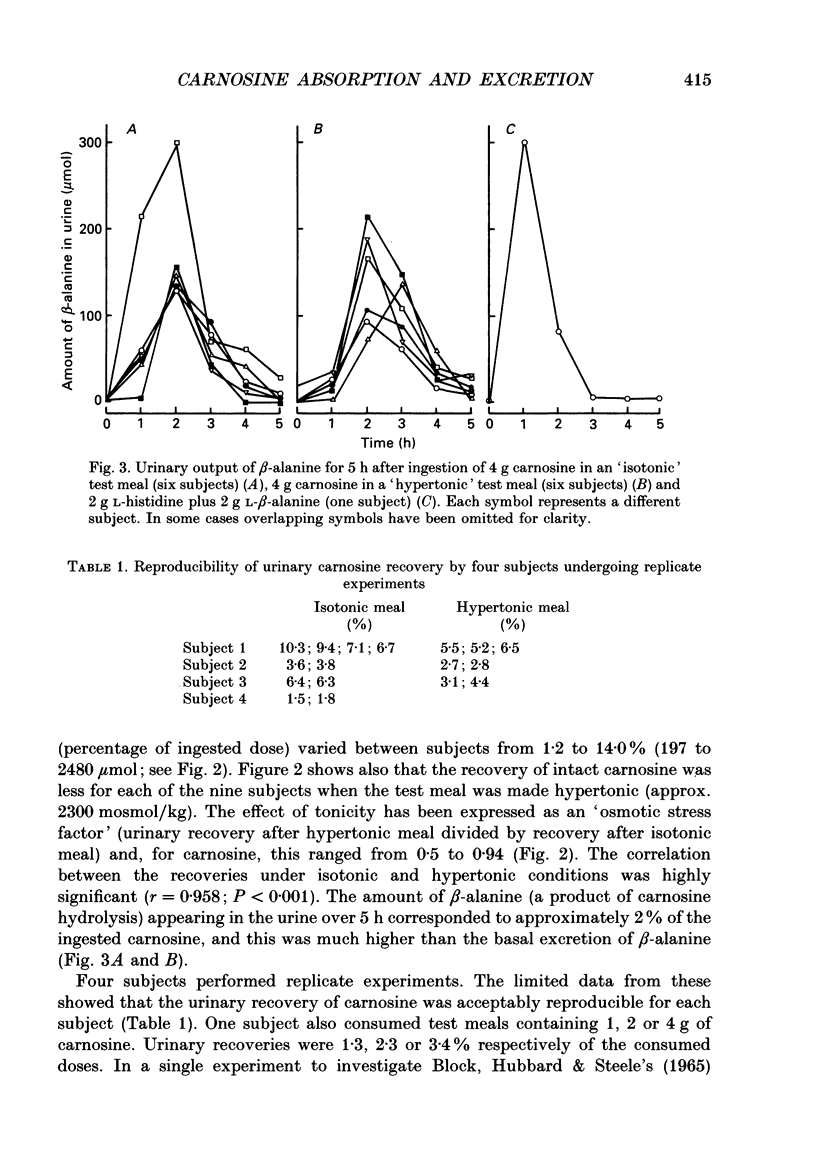
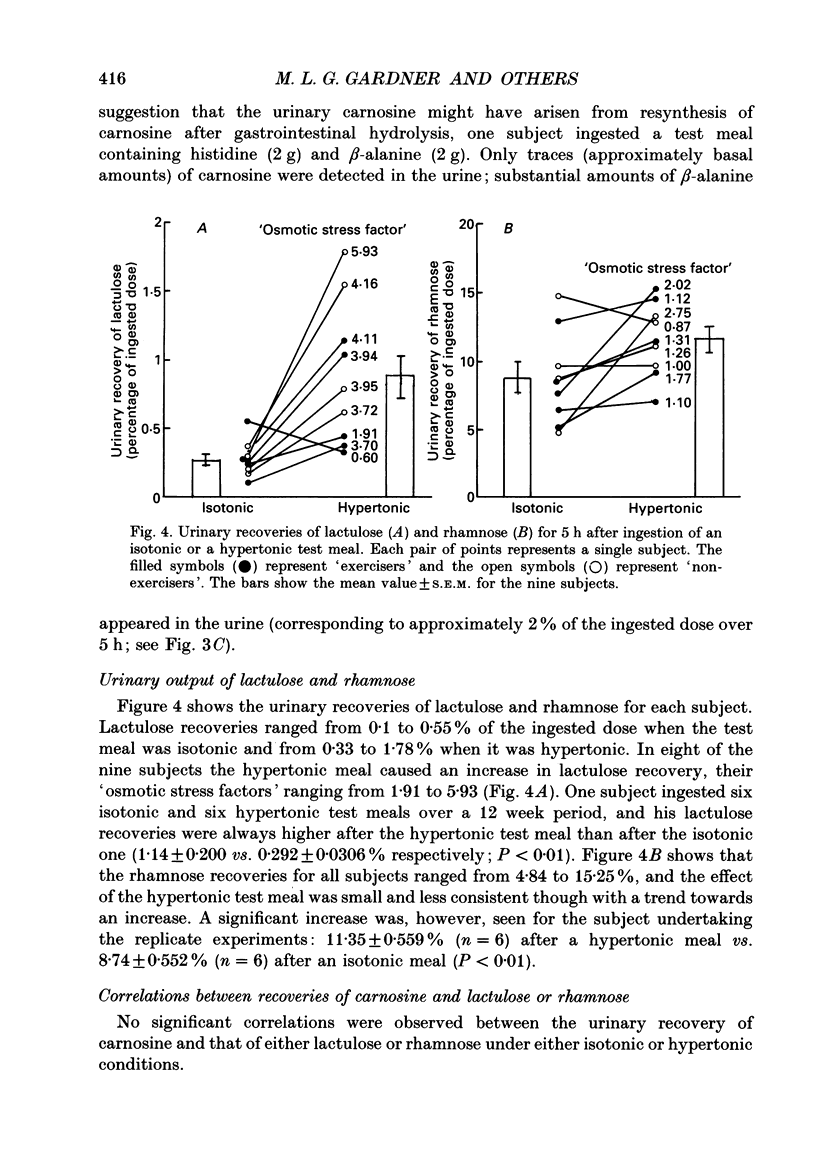
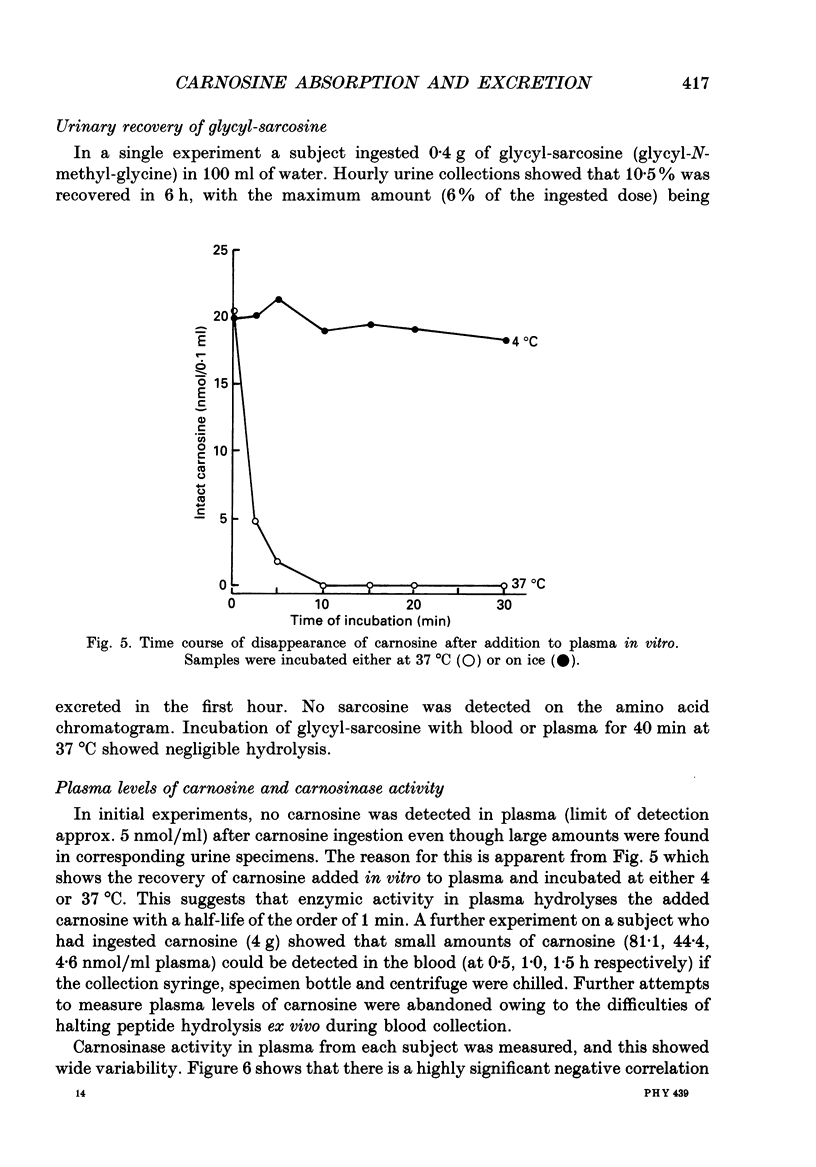
1. Healthy humans ingested the dipeptide carnosine (L-beta-alanyl-L-histidine). Their plasma levels and urinary outputs of carnosine and beta-alanine were monitored over the following 5 h. 2. Large amounts of intact carnosine (up to 14% of the ingested dose) were recovered in the urine over the 5 h after ingestion. However, carnosine was undetectable in the plasma unless precautions were taken to inhibit blood carnosinase activity ex vivo during and after blood collection. 3. The amount of carnosine recovered in urine varied substantially between subjects. It correlated negatively with carnosinase enzymic activity in the plasma. Highest carnosinase activities were observed in those subjects who regularly underwent physical training. 4. Urinary recovery of the disaccharide lactulose also varied considerably between subjects, but was substantially lower than that of carnosine. There was no significant correlation between the recoveries of carnosine and lactulose. 5. When lactulose was ingested with a hypertonic solution, the urinary recovery of lactulose was generally increased. When carnosine was ingested with a hypertonic solution, the urinary recovery of carnosine was reduced: hence the paracellular route probably is not dominant for absorption of intact carnosine. 6. Intact carnosine must have crossed the intestine to an extent much greater than hitherto recognized. Rapid post-absorptive hydrolysis is a severe obstacle to quantification of intact peptide absorption.