Abstract
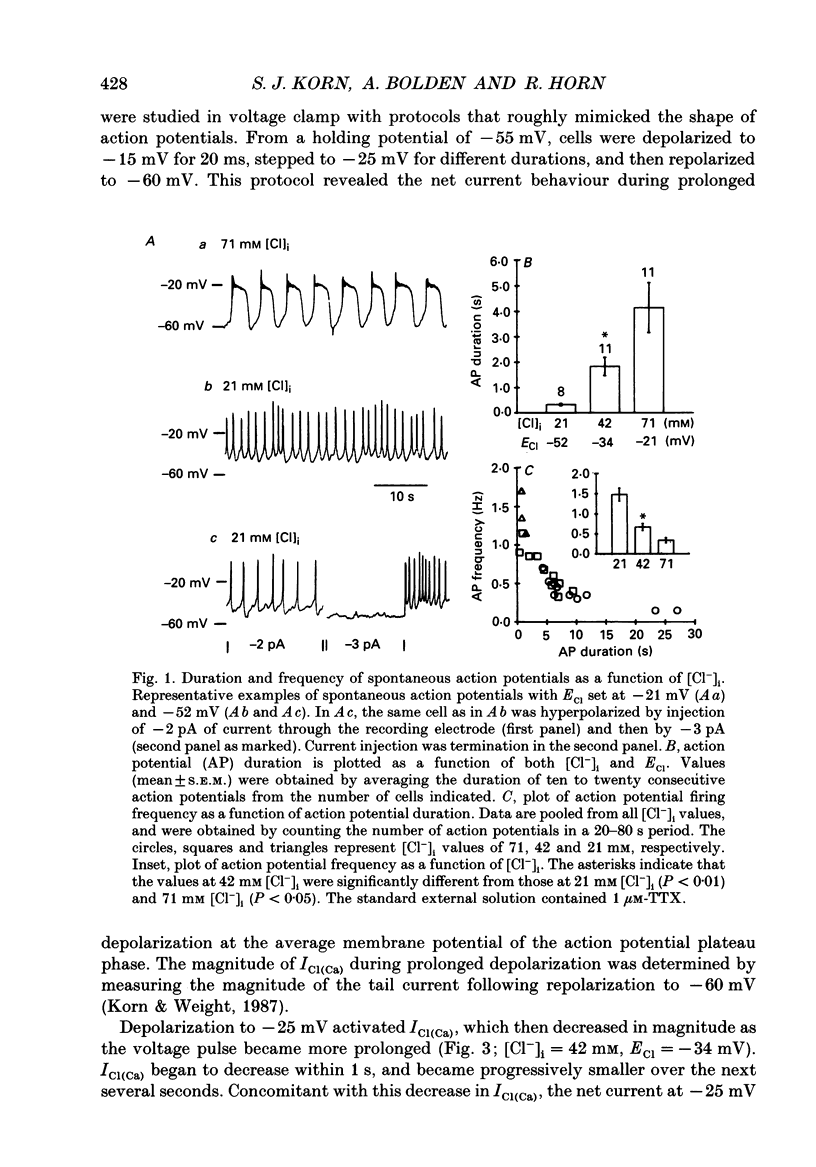
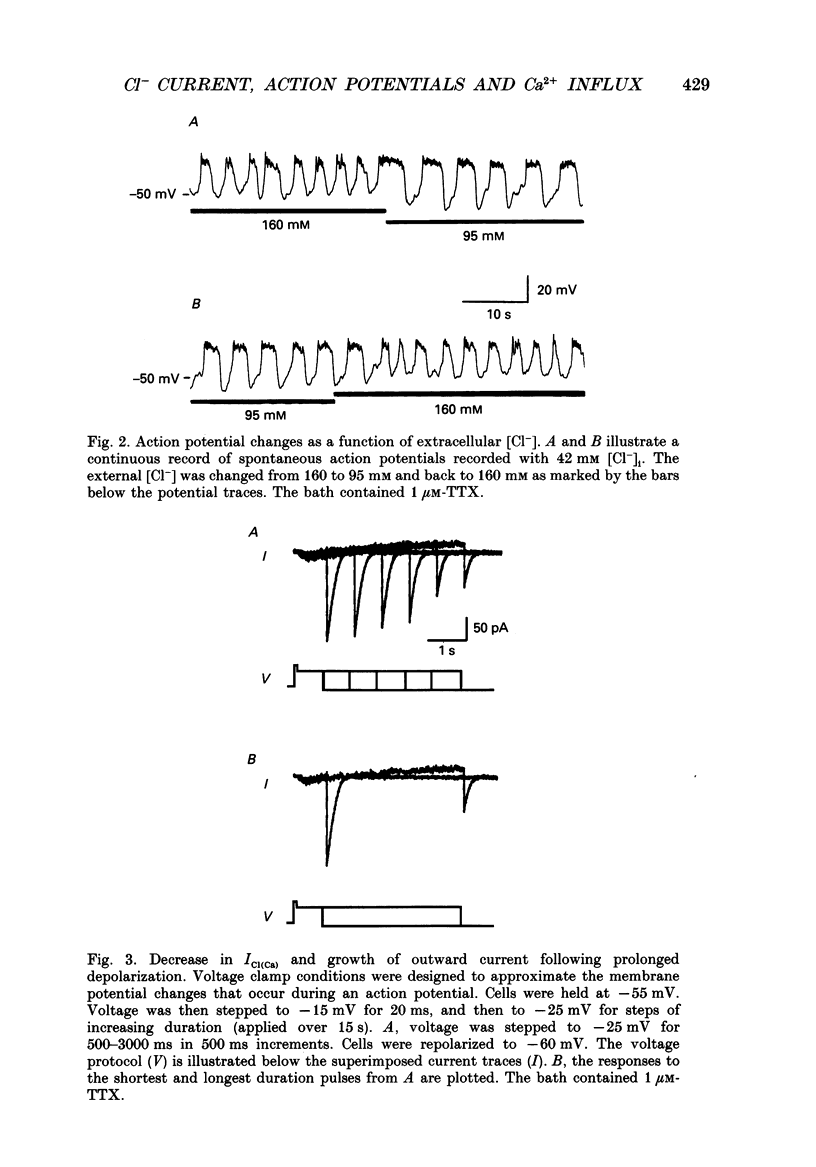
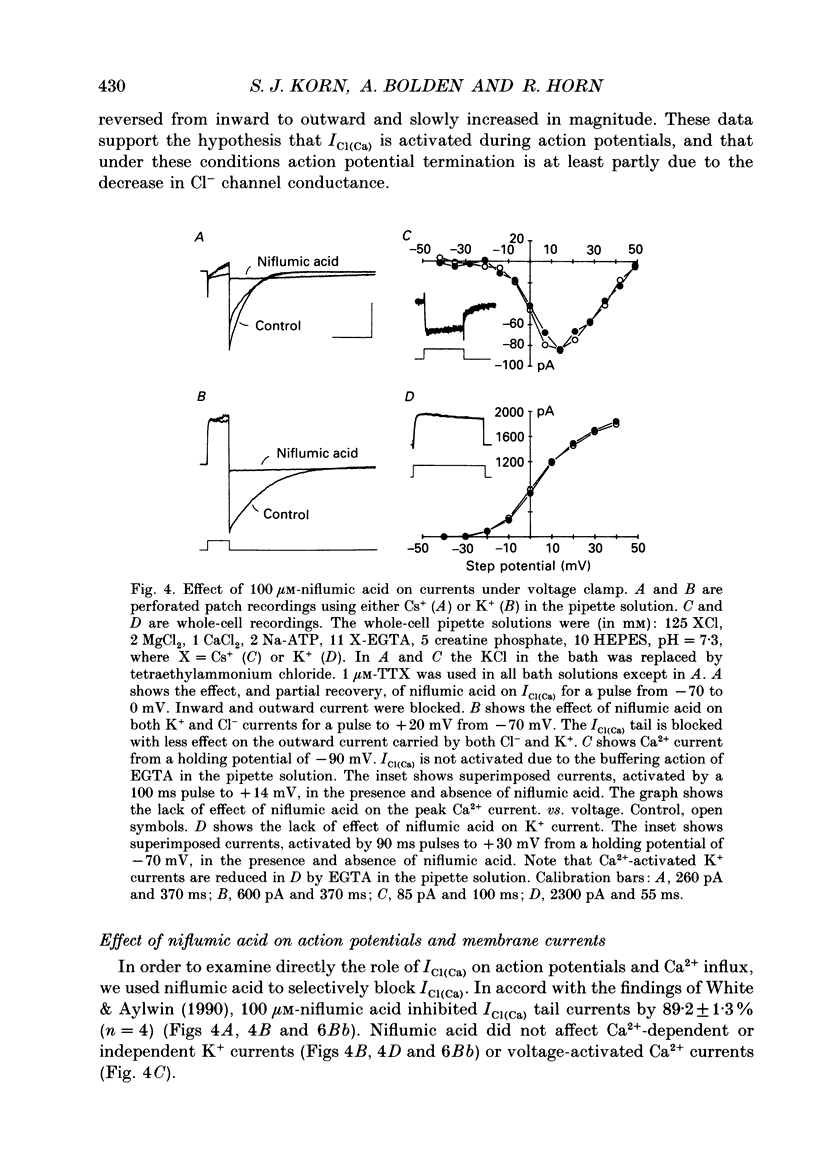
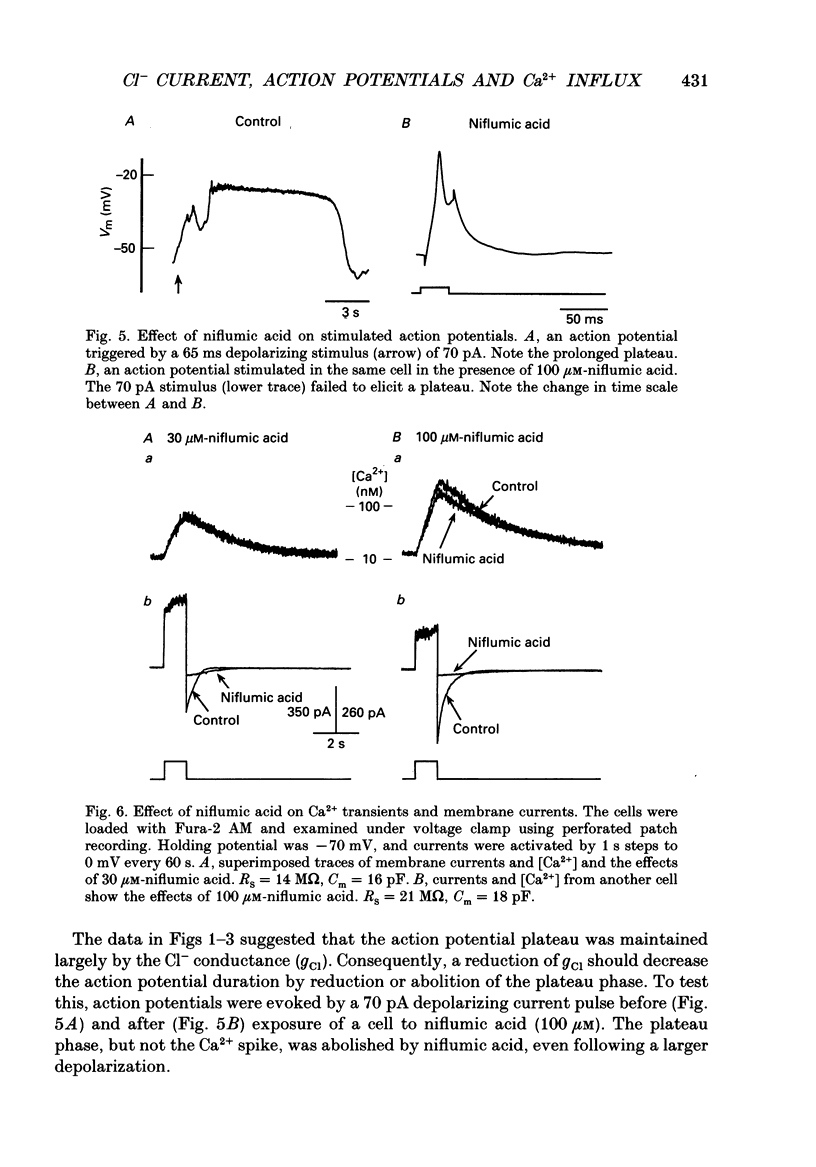
1. Perforated patch recording was used to examine the influence of the calcium-dependent chloride current (iCl(Ca)) on Ca2+ action potentials in AtT-20 pituitary cells. The calculated chloride equilibrium potential (ECl) was adjusted by changing either intracellular or extracellular [Cl-]. Action potential duration varied as a function of ECl. When ECl was set at -21 mV, both spontaneous and evoked action potentials displayed a long plateau phase between -20 and -25 mV, which typically lasted for several seconds. Setting ECl to more negative potentials resulted in briefer action potentials; at an ECl of -52 mV, no plateau phase was evident. Spontaneous depolarization and action potential firing still occurred when ECl was negative to firing threshold, which indicates that the slow depolarizing wave that precedes the firing of spontaneous action potentials does not require activation of ICl(Ca). 