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison J. M., Burston D., Matthews D. M. Evidence for active transport of the dipeptide glycylsarcosine by hamster jejunum in vitro. Clin Sci. 1972 Dec;43(6):907–911. doi: 10.1042/cs0430907. [DOI] [PubMed] [Google Scholar]
- Asatoor A. M., Bandoh J. K., Lant A. F., Milne M. D., Navab F. Intestinal absorption of carnosine and its constituent amino acids in man. Gut. 1970 Mar;11(3):250–254. doi: 10.1136/gut.11.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOCK W. D., HUBBARD R. W., STEELE B. F. EXCRETION OF HISTIDINE AND HISTIDINE DERIVATIVES BY HUMAN SUBJECTS INGESTING PROTEIN FROM DIFFERENT SOURCES. J Nutr. 1965 Apr;85:419–425. doi: 10.1093/jn/85.4.419. [DOI] [PubMed] [Google Scholar]
- Bando K., Ichihara K., Shimotsuji T., Toyoshima H., Koda K., Hayashi C., Miyai K. Reduced serum carnosinase activity in hypothyroidism. Ann Clin Biochem. 1986 Mar;23(Pt 2):190–194. doi: 10.1177/000456328602300208. [DOI] [PubMed] [Google Scholar]
- Bando K., Shimotsuji T., Toyoshima H., Hayashi C., Miyai K. Fluorometric assay of human serum carnosinase activity in normal children, adults and patients with myopathy. Ann Clin Biochem. 1984 Nov;21(Pt 6):510–514. doi: 10.1177/000456328402100613. [DOI] [PubMed] [Google Scholar]
- Duane P., Peters T. J. Serum carnosinase activities in patients with alcoholic chronic skeletal muscle myopathy. Clin Sci (Lond) 1988 Aug;75(2):185–190. doi: 10.1042/cs0750185. [DOI] [PubMed] [Google Scholar]