2. In voltage clamp experiments the magnitude of ICl(Ca) diminished slowly during a prolonged depolarization, over a time course that coincided with action potential termination. 3. Niflumic acid (100 microM) blocked ICl(Ca) by 90% but had no effect on either K+ or Ca2+ currents. This concentration of niflumic acid eliminated the plateau phase, but did not prevent the firing, of Ca2+ action potentials. 4. Internal [Ca2+] was measured photometrically after loading cells with the Ca2+ indicator dye, Fura-2. Under voltage clamp conditions, concentrations of niflumic acid (30-100 microM) that blocked depolarization-evoked ICl(Ca) had little or no effect on simultaneously recorded Ca2+ transients. Perforated patch recording from Fura-loaded cells showed that action potentials were temporally associated with transient increases in intracellular [Ca2+]. Niflumic acid (30-100 microM) disrupted the rhythmic firing of spontaneous action potentials and associated intracellular Ca2+ transients. 5. Fluorescent measurements of Ca2+ transients were also made in cells unperturbed by patch recording, and were used as a measure of action potential duration in the absence of experimental alteration of internal [Cl-]. Spontaneous Ca2+ transients were of long duration (approximately 2 s), which suggests that intracellular [Cl-] is relatively high (40-50 mM) in these cells. The spontaneous Ca2+ transients were inhibited by niflumic acid. 6. Niflumic acid up to 100 microM, had neglible effects on either basal or stimulated (by 2 microM-(+/-)-isoprenaline) hormone secretion, as shown by radioimmunoassay of adrenocortotrophic hormone release.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




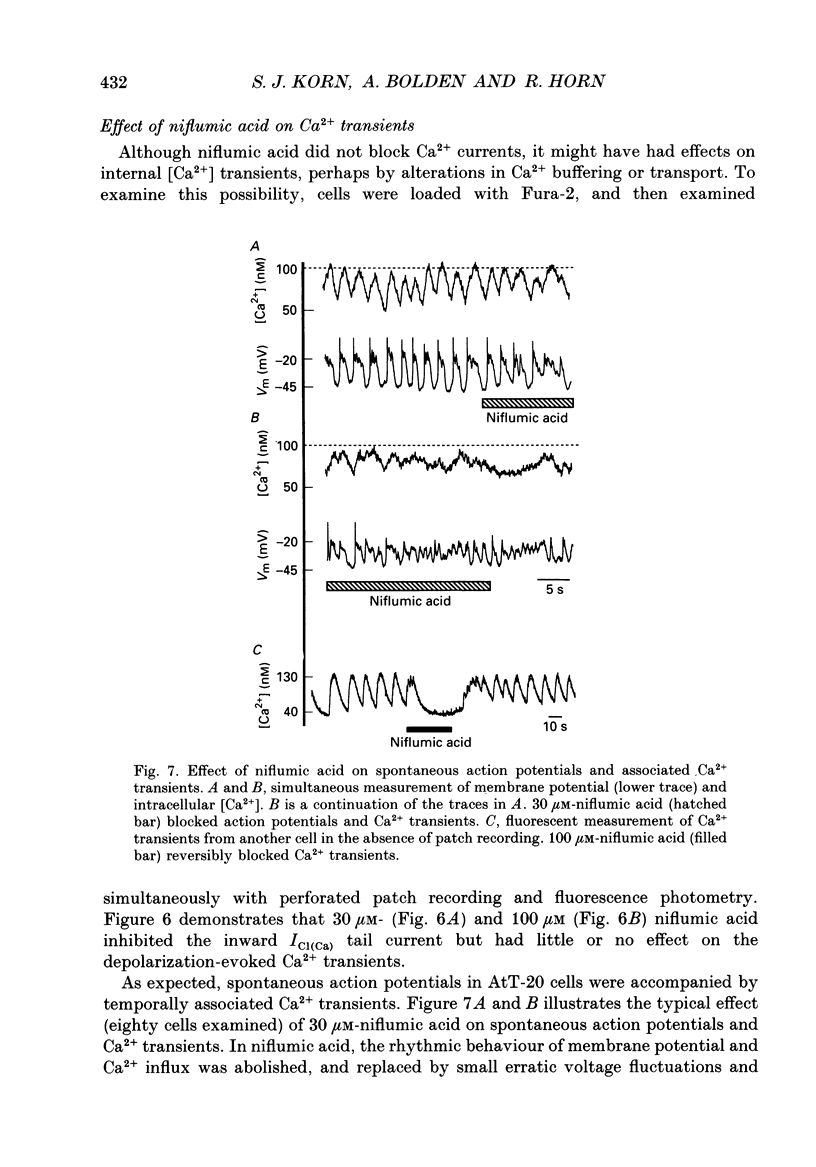

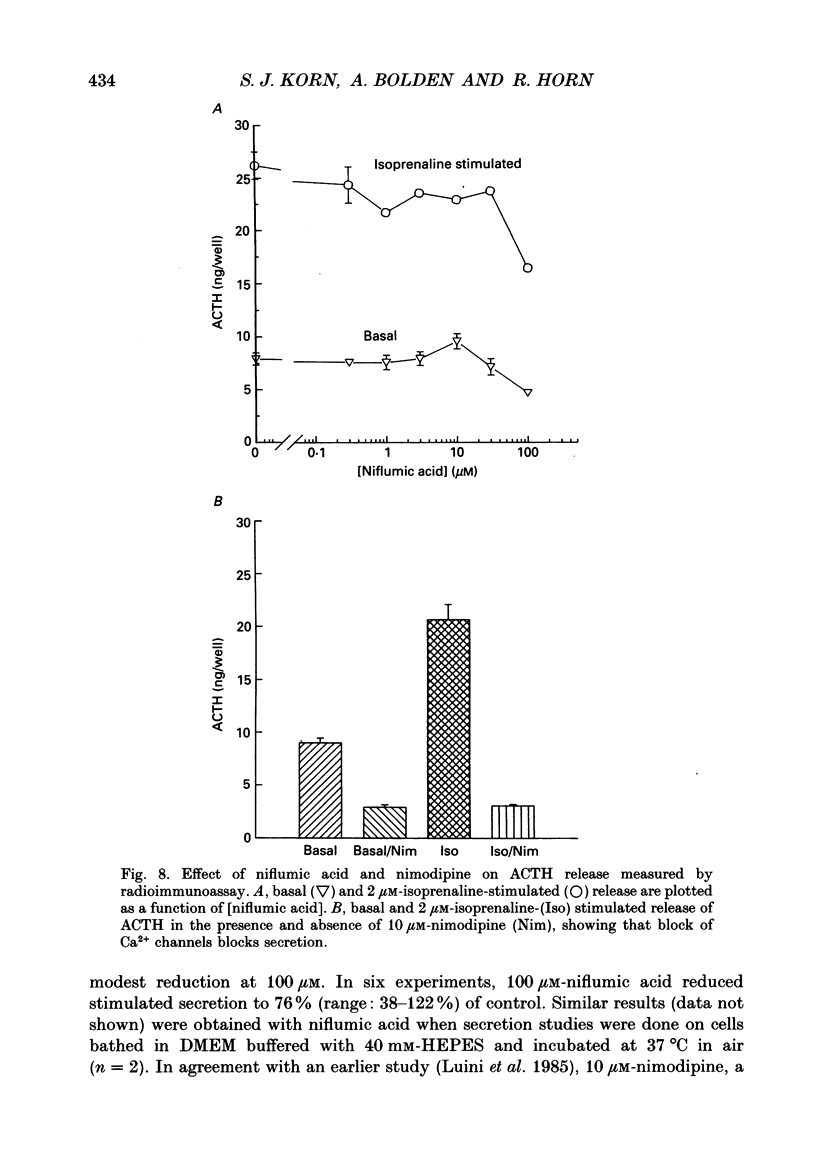



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler M., Wong B. S., Sabol S. L., Busis N., Jackson M. B., Weight F. F. Action potentials and membrane ion channels in clonal anterior pituitary cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2086–2090. doi: 10.1073/pnas.80.7.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J., Reisine T. D. Stress hormones: their interaction and regulation. Science. 1984 May 4;224(4648):452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harvey R. D., Clark C. D., Hume J. R. Chloride current in mammalian cardiac myocytes. Novel mechanism for autonomic regulation of action potential duration and resting membrane potential. J Gen Physiol. 1990 Jun;95(6):1077–1102. doi: 10.1085/jgp.95.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holl R. W., Thorner M. O., Leong D. A. Intracellular calcium concentration and growth hormone secretion in individual somatotropes: effects of growth hormone-releasing factor and somatostatin. Endocrinology. 1988 Jun;122(6):2927–2932. doi: 10.1210/endo-122-6-2927. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R. I., Thomas S. A. A calcium- and voltage-dependent chloride current in developing chick skeletal muscle. J Physiol. 1989 Oct;417:241–261. doi: 10.1113/jphysiol.1989.sp017799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y. Spontaneous calcium action potentials in a clonal pituitary cell line and their relationship to prolactin secretion. Nature. 1975 Dec 25;258(5537):741–742. doi: 10.1038/258741a0. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Giacchino J. L., Chamberlin N. L., Dingledine R. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol. 1987 Jan;57(1):325–340. doi: 10.1152/jn.1987.57.1.325. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Influence of sodium-calcium exchange on calcium current rundown and the duration of calcium-dependent chloride currents in pituitary cells, studied with whole cell and perforated patch recording. J Gen Physiol. 1989 Nov;94(5):789–812. doi: 10.1085/jgp.94.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Nordihydroguaiaretic acid inhibits voltage-activated Ca2+ currents independently of lipoxygenase inhibition. Mol Pharmacol. 1990 Oct;38(4):524–530. [PubMed] [Google Scholar]
- Luini A., Lewis D., Guild S., Corda D., Axelrod J. Hormone secretagogues increase cytosolic calcium by increasing cAMP in corticotropin-secreting cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8034–8038. doi: 10.1073/pnas.82.23.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Chloride conductance activated by external agonists and internal messengers in rat peritoneal mast cells. J Physiol. 1989 Nov;418:131–144. doi: 10.1113/jphysiol.1989.sp017831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollard P., Vacher P., Guerin J., Rogawski M. A., Dufy B. Electrical properties of cultured human adrenocorticotropin-secreting adenoma cells: effects of high K+, corticotropin-releasing factor, and angiotensin II. Endocrinology. 1987 Jul;121(1):395–405. doi: 10.1210/endo-121-1-395. [DOI] [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. A Ca-dependent Cl- conductance in cultured mouse spinal neurones. Nature. 1984 Oct 11;311(5986):567–570. doi: 10.1038/311567a0. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Srikant C. B. Somatostatin mediation of adenohypophysial secretion. Annu Rev Physiol. 1986;48:551–567. doi: 10.1146/annurev.ph.48.030186.003003. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Action potentials occur in cells of the normal anterior pituitary gland and are stimulated by the hypophysiotropic peptide thyrotropin-releasing hormone. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4064–4067. doi: 10.1073/pnas.74.9.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. M., Aylwin M. Niflumic and flufenamic acids are potent reversible blockers of Ca2(+)-activated Cl- channels in Xenopus oocytes. Mol Pharmacol. 1990 May;37(5):720–724. [PubMed] [Google Scholar]