- Dyer J., Beechey R. B., Gorvel J. P., Smith R. T., Wootton R., Shirazi-Beechey S. P. Glycyl-L-proline transport in rabbit enterocyte basolateral-membrane vesicles. Biochem J. 1990 Aug 1;269(3):565–571. doi: 10.1042/bj2690565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris R. P., Diamond J., Kwan W. W. Dietary regulation of intestinal transport of the dipeptide carnosine. Am J Physiol. 1988 Aug;255(2 Pt 1):G143–G150. doi: 10.1152/ajpgi.1988.255.2.G143. [DOI] [PubMed] [Google Scholar]
- Fuessl H. S., Domin J., Bloom S. R. Oral absorption of the somatostatin analogue SMS 201-995: theoretical and practical implications. Clin Sci (Lond) 1987 Feb;72(2):255–257. doi: 10.1042/cs0720255. [DOI] [PubMed] [Google Scholar]
- Gardner M. L. Intestinal assimilation of intact peptides and proteins from the diet--a neglected field? Biol Rev Camb Philos Soc. 1984 Aug;59(3):289–331. doi: 10.1111/j.1469-185x.1984.tb00708.x. [DOI] [PubMed] [Google Scholar]
- Gardner M. L., Wood D. Transport of peptides across the gastrointestinal tract. Biochem Soc Trans. 1989 Oct;17(5):934–937. doi: 10.1042/bst0170934. [DOI] [PubMed] [Google Scholar]
- Hama T., Tamaki N., Miyamoto F., Kita M., Tsunemori F. Intestinal absorption of beta-alanine, anserine and carnosine in rats. J Nutr Sci Vitaminol (Tokyo) 1976;22(2):147–157. doi: 10.3177/jnsv.22.147. [DOI] [PubMed] [Google Scholar]
- Laker M. F., Menzies I. S. Increase in human intestinal permeability following ingestion of hypertonic solutions. J Physiol. 1977 Mar;265(3):881–894. doi: 10.1113/jphysiol.1977.sp011750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. M., Addison J. M., Burston D. Evidence for active transport of the dipeptide carnosine (beta-alanyl-L-histidine) by hamster jejunum in vitro. Clin Sci Mol Med. 1974 Jun;46(6):693–705. doi: 10.1042/cs0460693. [DOI] [PubMed] [Google Scholar]
- Matthews D. M. Intestinal absorption of peptides. Physiol Rev. 1975 Oct;55(4):537–608. doi: 10.1152/physrev.1975.55.4.537. [DOI] [PubMed] [Google Scholar]
- Maxton D. G., Bjarnason I., Reynolds A. P., Catt S. D., Peters T. J., Menzies I. S. Lactulose, 51Cr-labelled ethylenediaminetetra-acetate, L-rhamnose and polyethyleneglycol 400 [corrected] as probe markers for assessment in vivo of human intestinal permeability. Clin Sci (Lond) 1986 Jul;71(1):71–80. doi: 10.1042/cs0710071. [DOI] [PubMed] [Google Scholar]
- Perry T. L., Hansen S., Tischler B., Bunting R., Berry K. Carnosinemia. A new metabolic disorder associated with neurologic disease and mental defect. N Engl J Med. 1967 Dec 7;277(23):1219–1227. doi: 10.1056/NEJM196712072772302. [DOI] [PubMed] [Google Scholar]
- Sadikali F., Darwish R., Watson W. C. Carnosinase activity of human gastrointestinal mucosa. Gut. 1975 Aug;16(8):585–589. doi: 10.1136/gut.16.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler P. G., Menzies I. S., Creamer B. Effect of hyperosmolar stimuli and coeliac disease on the permeability of the human gastrointestinal tract. Clin Sci Mol Med. 1978 May;54(5):495–501. doi: 10.1042/cs0540495. [DOI] [PubMed] [Google Scholar]
- Zioudrou C., Streaty R. A., Klee W. A. Opioid peptides derived from food proteins. The exorphins. J Biol Chem. 1979 Apr 10;254(7):2446–2449. [PubMed] [Google Scholar]
- van Hoogdalem E. J., de Boer A. G., Breimer D. D. Intestinal drug absorption enhancement: an overview. Pharmacol Ther. 1989;44(3):407–443. doi: 10.1016/0163-7258(89)90009-0. [DOI] [PubMed] [Google Scholar]


